Nuclear Molecular Imaging of Disease Burden and Response to Treatment for Cardiac Amyloidosis
Abstract
Simple Summary
Abstract
1. Introduction
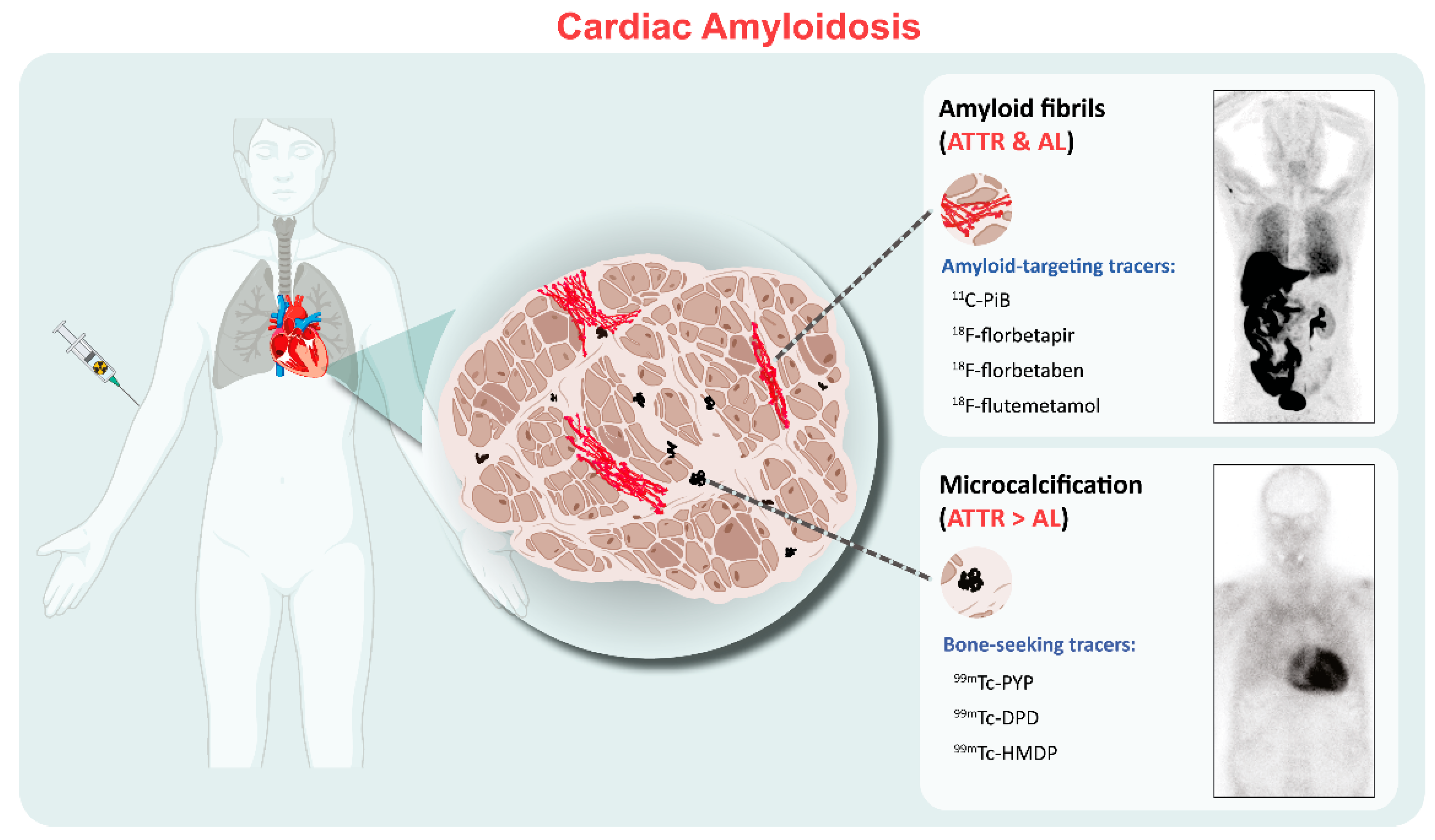
2. Binding Properties of Molecular Imaging in CA
3. Bone Scintigraphy
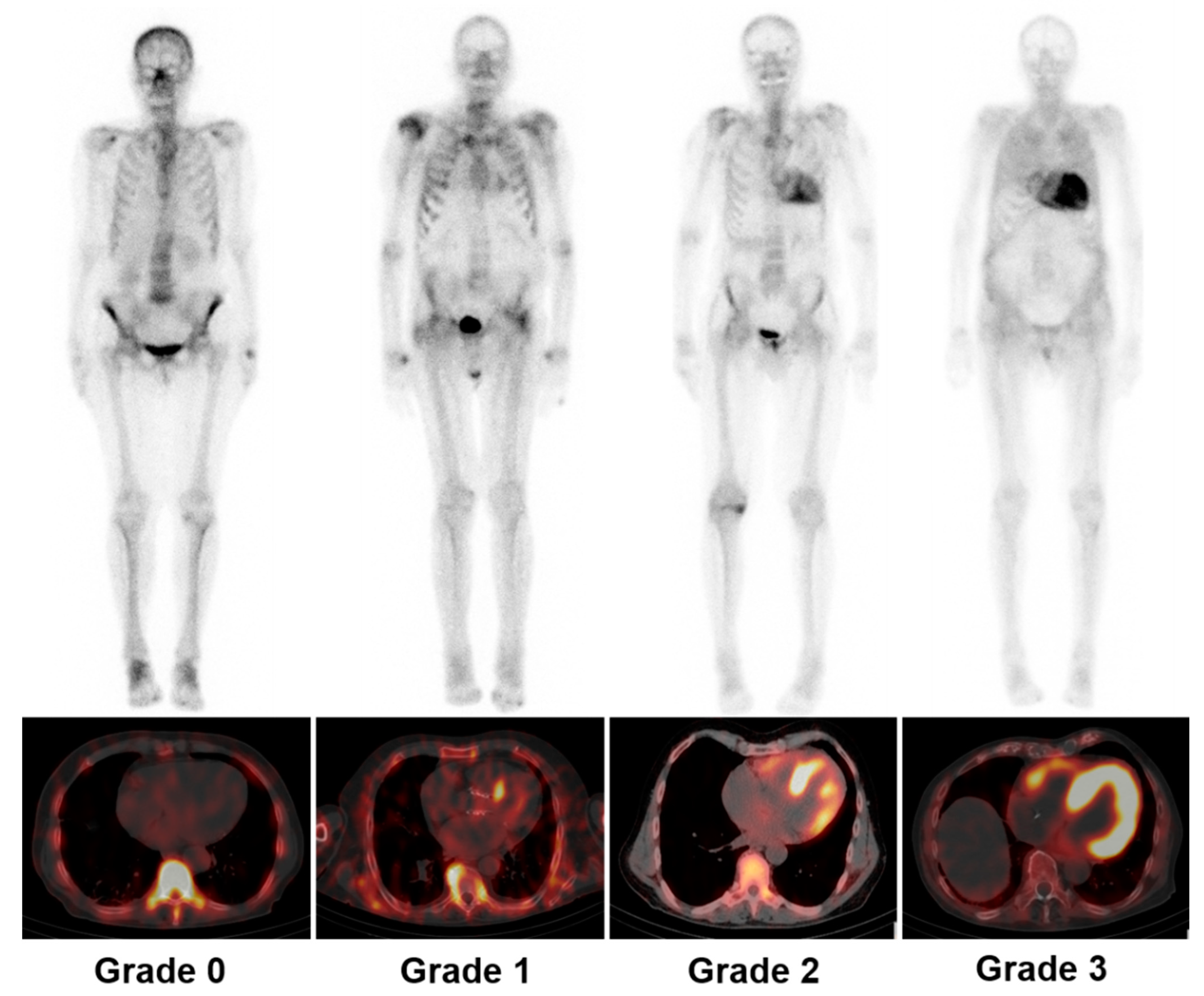
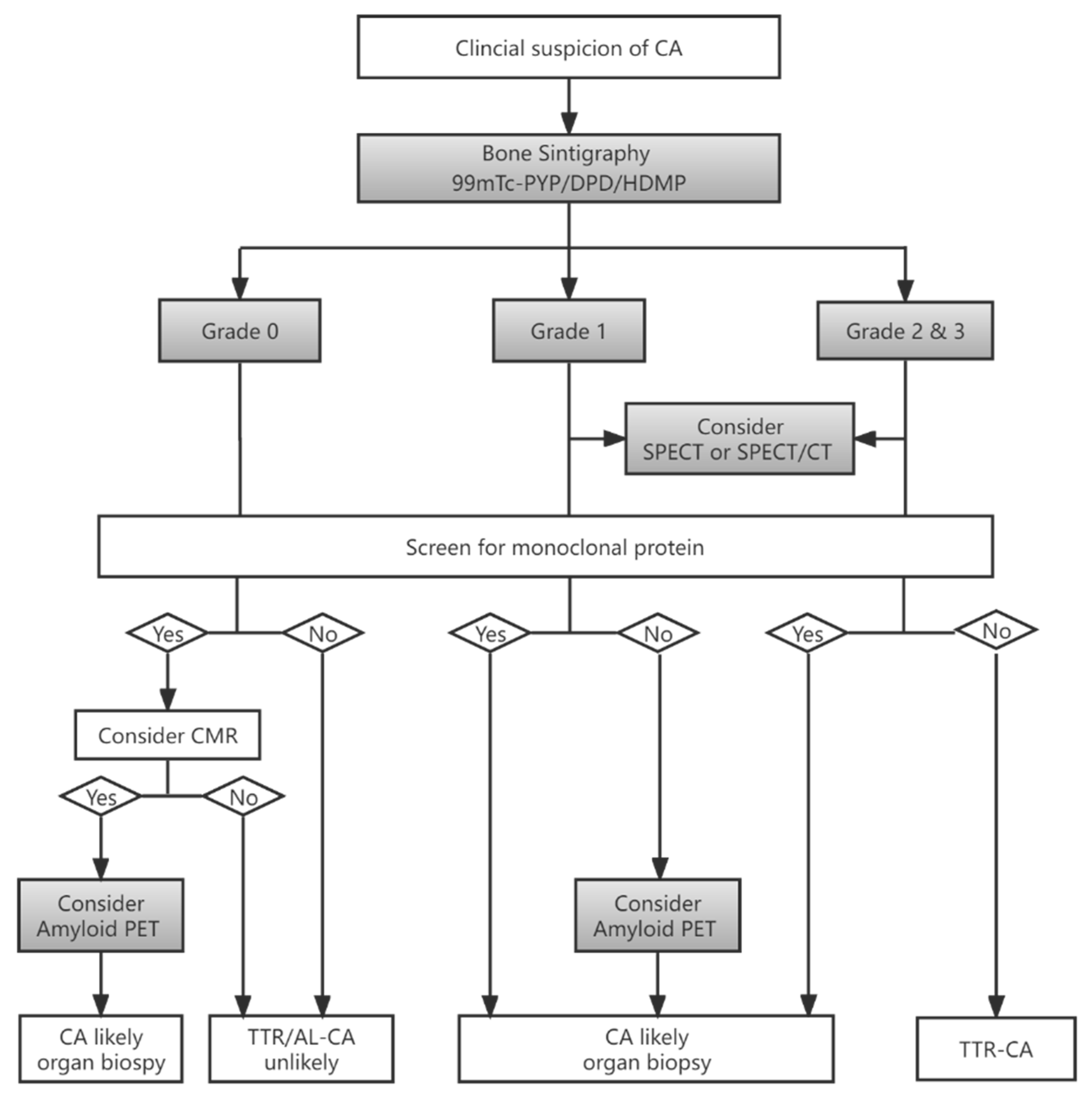
3.1. Diagnosis
3.2. Quantitative SPECT/CT
3.3. Differentiation between AL and ATTR by SPECT
3.4. Prognostic Value
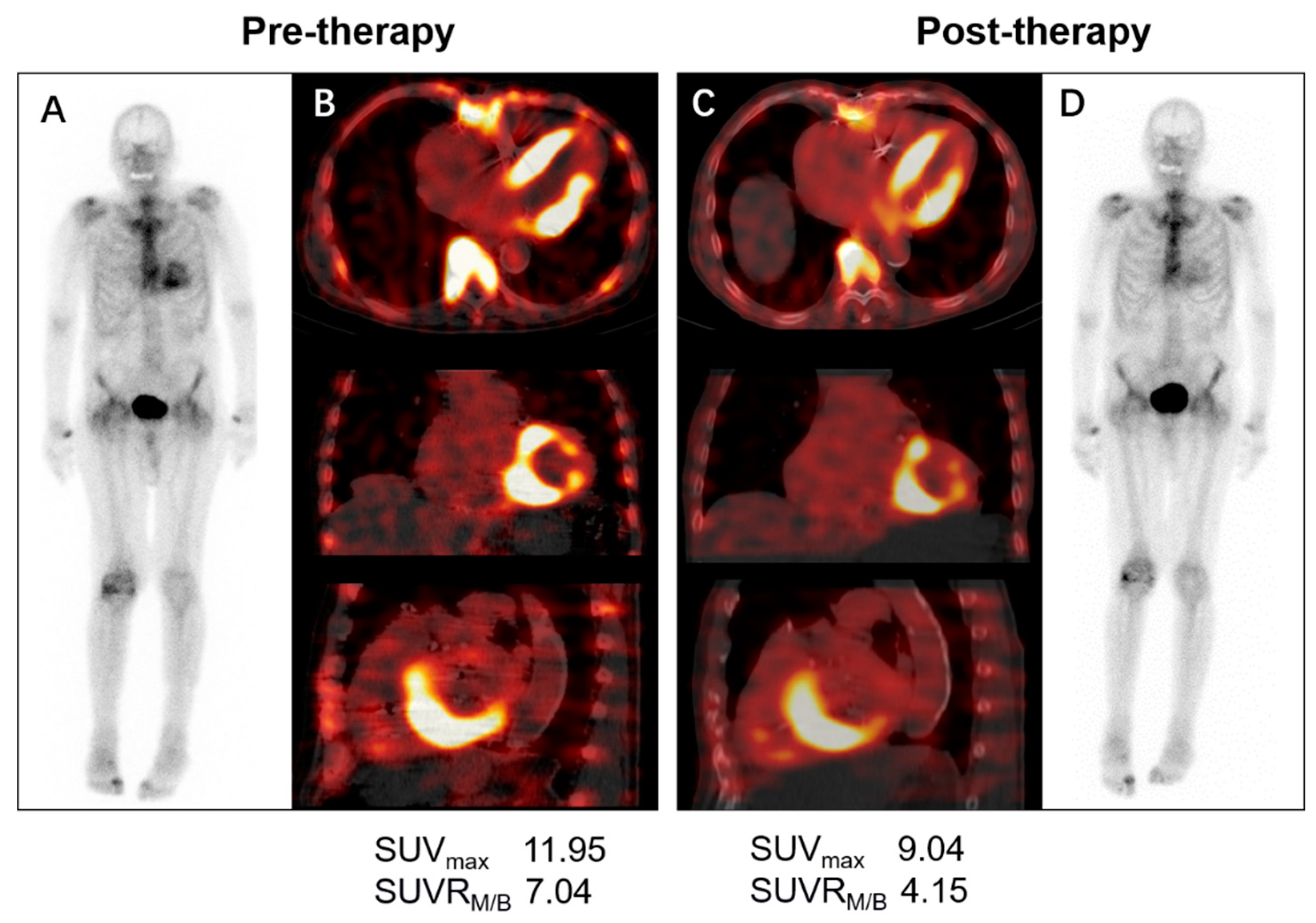
3.5. Monitoring Therapy Response
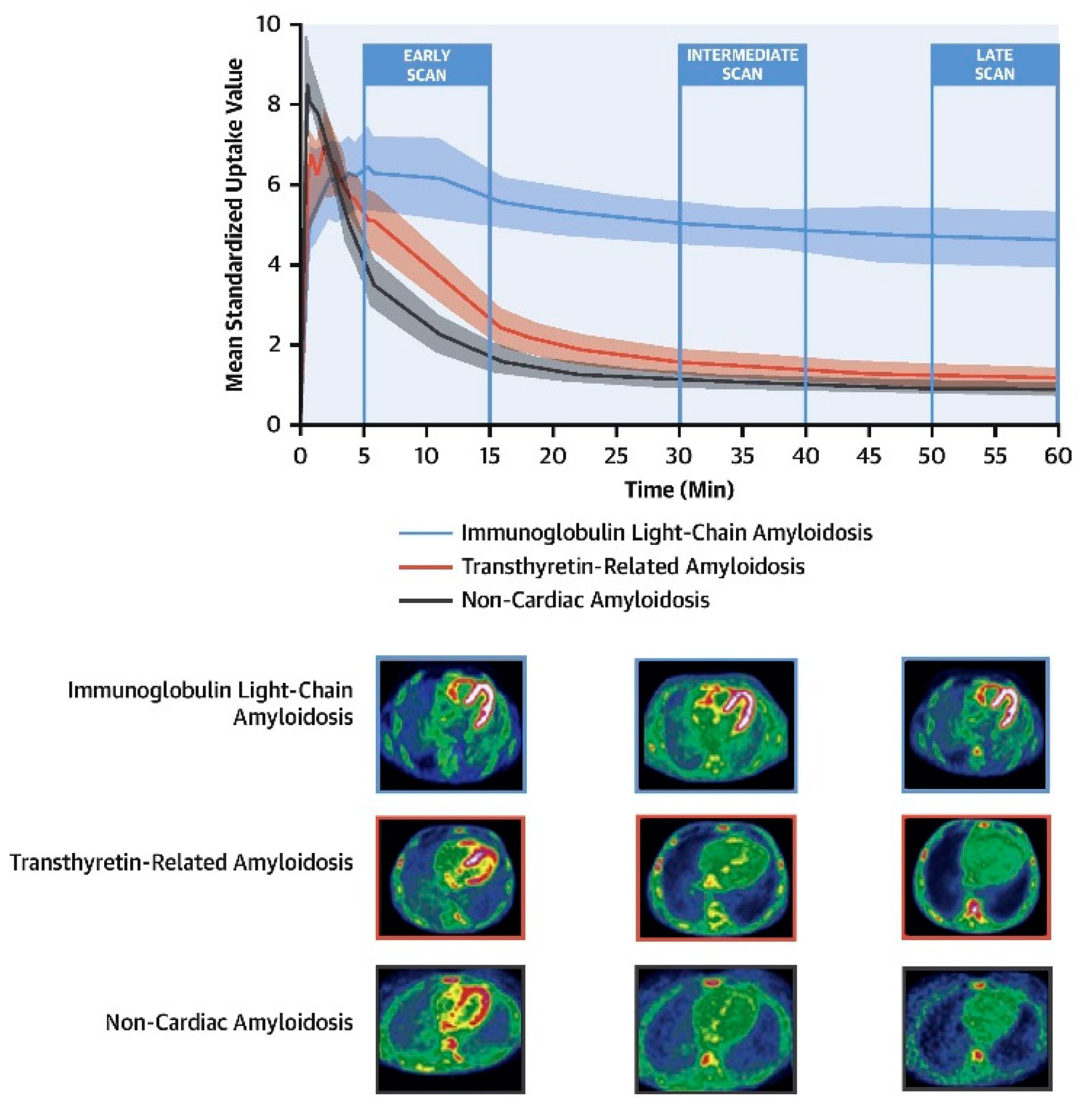
4. Amyloid-Targeting PET Imaging
4.1. Diagnosis
4.2. Differentiation between AL and ATTR by PET
4.3. Prognostic Value
4.4. Therapy Response Evaluation
5. Future Perspective and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maurer, M.S.; Elliott, P.; Comenzo, R.; Semigran, M.; Rapezzi, C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation 2017, 135, 1357–1377. [Google Scholar] [CrossRef]
- Bokhari, S.; Shahzad, R.; Castano, A.; Maurer, M.S. Nuclear imaging modalities for cardiac amyloidosis. J. Nucl. Cardiol. 2014, 21, 175–184. [Google Scholar] [CrossRef]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation 2007, 116, 2216–2233. [Google Scholar] [CrossRef]
- Yilmaz, A.; Kindermann, I.; Kindermann, M.; Mahfoud, F.; Ukena, C.; Athanasiadis, A.; Hill, S.; Mahrholdt, H.; Voehringer, M.; Schieber, M.; et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: Differences in complication rate and diagnostic performance. Circulation 2010, 122, 900–909. [Google Scholar] [CrossRef]
- Hasib Sidiqi, M.; Gertz, M.A. Immunoglobulin light chain amyloidosis diagnosis and treatment algorithm 2021. Blood Cancer J. 2021, 11, 90. [Google Scholar] [CrossRef]
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2-evidence base and standardized methods of imaging. J. Nucl. Cardiol. 2019, 26, 2065–2123. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef]
- Treglia, G.; Glaudemans, A.; Bertagna, F.; Hazenberg, B.P.C.; Erba, P.A.; Giubbini, R.; Ceriani, L.; Prior, J.O.; Giovanella, L.; Slart, R. Diagnostic accuracy of bone scintigraphy in the assessment of cardiac transthyretin-related amyloidosis: A bivariate meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1945–1955. [Google Scholar] [CrossRef]
