Physiotherapeutic Protocol and ZnO Nanoparticles: A Combined Novel Treatment Program against Bacterial Pyomyositis
Abstract
Simple Summary
Abstract
1. Introduction
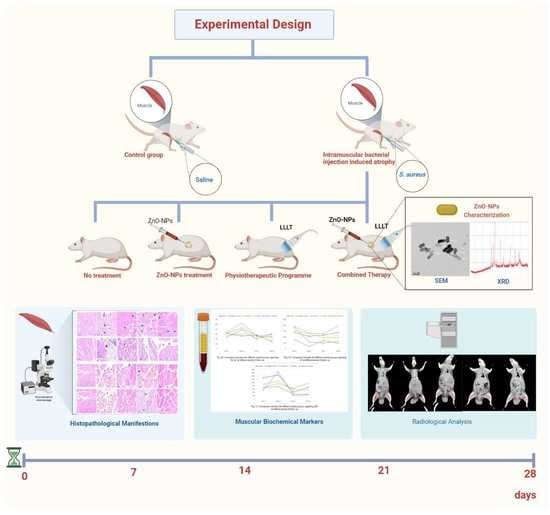
2. Materials and Methods
2.1. Bacterial Sample Collection and Identification
2.2. ZnO Nanoparticle Preparation
Physicochemical Characterization of Zinc Oxide Nanoparticles
2.3. In Vitro Studies
2.3.1. Antimicrobial Activity of the Synthesized ZnO-NPs
2.3.2. Cytotoxicity of the Synthesized ZnO-NPs
2.4. In Vivo study
2.4.1. Experimental Animals
2.4.2. Histopathological Study
2.4.3. Blood Hematological and Biochemical Analyses
2.4.4. Radiological Analysis
Computed Tomography (CT) Imaging Technique
2.5. Statistical Analysis
3. Results
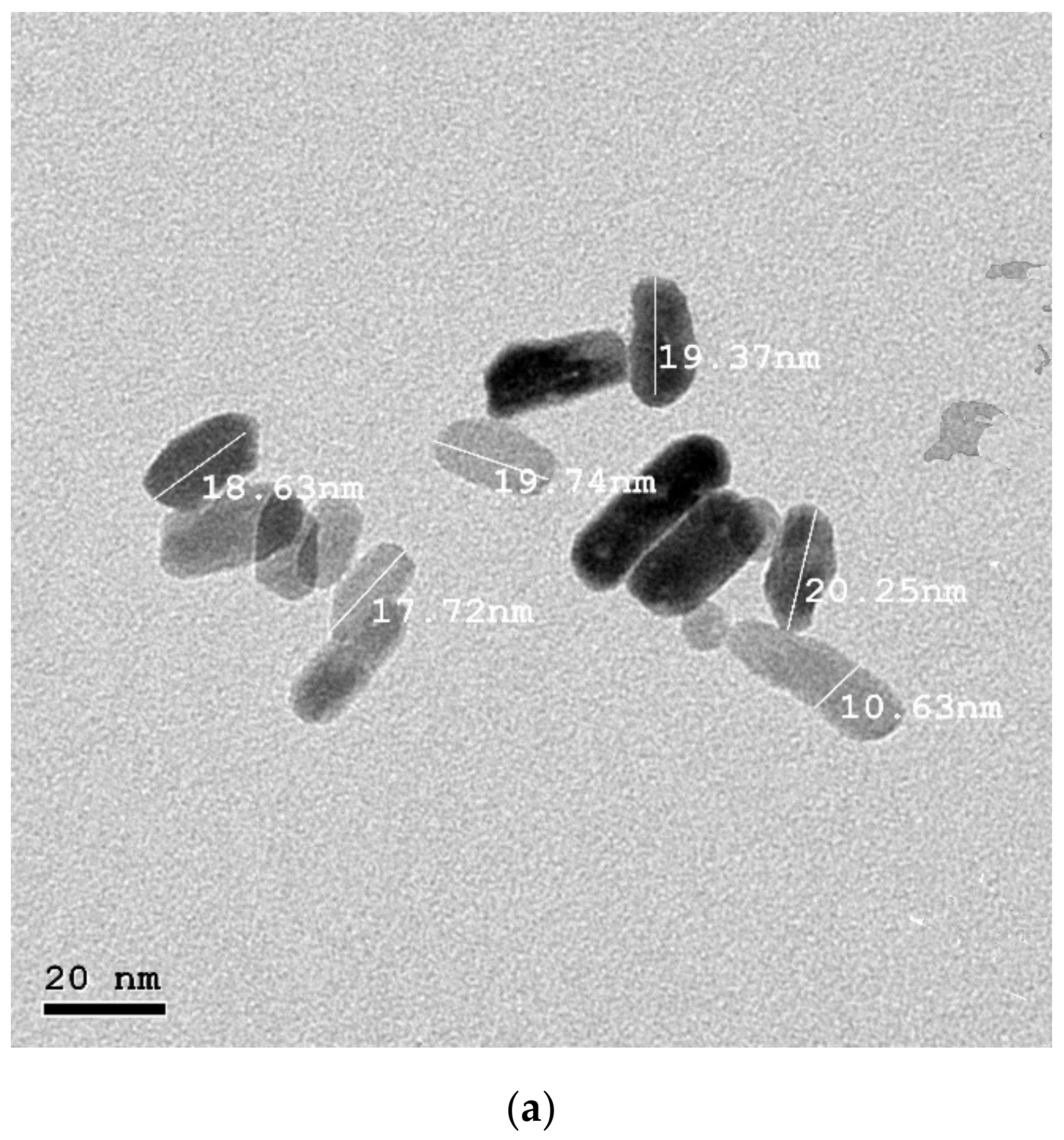
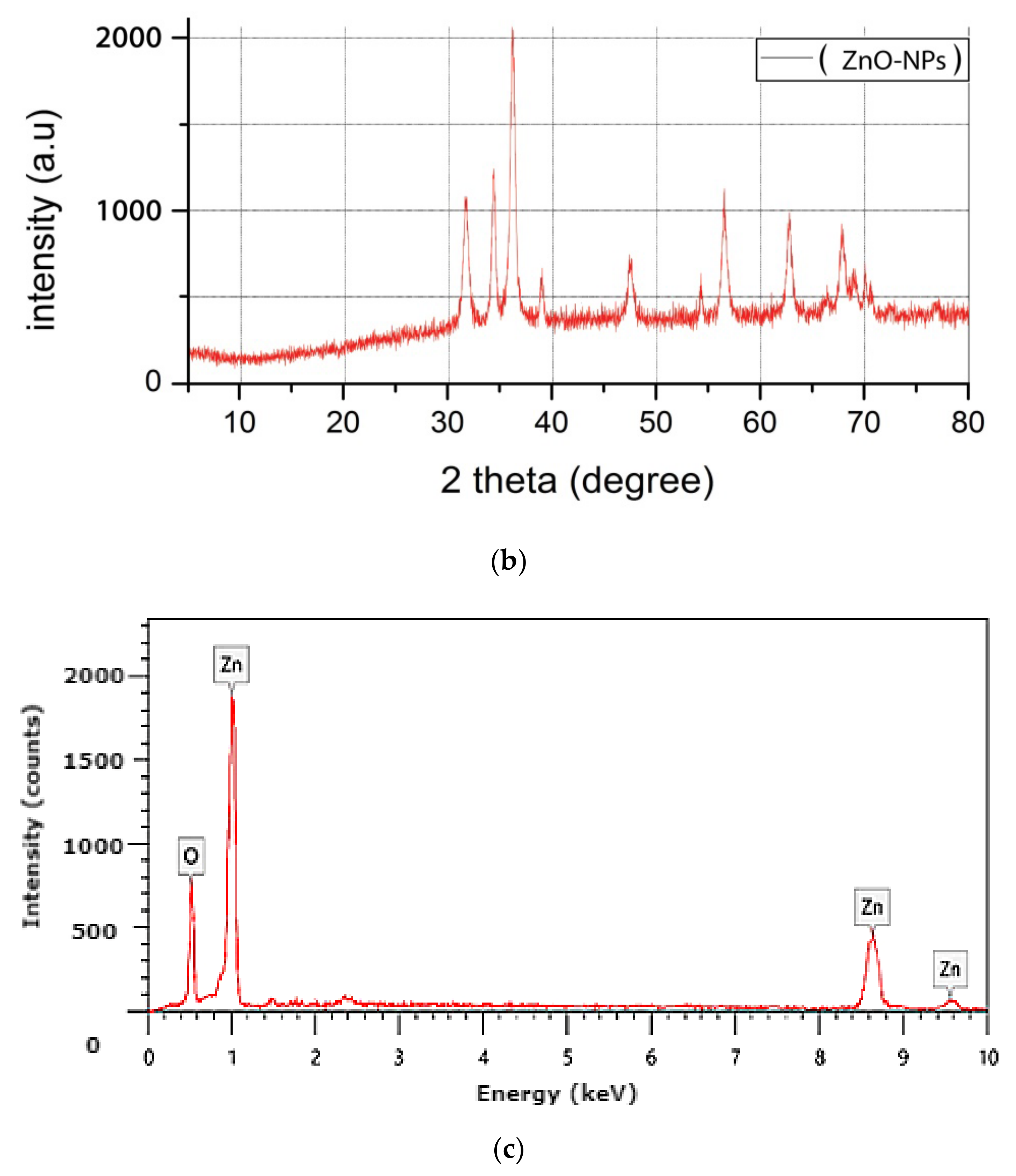
3.1. Nanoparticles Characterization
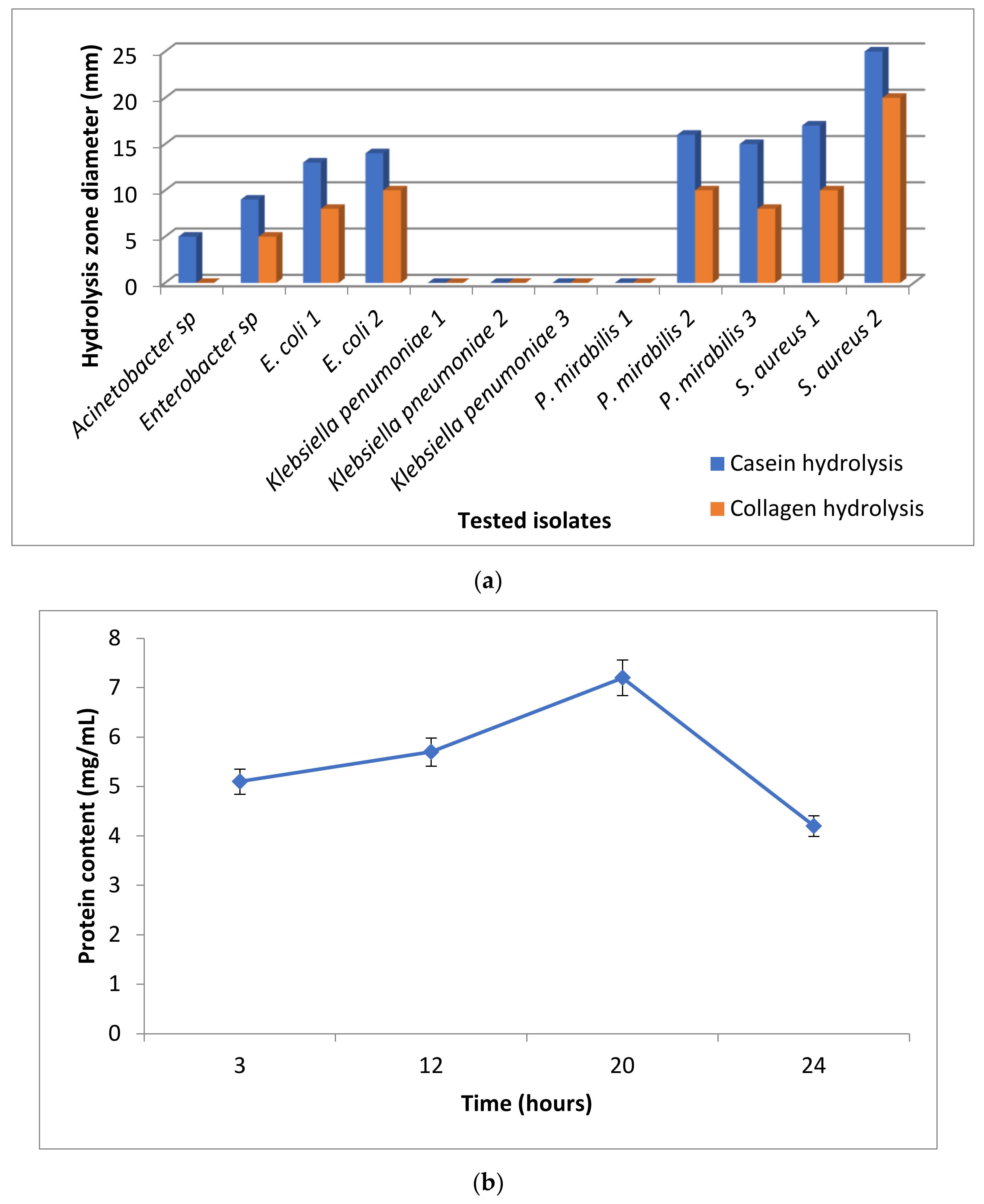
3.2. Proteolytic Enzyme Activity
3.3. In Vitro Studies
3.3.1. Antibacterial Activity of the Prepared Nanoparticles against the Potent Proteolytic Bacteria
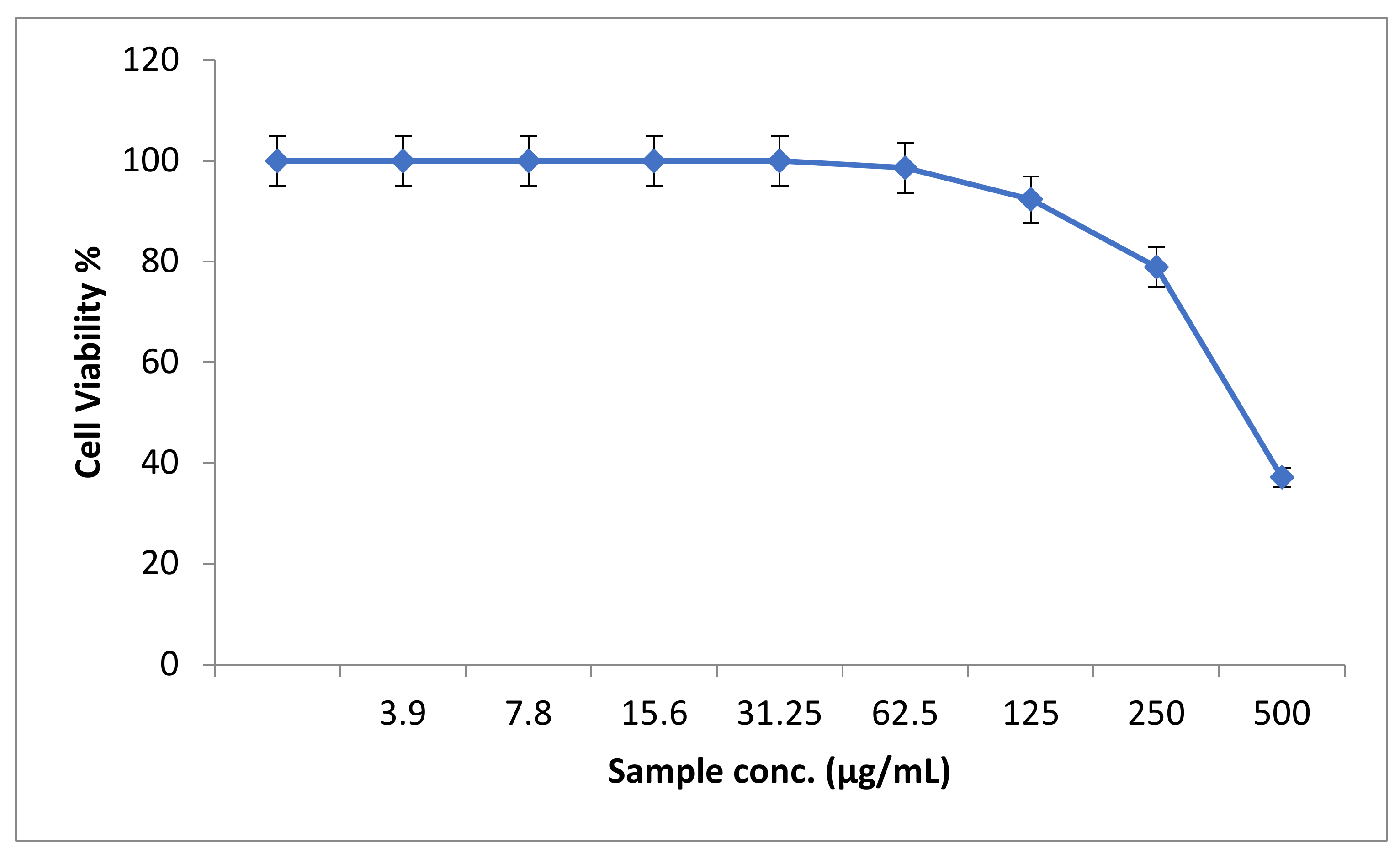
3.3.2. Cytotoxic Effect of the Synthesized ZnO Nanoparticles
3.4. In Vivo Study
3.4.1. Histopathological Study
3.4.2. Blood Biochemical Analysis
3.4.3. Radiological Analysis Results
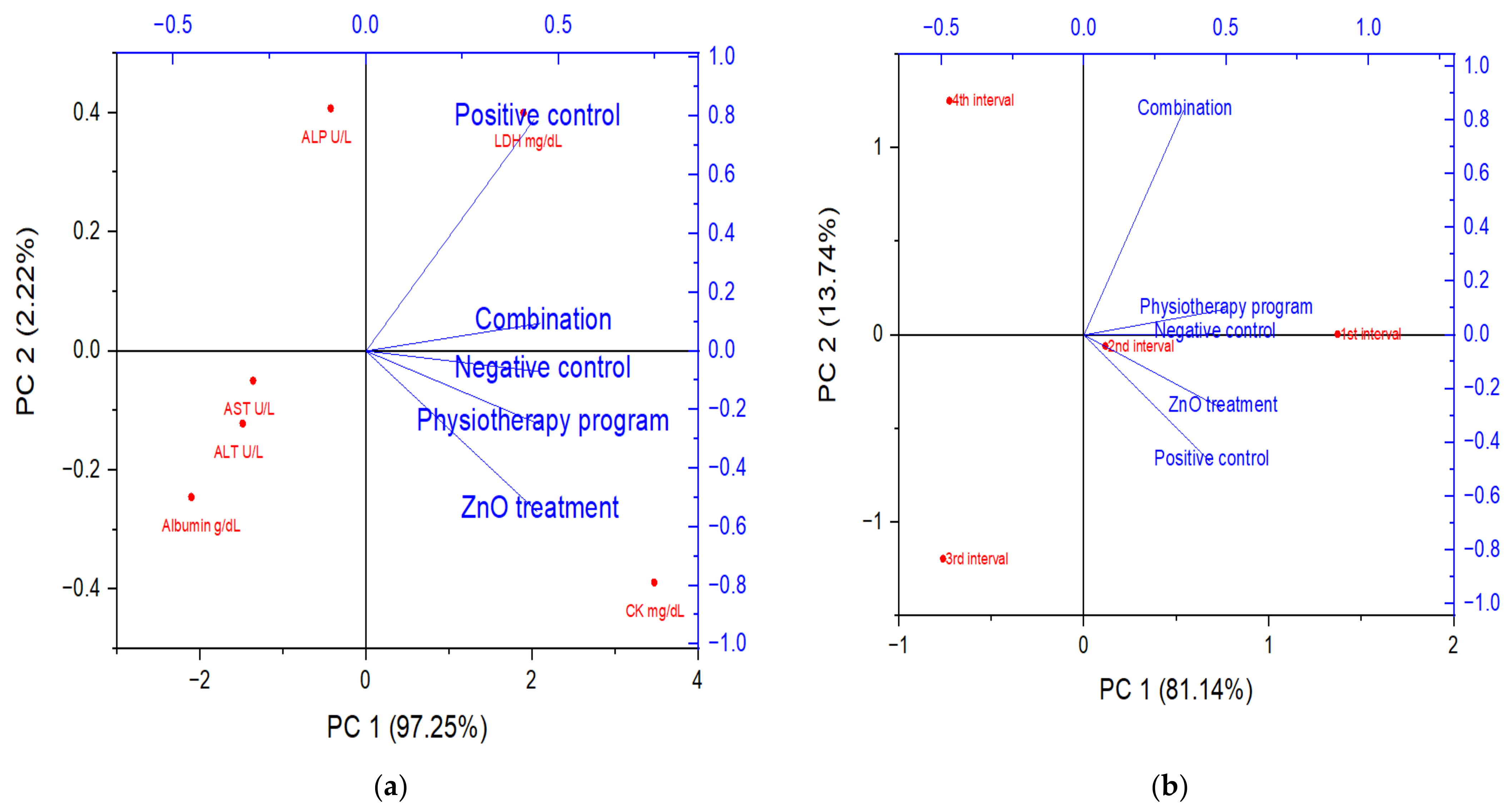
3.5. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, F.A.; Quadrilatero, J. Mitochondrial-apoptotic signaling involvement in remodeling during myogenesis and skeletal muscle atrophy. In Seminars in Cell Developmental Biology; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Models Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef]
- Ozaki, Y.; Ohashi, K.; Otaka, N.; Ogawa, H.; Kawanishi, H.; Takikawa, T.; Fang, L.; Tatsumi, M.; Takefuji, M.; Enomoto, T.; et al. Neuron-derived neurotrophic factor protects against dexamethasone-induced skeletal muscle atrophy. Biochem. Biophys. Res. Commun. 2022, 593, 5–12. [Google Scholar] [CrossRef]
- Biduski, G.M.; Bertoli, J.; Sousa, M.V.; Diefenthaeler, F.; de la Rocha Freitas, C. Effects of 6-weeks of detraining on functional capacity and rapid torque production in older women. J. Bodyw. Mov. Ther. 2022, 29, 167–173. [Google Scholar] [CrossRef]