- Yang, J.C.; Fox, J.; Chen, C.; Yu, A.F. Cardiac ATTR amyloid nuclear imaging-not all bone scintigraphy radionuclide tracers are created equal. J. Nucl. Cardiol. 2018, 25, 1879–1884. [Google Scholar] [CrossRef]
- Rapezzi, C.; Quarta, C.C.; Guidalotti, P.L.; Longhi, S.; Pettinato, C.; Leone, O.; Ferlini, A.; Salvi, F.; Gallo, P.; Gagliardi, C.; et al. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 470–478. [Google Scholar] [CrossRef]
- Stats, M.A.; Stone, J.R. Varying levels of small microcalcifications and macrophages in ATTR and AL cardiac amyloidosis: Implications for utilizing nuclear medicine studies to subtype amyloidosis. Cardiovasc. Pathol. 2016, 25, 413–417. [Google Scholar] [CrossRef]
- Musumeci, M.B.; Cappelli, F.; Russo, D.; Tini, G.; Canepa, M.; Milandri, A.; Bonfiglioli, R.; Di Bella, G.; My, F.; Luigetti, M.; et al. Low sensitivity of bone scintigraphy in detecting Phe64Leu mutation-related transthyretin cardiac amyloidosis. JACC Cardiovasc. Imaging 2020, 13, 1314–1321. [Google Scholar] [CrossRef]
- Park, M.A.; Padera, R.F.; Belanger, A.; Dubey, S.; Hwang, D.H.; Veeranna, V.; Falk, R.H.; Di Carli, M.F.; Dorbala, S. 18F-florbetapir binds specifically to myocardial light chain and transthyretin amyloid deposits: Autoradiography study. Circ. Cardiovasc Imaging 2015, 8, e002954. [Google Scholar] [CrossRef]
- Ruberg, F.L.; Grogan, M.; Hanna, M.; Kelly, J.W.; Maurer, M.S. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 2872–2891. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; BozkurtChair, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. AM. Coll Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Kitaoka, H.; Izumi, C.; Izumiya, Y.; Inomata, T.; Ueda, M.; Kubo, T.; Koyama, J.; Sano, M.; Sekijima, Y.; Tahara, N.; et al. JCS 2020 guideline on diagnosis and treatment of cardiac amyloidosis. Circ. J. 2020, 84, 1610–1671. [Google Scholar] [CrossRef]
- Sperry, B.W.; Gonzalez, M.H.; Brunken, R.; Cerqueira, M.D.; Hanna, M.; Jaber, W.A. Non-cardiac uptake of technetium-99m pyrophosphate in transthyretin cardiac amyloidosis. J. Nucl. Cardiol. 2019, 26, 1630–1637. [Google Scholar] [CrossRef]