- Fernández-Costa, J.M.; Fernández-Garibay, X.; Velasco-Mallorquí, F.; Ramón-Azcón, J. Bioengineered in vitro skeletal muscles as new tools for muscular dystrophies preclinical studies. J. Tissue Eng. 2021, 12, 2041731420981339. [Google Scholar] [CrossRef]
- Ngor, C.; Hall, L.; Dean, J.A.; Gilks, C.F. Factors associated with pyomyositis: A systematic review and meta-analysis. Trop. Med. Int. Health 2021, 26, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; El-Khatib, A.; El-khatib, M. Synthesis of hexagonal nanozinc by arc discharge for antibacterial water treatment. Surf. Innov. 2020, 8, 165–171. [Google Scholar] [CrossRef]
- Singh, T.A.; Sharma, A.; Tejwan, N.; Ghosh, N.; Das, J.; Sil, P.C. A state of the art review on the synthesis, antibacterial, antioxidant, antidiabetic and tissue regeneration activities of zinc oxide nanoparticles. Adv. Colloid Interface Sci. 2021, 295, 102495. [Google Scholar] [CrossRef] [PubMed]
- Gueron, L.J.P.; Amoyi, A.; Chao, W.; Chepngetich, J.; Kibet, J.J.; Nyambok, S.; Wesonga, J. Group physiotherapy with survivors of torture in urban and camp settings in Jordan and Kenya. Torture J. 2021, 30, 27–42. [Google Scholar] [CrossRef]
- Bhan, K.; Hasan, K.; Pawar, A.S.; Patel, R. Rehabilitation Following Surgically Treated Distal Radius Fractures: Do Immobilization and Physiotherapy Affect the Outcome? Cureus 2021, 13, e16230. [Google Scholar] [CrossRef]
- Alsofi, L.; Khalil, W.; Binmadi, N.O.; Al-Habib, M.A.; Alharbi, H. Pulpal and periapical tissue response after direct pulp capping with endosequence root repair material and low-level laser application. BMC Oral Health 2022, 22, 57. [Google Scholar] [CrossRef]
- Kamel, N.M.; Toson, R.A.; Elsayeh, S.M. Response of Aerobic Capacity to Low-Level Laser Therapy in Burned Patients. J. Burn. Care Res. 2021, 43, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Elwakil, B.H.; Awad, D.; Hussein, A.A.; Harfoush, R.A.; Gohar, Y.M. Chitosan and Liposomes Nanoparticles Encapsulated Cinnamon Extract: Antiproteolytic Activity and Wound Healing Efficiency of Diabetic Rats. CMU J. Nat. Sci. 2020, 19, 595–611. [Google Scholar]
- El-Khatib, A.M.; Yousef, N.S.; Ghatass, Z.F.; Badawi, M.S.; Mohamed, M.M.; Elkhatib, M. Synthesized silver carbon nanotubes and zinc oxide nanoparticles and their ability to remove methylene blue dye. J. Nano Res. 2019, 56, 1–16. [Google Scholar] [CrossRef]
- Hamed, M.T.; Bakr, B.A.; Shahin, Y.H.; Elwakil, B.H.; Abu-Serie, M.M.; Aljohani, F.S.; Bekhit, A.A. Novel Synthesis of Titanium Oxide Nanoparticles: Biological Activity and Acute Toxicity Study. Bioinorg. Chem. Appl. 2021, 2021, 8171786. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Lakyová, L.; Toporcer, T.; Tomečková, V.; Sabo, J.; Radoňak, J. Low-level laser therapy for protection against skeletal muscle damage after ischemia–reperfusion injury in rat hindlimbs. Lasers Surg. Med. 2010, 42, 825–832. [Google Scholar] [CrossRef]
- Schrauwen-Hinderling, V.B.; Hesselink, M.K.; Schrauwen, P.; Kooi, M.E. Intramyocellular lipid content in human skeletal muscle. Obesity 2006, 143, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Stray-Gundersen, J.; Parsons, D.; Bertocci, L.A. Skeletal muscle morphology and exercise response in congenital generalized lipodystrophy. Diabetes Care 2000, 23, 1545–1550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dunyach-Remy, C.; Ngba Essebe, C.; Sotto, A.; Lavigne, J.P. Staphylococcus aureus toxins and diabetic foot ulcers: Role in pathogenesis and interest in diagnosis. Toxins 2016, 8, 209. [Google Scholar] [CrossRef] [PubMed]
- John, A.T.; Sharkey, M. Diabetic foot infections. In Emergency Management of Infectious Diseases; Cambridge University Press: Cambridge, UK, 2018; pp. 228–232. [Google Scholar] [CrossRef]
- Serpe, L.; Foglietta, F.; Canaparo, R. Nanosonotechnology: The next challenge in cancer sonodynamic therapy. Nanotechnol. Rev. 2012, 1, 173–182. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Sleep, D.; Cameron, J.; Evans, L.R. Albumin as a versatile platform for drug half-life extension. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2013, 1830, 5526–5534. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. In Nano-Enabled Medical Applications; Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 61–91. [Google Scholar]
- Ouyang, J.; Tang, Z.; Farokhzad, N.; Kong, N.; Kim, N.Y.; Feng, C.; Blake, S.; Xiao, Y.; Liu, C.; Xie, T.; et al. Ultrasound mediated therapy: Recent progress and challenges in nanoscience. Nano Today 2020, 35, 100949. [Google Scholar] [CrossRef]