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Bacchi-Reggiani, L.; et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J. Am. Coll. Cardiol. 2005, 46, 1076–1084. [Google Scholar] [CrossRef]
- Bokhari, S.; Castano, A.; Pozniakoff, T.; Deslisle, S.; Latif, F.; Maurer, M.S. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ. Cardiovasc. Imaging 2013, 6, 195–201. [Google Scholar] [CrossRef]
- Pelletier-Galarneau, M.; Abikhzer, G.; Giraldeau, G.; Harel, F. Molecular imaging of cardiac amyloidosis. Curr. Cardiol. Rep. 2019, 21, 12. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, C. Diagnostic performance of CMR, SPECT, and PET imaging for the detection of cardiac amyloidosis: A meta-analysis. BMC Cardiovasc. Disord 2021, 21, 482. [Google Scholar] [CrossRef]
- Caobelli, F.; Braun, M.; Haaf, P.; Wild, D.; Zellweger, M.J. Quantitative (99m)Tc-DPD SPECT/CT in patients with suspected ATTR cardiac amyloidosis: Feasibility and correlation with visual scores. J. Nucl. Cardiol. 2020, 27, 1456–1463. [Google Scholar] [CrossRef]
- Scully, P.R.; Morris, E.; Patel, K.P.; Treibel, T.A.; Burniston, M.; Klotz, E.; Newton, J.D.; Sabharwal, N.; Kelion, A.; Manisty, C.; et al. DPD Quantification in cardiac amyloidosis: A novel imaging biomarker. JACC Cardiovasc. Imaging 2020, 13, 1353–1363. [Google Scholar] [CrossRef]
- Wollenweber, T.; Rettl, R.; Kretschmer-Chott, E.; Rasul, S.; Kulterer, O.; Rainer, E.; Raidl, M.; Schaffarich, M.P.; Matschitsch, S.; Stadler, M.; et al. In vivo quantification of myocardial amyloid deposits in patients with suspected transthyretin-related amyloidosis (ATTR). J. Clin. Med. 2020, 9, 3446. [Google Scholar] [CrossRef]
- Castano, A.; Haq, M.; Narotsky, D.L.; Goldsmith, J.; Weinberg, R.L.; Morgenstern, R.; Pozniakoff, T.; Ruberg, F.L.; Miller, E.J.; Berk, J.L.; et al. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: Predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol. 2016, 1, 880–889. [Google Scholar] [CrossRef]
- Quarta, C.C.; Zheng, J.; Hutt, D.; Grigore, S.F.; Manwani, R.; Sachchithanantham, S.; Mahmood, S.A.; Whelan, C.J.; Fontana, M.; Martinez-Naharro, A.; et al. 99mTc-DPD scintigraphy in immunoglobulin light chain (AL) cardiac amyloidosis. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1304–1311. [Google Scholar] [CrossRef]