- Anugrahwidya, R.; Armynah, B.; Tahir, D. Composites Bioplastic Film for Various Concentration of Zinc Oxide (ZnO) Nanocrystals Towards Physical Properties for High Biodegradability in Soil and Seawater. J. Polym. Environ. 2022, 30, 2589–2601. [Google Scholar] [CrossRef]
- Chen, M.C.; Koh, P.W.; Ponnusamy, V.K.; Lee, S.L. Titanium dioxide and other nanomaterials based antimicrobial additives in functional paints and coatings. Prog. Org. Coat. 2022, 163, 106660. [Google Scholar] [CrossRef]
- Sanjivkumar, M.; Silambarasan, T.; Ananthi, S.; Thanga Tharani, K. Biosynthesis and characterization of zinc oxide nanoparticles from an estuarine-associated actinobacterium Streptomyces spp. and its biotherapeutic applications. Arch. Microbiol. 2022, 204, 17. [Google Scholar] [CrossRef]
- Aqib, A.I.; Muzammil, I.; Ahmad, S.; Sohail, M.L.; Ali, A.; Prince, K.; Sajid, H.A. Metal Nanoparticles against Bacteria. In Nanomaterials in the Battle against Pathogens and Disease Vectors; CRC Press: Boca Raton, FL, USA, 2022; pp. 119–160. [Google Scholar]
- Baláž, M.; Bedlovičová, Z.; Daneu, N.; Siksa, P.; Sokoli, L.; Tkáčiková, Ľ.; Salayová, A.; Džunda, R.; Kováčová, M.; Bureš, R.; et al. Mechanochemistry as an alternative method of green synthesis of silver nanoparticles with antibacterial activity: A Comparative Study. Nanomaterials 2021, 11, 1139. [Google Scholar] [CrossRef]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A.; Khaniabadi, P.M.; Kareem, A.A.; Alrosan, M.; Ali, A.T.; Rabeea, M.A.; Mehrdel, B. Mycosynthesis of ultrasonically-assisted uniform cubic silver nanoparticles by isolated phenols from Agaricus bisporus and its antibacterial activity. Surf. Interfaces 2022, 29, 101774. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A mini review of antibacterial properties of ZnO nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.D.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Renato Nallin Montagnolli, H.F.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Liu, F.; Hong, T.; Xie, J.; Zhan, X.; Wang, Y. Application of Reactive Oxygen Species-Based Nanomaterials in Dentistry: A Review. Crystals 2021, 11, 266. [Google Scholar] [CrossRef]
- Fatima, F.; Siddiqui, S.; Khan, W.A. Nanoparticles as novel emerging therapeutic antibacterial agents in the antibiotics resistant era. Biol. Trace Elem. Res. 2021, 199, 2552–2564. [Google Scholar] [CrossRef] [PubMed]
- Hamm, S.E.; Fathalikhani, D.D.; Bukovec, K.E.; Addington, A.K.; Zhang, H.; Perry, J.B.; McMillan, R.P.; Lawlor, M.W.; Prom, M.J.; Avond, M.A.V.; et al. Voluntary wheel running complements microdystrophin gene therapy to improve muscle function in mdx mice. Mol. Ther.—Methods Clin. Dev. 2021, 21, 144–160. [Google Scholar] [CrossRef]
- Kimura, S.; Ikezawa, M.; Nomura, K.; Ito, K.; Ozasa, S.; Ueno, H.; McMillan, R.P.; Lawlor, M.W.; Prom, M.J.; Avond, M.A.V.; et al. Immobility reduces muscle fiber necrosis in dystrophin deficient muscular dystrophy. Brain Dev. 2006, 28, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Pereira, M.; Cooper, T.A.; Gourdon, G. Myotonic dystrophy mouse models: Towards rational therapy development. Trends Mol. Med. 2011, 17, 506–517. [Google Scholar] [CrossRef]
- Diong, J.; Carden, P.C.; O’Sullivan, K.; Sherrington, C.; Reed, D.S. Eccentric exercise improves joint flexibility in adults: A systematic review update and meta-analysis. Musculoskelet. Sci. Pract. 2022, 60, 102556. [Google Scholar] [CrossRef]
- Lopes-Martins, R.A.B.; Albertini, R.; Lopes, P.S.L.; Bjordal, J.M.; Neto, H.C.C.F. Spontaneous effects of low-level laser therapy (650 nm) in acute inflammatory mouse pleurisy induced by carrageenan. Photomed. Laser Ther. 2005, 23, 377–381. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Kearns, A.K.; Rouzier, P.; Rubin, R.; Thompson, P.D. Serum creatine kinase levels and renal function measures in exertional muscle damage. Med. Sci. Sports Exerc. 2006, 38, 623. [Google Scholar] [CrossRef]
- Kindermann, W. Creatine kinase levels after exercise. Dtsch. Ärzteblatt Int. 2016, 113, 344. [Google Scholar] [CrossRef]
- Fillipin, L.I.; Mauriz, J.L.; Vedovelli, K.; Moreira, A.J.; Zettler, C.G.; Lech, O.; Marroni, N.P.; González-Gallego, J. Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 2005, 37, 293–300. [Google Scholar] [CrossRef]
- Smerdu, V.; Perše, M. Effect of high-fat mixed lipid diet and swimming on fibre types in skeletal muscles of rats with colon tumours. Eur. J. Histochem. 2018, 62, 2945. [Google Scholar] [CrossRef] [PubMed]