- Hutt, D.F.; Fontana, M.; Burniston, M.; Quigley, A.M.; Petrie, A.; Ross, J.C.; Page, J.; Martinez-Naharro, A.; Wechalekar, A.D.; Lachmann, H.J.; et al. Prognostic utility of the Perugini grading of 99mTc-DPD scintigraphy in transthyretin (ATTR) amyloidosis and its relationship with skeletal muscle and soft tissue amyloid. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1344–1350. [Google Scholar] [CrossRef]
- Vranian, M.N.; Sperry, B.W.; Hanna, M.; Hachamovitch, R.; Ikram, A.; Brunken, R.C.; Jaber, W.A. Technetium pyrophosphate uptake in transthyretin cardiac amyloidosis: Associations with echocardiographic disease severity and outcomes. J. Nucl. Cardiol. 2018, 25, 1247–1256. [Google Scholar] [CrossRef]
- Rapezzi, C.; Quarta, C.C.; Guidalotti, P.L.; Pettinato, C.; Fanti, S.; Leone, O.; Ferlini, A.; Longhi, S.; Lorenzini, M.; Reggiani, L.B.; et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging 2011, 4, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Sperry, B.W.; Vranian, M.N.; Tower-Rader, A.; Hachamovitch, R.; Hanna, M.; Brunken, R.; Phelan, D.; Cerqueira, M.D.; Jaber, W.A. Regional variation in technetium pyrophosphate uptake in transthyretin cardiac amyloidosis and impact on mortality. JACC Cardiovasc Imaging 2018, 11, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, K.; Shiraishi, S.; Tsuda, N.; Sakamoto, F.; Oda, S.; Takashio, S.; Tsujita, K.; Hirai, T. Usefulness of quantitative (99m)Tc-pyrophosphate SPECT/CT for predicting the prognosis of patients with wild-type transthyretin cardiac amyloidosis. Jpn J. Radiol. 2022, 40, 508–517. [Google Scholar] [CrossRef]
- Giblin, G.T.; Cuddy, S.A.M.; Gonzalez-Lopez, E.; Sewell, A.; Murphy, A.; Dorbala, S.; Falk, R.H. Effect of tafamidis on global longitudinal strain and myocardial work in transthyretin cardiac amyloidosis. Eur. Heart J. Cardiovasc Imaging 2022, 23, 1029–1039. [Google Scholar] [CrossRef]
- Rettl, R.; Mann, C.; Duca, F.; Dachs, T.M.; Binder, C.; Ligios, L.C.; Schrutka, L.; Dalos, D.; Koschutnik, M.; Dona, C.; et al. Tafamidis treatment delays structural and functional changes of the left ventricle in patients with transthyretin amyloid cardiomyopathy. Eur. Heart J. Cardiovasc Imaging 2021, 23, 767–780. [Google Scholar] [CrossRef]