- Falcai, M.J.; Zamarioli, A.; Leoni, G.B.; Sousa Neto, M.D.D.; Volpon, J.B. Swimming activity prevents the unloading induced loss of bone mass, architecture, and strength in rats. BioMed Res. Int. 2015, 2015, 507848. [Google Scholar] [CrossRef] [PubMed]
- Damm, T.B.; Egli, M. Calcium’s role in mechanotransduction during muscle development. Cell. Physiol. Biochem. 2014, 33, 249–272. [Google Scholar] [CrossRef]
- Orengo, J.P.; Chambon, P.; Metzger, D.; Mosier, D.R.; Snipes, G.J.; Cooper, T.A. Expanded CTG repeats within the DMPK 3′ UTR causes severe skeletal muscle wasting in an inducible mouse model for myotonic dystrophy. Proc. Natl. Acad. Sci. USA 2008, 105, 2646–2651. [Google Scholar] [CrossRef]
- Nagy, J.A.; Semple, C.; Riveros, D.; Sanchez, B.; Rutkove, S.B. Altered electrical properties in skeletal muscle of mice with glycogen storage disease type II. Sci. Rep. 2022, 12, 5327. [Google Scholar] [CrossRef]
- Bonnefoy, J.; Baselet, B.; Moser, D.; Ghislin, S.; Miranda, S.; Riant, E.; Vermeesen, R.; Keiler, A.M.; Baatout, S.; Choukér, A.; et al. B-Cell Homeostasis Is Maintained During Two Months of Head-Down Tilt Bed Rest with or without Antioxidant Supplementation. Front. Immunol. 2022, 13, 830662. [Google Scholar] [CrossRef]
- Kwon, D.; Kim, C.; Woo, Y.K.; Hwang, J.K. Inhibitory Effects of Chrysanthemum (Chrysanthemum morifolium Ramat.) Extract and Its Active Compound Isochlorogenic Acid A on Sarcopenia. Prev. Nutr. Food Sci. 2021, 26, 408. [Google Scholar] [CrossRef]
- Wang, W.W.; Wu, L.; Lu, W.; Chen, W.; Yan, W.; Qi, C.; Xuan, S.; Shang, A. Lipopolysaccharides increase the risk of colorectal cancer recurrence and metastasis due to the induction of neutrophil extracellular traps after curative resection. J. Cancer Res. Clin. Oncol. 2021, 147, 2609–2619. [Google Scholar] [CrossRef] [PubMed]
- Utari, A.U.; Djabir, Y.Y.; Palinggi, B.P.; Djabir, Y.Y. A combination of virgin coconut oil and extra virgin olive oil elicits superior protection against doxorubicin cardiotoxicity in rats. Turk. J. Pharm. Sci. 2021, 19, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, B.O.M.; Preciado, A.R.F.; Emiliano, J.R.; Garza, S.M.; Rodríguez, E.R.; de Alba Macías, L.A. Recovery of bone and muscle mass in patients with chronic kidney disease and iron overload on hemodialysis and taking combined supplementation with curcumin and resveratrol. Clin. Interv. Aging 2019, 14, 2055. [Google Scholar] [CrossRef]