- Bellevre, D.; Bailliez, A.; Marechaux, S.; Manrique, A.; Mouquet, F. First follow-up of cardiac amyloidosis treated by tafamidis, evaluated by absolute quantification in bone scintigraphy. JACC Case Rep. 2021, 3, 133–135. [Google Scholar] [CrossRef]
- Slart, R.; Glaudemans, A.; Gheysens, O.; Lubberink, M.; Kero, T.; Dweck, M.R.; Habib, G.; Gaemperli, O.; Saraste, A.; Gimelli, A.; et al. Procedural recommendations of cardiac PET/CT imaging: Standardization in inflammatory-, infective-, infiltrative-, and innervation- (4Is) related cardiovascular diseases: A joint collaboration of the EACVI and the EANM: Summary. Eur. Heart J. Cardiovasc Imaging 2020, 21, 1320–1330. [Google Scholar] [CrossRef]
- Dorbala, S.; Vangala, D.; Semer, J.; Strader, C.; Bruyere, J.R., Jr.; Di Carli, M.F.; Moore, S.C.; Falk, R.H. Imaging cardiac amyloidosis: A pilot study using (1)(8)F-florbetapir positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1652–1662. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ha, S.; Kim, Y.I. Cardiac amyloidosis imaging with amyloid positron emission tomography: A systematic review and meta-analysis. J. Nucl. Cardiol. 2020, 27, 123–132. [Google Scholar] [CrossRef]
- Lee, S.P.; Lee, E.S.; Choi, H.; Im, H.J.; Koh, Y.; Lee, M.H.; Kwon, J.H.; Paeng, J.C.; Kim, H.K.; Cheon, G.J.; et al. 11C-Pittsburgh B PET imaging in cardiac amyloidosis. JACC Cardiovasc Imaging 2015, 8, 50–59. [Google Scholar] [CrossRef]
- Law, W.P.; Wang, W.Y.; Moore, P.T.; Mollee, P.N.; Ng, A.C. Cardiac amyloid imaging with 18F-florbetaben PET: A pilot study. J. Nucl. Med. 2016, 57, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, M.; Kessler, L.; Carpinteiro, A.; Hagenacker, T.; Nensa, F.; Umutlu, L.; Forsting, M.; Brainman, A.; Kleinschnitz, C.; Antoch, G.; et al. (18)F-flutemetamol positron emission tomography in cardiac amyloidosis. J. Nucl. Cardiol. 