- Koju, N.; Qin, Z.H.; Sheng, R. Reduced nicotinamide adenine dinucleotide phosphate in redox balance and diseases: A friend or foe? Acta Pharmacol. Sin. 2022, 43, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Qin, J.; Hao, Y.; Shi, Y.; Fu, L. Structural and functional changes of gut microbiota in ovariectomized rats and their correlations with altered bone mass. Aging 2020, 12, 10736. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, R.; Beavis, C.; Obaid, H.; Farthing, J.P.; Kim, S.Y. Surgical repair of the supraspinatus: Pre-and postoperative architectural changes in the muscle. Singapore Med. J. 2022, 63, 97–104. [Google Scholar] [CrossRef] [PubMed]


| Hematological Parameters | Negative Control | Physiotherapeutic Program | Combination | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | Week 1 | Week 2 | Week 3 | Week 4 | Week 1 | Week 2 | Week 3 | Week 4 | |
| WBCs | 8700 ± 669.2 | 7200 ± 480.0 | 8300 ± 592.9 | 8800 ± 586.7 | 5500 ± 343.8 | 10,400 ± 742.9 | 10,200 ± 680.0 | 10,350 ± 646.9 | 4400 ± 366.7 | 6500 ± 500.0 | 7000 ± 583.3 | 12,800 ± 752.9 |
| Eosinophile | 3 ± 0.30 | 2 ± 0.29 | 3 ± 0.43 | 2 ± 0.20 | 4 ± 0.50 | 4 ± 0.33 | 4 ± 0.40 | 3 ± 0.25 | 3 ± 0.25 | 2 ± 0.29 | 2 ± 0.18 | 2 ± 0.22 |
| Neutrophile | 40 ± 3.08 | 44 ± 4.00 | 45 ± 4.50 | 32 ± 3.56 | 41 ± 3.15 | 57 ± 5.70 | 58 ± 5.80 | 60 ± 5.00 | 41 ± 3.15 | 40 ± 4.00 | 41 ± 4.10 | 36 ± 2.77 |
| Lymphocyte | 52 ± 4.73 | 51 ± 5.10 | 56 ± 4.67 | 62 ± 4.77 | 52 ± 5.20 | 37 ± 2.85 | 55 ± 4.58 | 59 ± 4.92 | 52 ± 4.00 | 58 ± 4.83 | 58 ± 4.14 | 59 ± 4.92 |
| Monocyte | 5 ± 0.45 | 3 ± 0.27 | 5 ± 0.50 | 4 ± 0.44 | 3 ± 0.33 | 2 ± 0.22 | 3 ± 0.33 | 3 ± 0.33 | 4 ± 0.36 | 3 ± 0.27 | 3 ± 0.33 | 3 ± 0.30 |
| Biochemical Parameters | Negative Control | Physiotherapeutic Program | Combination | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | Week 1 | Week 2 | Week 3 | Week 4 | Week 1 | Week 2 | Week 3 | Week 4 | |
| LDH mg/dL | 1295 ± 86.3 | 1467 ± 97.8 | 417 ± 27.8 | 700 ± 53.8 | 894 ± 74.5 | 1091 ± 99.2 | 584 ± 41.7 | 540 ± 45.0 | 558 ± 39.9 | 1647 ± 137.3 | 221 ± 18.4 | 260 ± 21.7 |
| Albumin g/dL | 4.2 ± 0.47 | 3.6 ± 0.30 | 2.3 ± 0.23 | 3.8 ± 0.42 | 3.3 ± 0.37 | 1.7 ± 0.17 | 2 ± 0.22 | 3.5 ± 0.32 | 3.8 ± 0.35 | 3.8 ± 0.35 | 3.3 ± 0.33 | 3.6 ± 0.36 |