2022, 29, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Dietemann, S.; Nkoulou, R. Amyloid PET imaging in cardiac amyloidosis: A pilot study using (18)F-flutemetamol positron emission tomography. Ann. Nucl. Med. 2019, 33, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Antoni, G.; Lubberink, M.; Estrada, S.; Axelsson, J.; Carlson, K.; Lindsjo, L.; Kero, T.; Langstrom, B.; Granstam, S.O.; Rosengren, S.; et al. In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J. Nucl. Med. 2013, 54, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Pilebro, B.; Arvidsson, S.; Lindqvist, P.; Sundstrom, T.; Westermark, P.; Antoni, G.; Suhr, O.; Sorensen, J. Positron emission tomography (PET) utilizing Pittsburgh compound B (PIB) for detection of amyloid heart deposits in hereditary transthyretin amyloidosis (ATTR). J. Nucl. Cardiol. 2018, 25, 240–248. [Google Scholar] [CrossRef]
- Rosengren, S.; Skibsted Clemmensen, T.; Tolbod, L.; Granstam, S.O.; Eiskjaer, H.; Wikstrom, G.; Vedin, O.; Kero, T.; Lubberink, M.; Harms, H.J.; et al. Diagnostic accuracy of [(11)C]PIB positron emission tomography for detection of cardiac amyloidosis. JACC Cardiovasc Imaging 2020, 13, 1337–1347. [Google Scholar] [CrossRef]
- Ezawa, N.; Katoh, N.; Oguchi, K.; Yoshinaga, T.; Yazaki, M.; Sekijima, Y. Visualization of multiple organ amyloid involvement in systemic amyloidosis using (11)C-PiB PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 452–461. [Google Scholar] [CrossRef]
- Genovesi, D.; Vergaro, G.; Giorgetti, A.; Marzullo, P.; Scipioni, M.; Santarelli, M.F.; Pucci, A.; Buda, G.; Volpi, E.; Emdin, M. [18F]-florbetaben PET/CT for Differential diagnosis among cardiac immunoglobulin light chain, transthyretin amyloidosis, and mimicking conditions. JACC Cardiovasc Imaging 2021, 14, 246–255. [Google Scholar] [CrossRef]
- Santarelli, M.F.; Genovesi, D.; Scipioni, M.; Positano, V.; Favilli, B.; Giorgetti, A.; Vergaro, G.; Landini, L.; Emdin, M.; Marzullo, P. Cardiac amyloidosis characterization by kinetic model fitting on [18F]florbetaben PET images. J. Nucl Cardiol 2022, 29, 1919–1932. [Google Scholar] [CrossRef]
- Santarelli, M.F.; Genovesi, D.; Positano, V.; Scipioni, M.; Vergaro, G.; Favilli, B.; Giorgetti, A.; Emdin, M.; Landini, L.; Marzullo, P. Deep-learning-based cardiac amyloidosis classification from early acquired pet images. Int J. Cardiovasc Imaging 2021, 37, 2327–2335. [Google Scholar] [CrossRef]
- Choi, Y.J.; Koh, Y.; Lee, H.J.; Hwang, I.C.; Park, J.B.; Yoon, Y.E.; Kim, H.L.; Kim, H.K.; Kim, Y.J.; Cho, G.Y.; et al. Independent prognostic utility of (11)C-pittsburgh compound B PET in patients with light-chain cardiac amyloidosis. J. Nucl. Med. 2022, 63, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Merlini, G.; Lousada, I.; Ando, Y.; Dispenzieri, A.; Gertz, M.A.; Grogan, M.; Maurer, M.S.; Sanchorawala, V.; Wechalekar, A.; Palladini, G.; et al. Rationale, application and clinical qualification for NT-proBNP as a surrogate end point in pivotal clinical trials in patients with AL amyloidosis. Leukemia 2016, 30, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, J.A.; Alemayehu, W.; Rathwell, S.; Grant, A.D.; Fiuzat, M.; Whellan, D.J.; Ahmad, T.; Adams, K.; Pina, I.L.; Cooper, L.S.; et al. The influence of comorbidities on achieving an N-terminal pro-b-type natriuretic peptide target: A secondary analysis of the GUIDE-IT trial. ESC Heart Fail. 2022, 9, 77–86. [Google Scholar] [CrossRef]
- Manwani, R.; Page, J.; Lane, T.; Burniston, M.; Skillen, A.; Lachmann, H.J.; Gillmore, J.D.; Fontana, M.; Whelan, C.; Hawkins, P.N.; et al. A pilot study demonstrating cardiac uptake with 18F-florbetapir PET in AL amyloidosis patients with cardiac involvement. Amyloid 2018, 25, 247–252. [Google Scholar] [CrossRef]
- Ehman, E.C.; El-Sady, M.S.; Kijewski, M.F.; Khor, Y.M.; Jacob, S.; Ruberg, F.L.; Sanchorawala, V.; Landau, H.; Yee, A.J.; Bianchi, G.; et al. Early detection of multiorgan light-chain amyloidosis by whole-body (18)F-florbetapir PET/CT. J. Nucl. Med. 2019, 60, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.; Ihne, S.; Brumberg, J.; Morbach, C.; Knop, S.; Kortum, K.M.; Stork, S.; Buck, A.K.; Reiter, T.; Bauer, W.R.; et al. Detection of cardiac amyloidosis with (18)F-Florbetaben-PET/CT in comparison to echocardiography, cardiac MRI and DPD-scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1407–1416. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, Y.S.; Kim, S.J. Diagnostic performance of PET for detection of cardiac amyloidosis: A systematic review and meta-analysis. J. Cardiol. 2020, 76, 618–625. [Google Scholar] [CrossRef]
- Guo, W.; Chen, H. (68)Ga FAPI PET/MRI in cardiac amyloidosis. Radiology 2022, 303, 51. [Google Scholar] [CrossRef]