| CK mg/dL | 1395 ± 99.6 | 1070 ± 89.2 | 1170 ± 90.0 | 1389 ± 81.7 | 1507 ± 115.9 | 1312 ± 93.7 | 970 ± 88.2 | 960 ± 68.6 | 1828 ± 140.6 | 586 ± 45.1 | 506 ± 36.1 | 628 ± 44.9 |
| ALT U/L | 99 ± 6.6 | 237 ± 21.5 | 77 ± 5.9 | 104 ± 9.5 | 100 ± 7.7 | 111 ± 7.9 | 112 ± 8.0 | 115 ± 9.6 | 51 ± 3.9 | 262 ± 23.8 | 95 ± 6.3 | 56 ± 3.7 |
| AST U/L | 123 ± 7.7 | 140 ± 8.2 | 135 ± 10.4 | 77 ± 5.9 | 140 ± 10.0 | 171 ± 15.5 | 172 ± 11.5 | 175 ± 13.5 | 110 ± 9.2 | 219 ± 12.9 | 91 ± 5.7 | 81 ± 6.2 |
| ALP U/L | 319 ± 26.6 | 261 ± 16.3 | 281 ± 18.7 | 153 ± 13.9 | 265 ± 24.1 | 311 ± 19.4 | 240 ± 16.0 | 255 ± 15.0 | 235 ± 13.8 | 386 ± 27.6 | 199 ± 18.1 | 298 ± 21.3 |
| Time Intervals | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
|---|---|---|---|---|---|
| First interval | 6.7 | 6.5 | 6.9 | 6.7 | 6.4 |
| Second interval | 6.5 | 5.9 | 6.5 | 6.1 | 6.3 |
| Third interval | 6.5 | 5.1 | 6.2 | 5.7 | 6 |
| Fourth interval | 6.5 | 4.2 | 6 | 5.8 | 6.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Shaer, H.; Elwakil, B.H.; Bakr, B.A.; Eldrieny, A.M.; El-Khatib, M.; Chong, K.P.; Abo Gazia, A.A. Physiotherapeutic Protocol and ZnO Nanoparticles: A Combined Novel Treatment Program against Bacterial Pyomyositis. Biology 2022, 11, 1393. https://doi.org/10.3390/biology11101393
El-Shaer H, Elwakil BH, Bakr BA, Eldrieny AM, El-Khatib M, Chong KP, Abo Gazia AA. Physiotherapeutic Protocol and ZnO Nanoparticles: A Combined Novel Treatment Program against Bacterial Pyomyositis. Biology. 2022; 11(10):1393. https://doi.org/10.3390/biology11101393
Chicago/Turabian StyleEl-Shaer, Hesham, Bassma H. Elwakil, Basant A. Bakr, Ahmed M. Eldrieny, Mostafa El-Khatib, Khim Phin Chong, and Amr A. Abo Gazia. 2022. "Physiotherapeutic Protocol and ZnO Nanoparticles: A Combined Novel Treatment Program against Bacterial Pyomyositis" Biology 11, no. 10: 1393. https://doi.org/10.3390/biology11101393
APA StyleEl-Shaer, H., Elwakil, B. H., Bakr, B. A., Eldrieny, A. M., El-Khatib, M., Chong, K. P., & Abo Gazia, A. A. (2022). Physiotherapeutic Protocol and ZnO Nanoparticles: A Combined Novel Treatment Program against Bacterial Pyomyositis. Biology, 11(10), 1393. https://doi.org/10.3390/biology11101393