- Zhang, L.X.; Martineau, P.; Finnerty, V.; Giraldeau, G.; Parent, M.C.; Harel, F.; Pelletier-Galarneau, M. Comparison of 18F-sodium fluoride positron emission tomography imaging and 99mTc-pyrophosphate in cardiac amyloidosis. J. Nucl. Cardiol. 2020, 29, 1132–1140. [Google Scholar] [CrossRef]
- Abulizi, M.; Sifaoui, I.; Wuliya-Gariepy, M.; Kharoubi, M.; Israel, J.M.; Emsen, B.; Bodez, D.; Monnet, A.; Didierlaurent, D.; Tacher, V.; et al. (18)F-sodium fluoride PET/MRI myocardial imaging in patients with suspected cardiac amyloidosis. J. Nucl. Cardiol. 2021, 28, 1586–1595. [Google Scholar] [CrossRef]
- Martineau, P.; Finnerty, V.; Giraldeau, G.; Authier, S.; Harel, F.; Pelletier-Galarneau, M. Examining the sensitivity of 18F-NaF PET for the imaging of cardiac amyloidosis. J. Nucl. Cardiol. 2021, 28, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Manabe, O.; Oyama-Manabe, N.; Tamaki, N. Positron emission tomography/MRI for cardiac diseases assessment. Br. J. Radiol. 2020, 93, 20190836. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Xu, B.; Liu, J.; Wang, G.; An, J.; Zhang, X.; Wang, R.; Dong, W.; Guan, Z. Diagnostic value of (11)C-PIB PET/MR in cardiac amyloidosis. Front. Cardiovasc Med. 2022, 9, 830572. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Calabretta, R.; Yu, J.; Binder, P.; Hu, S.; Hacker, M.; Li, X. Nuclear Molecular Imaging of Disease Burden and Response to Treatment for Cardiac Amyloidosis. Biology 2022, 11, 1395. https://doi.org/10.3390/biology11101395
Zhao M, Calabretta R, Yu J, Binder P, Hu S, Hacker M, Li X. Nuclear Molecular Imaging of Disease Burden and Response to Treatment for Cardiac Amyloidosis. Biology. 2022; 11(10):1395. https://doi.org/10.3390/biology11101395
Chicago/Turabian StyleZhao, Min, Raffaella Calabretta, Josef Yu, Patrick Binder, Shuo Hu, Marcus Hacker, and Xiang Li. 2022. "Nuclear Molecular Imaging of Disease Burden and Response to Treatment for Cardiac Amyloidosis" Biology 11, no. 10: 1395. https://doi.org/10.3390/biology11101395
APA StyleZhao, M., Calabretta, R., Yu, J., Binder, P., Hu, S., Hacker, M., & Li, X. (2022). Nuclear Molecular Imaging of Disease Burden and Response to Treatment for Cardiac Amyloidosis. Biology, 11(10), 1395. https://doi.org/10.3390/biology11101395






