Added Value of Abnormal Lymph Nodes Detected with FDG-PET/CT in Suspected Vascular Graft Infection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
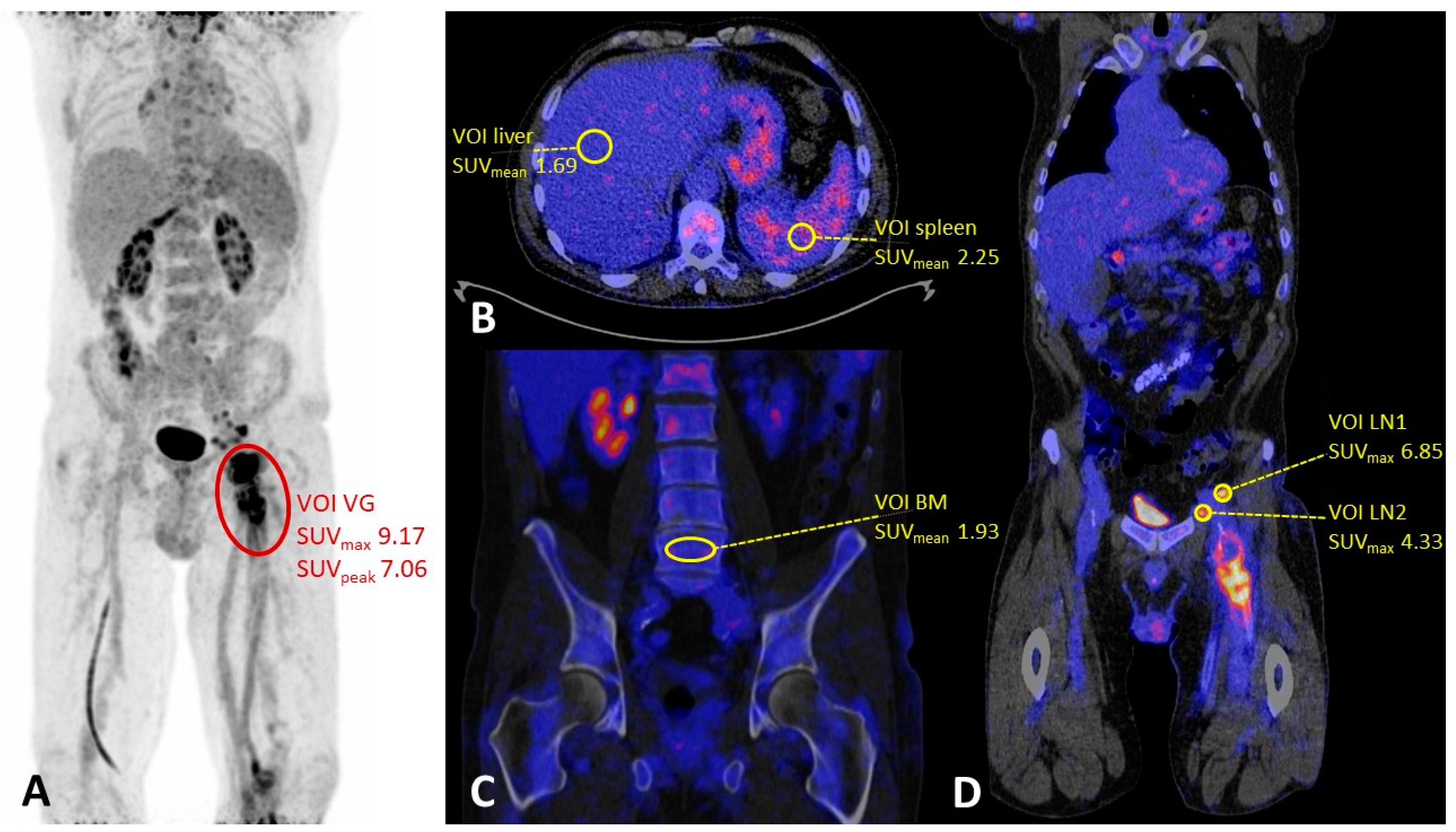
2.2. PET/CT Acquisition and Image Analysis
2.3. Statistical Analysis
3. Results
3.1. General
3.2. Qualitative Assessment
3.3. Semi-Quantitative Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reinders Folmer, E.I.; von Meijenfeldt, G.C.I.; te Riet ook genaamd Scholten, R.S.; van der Laan, M.J.; Glaudemans, A.W.J.M.; Slart, R.H.J.A.; Zeebregts, C.J.; Saleem, B.R. A systematic review and meta-analysis of 18F-fluoro-d-deoxyglucose positron emission tomography interpretation methods in vascular graft and endograft infection. J. Vasc. Surg. 2020, 72, 2174.e2–2185.e2. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Bower, T.C.; Creager, M.A.; Amin-Hanjani, S.; O’Gara, P.T.; Lockhart, P.B.; Darouiche, O.R.; Ramlawi, B.; Derdeyn, C.P.’; Bolger, A.F.; et al. Vascular Graft Infections, Mycotic Aneurysms, and Endovascular Infections: A Scientific Statement from the American Heart Association. Circulation 2016, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Gharamti, A.; Kanafani, Z.A. Vascular Graft Infections. Infect. Dis. Clin. N. Am. 2018, 32, 789–809. [Google Scholar] [CrossRef] [PubMed]
- Chakfé, N.; Diener, H.; Lejay, A.; Assadian, O.; Berard, X.; Caillon, J.; Fourneau, I.; Glaudemans, A.W.J.M.; Koncar, I.; Lindholt, J.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Vascular Graft and Endograft Infections. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 339–384. [Google Scholar] [CrossRef] [PubMed]
- Wouthuyzen-Bakker, M.; van Oosten, M.; Bierman, W.; Winter, R.; Glaudemans, A.; Slart, R.; Toren-Wielema, M.; Tielliu, I.; Zeebregts, C.J.; Prakken, N.H.J.; et al. Diagnosis and treatment of vascular graft and endograft infections: A structured clinical approach. Int. J. Infect. Dis. 2023, 126, 22–27. [Google Scholar] [CrossRef]
- Arnon-Sheleg, E.; Keidar, Z. Vascular Graft Infection Imaging. Semin. Nucl. Med. 2023, 53, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Jamar, F.; Buscombe, J.; Chiti, A.; Christian, P.E.; Delbeke, D.; Donohoe, K.J.; Israel, O.; Martin-Comin, J.; Signore, A. EANM/SNMMI Guideline for 18F-FDG Use in Inflammation and Infection. J. Nucl. Med. 2013, 54, 647–658. [Google Scholar] [CrossRef]
- Nakagawa, T.; Yamada, M.; Suzuki, Y. 18F-FDG Uptake in Reactive Neck Lymph Nodes of Oral Cancer: Relationship to Lymphoid Follicles. J. Nucl. Med. 2008, 49, 1053–1059. [Google Scholar] [CrossRef]
- Kostakoglu, L.; Agress, H.; Goldsmith, S.J. Clinical Role of FDG PET in Evaluation of Cancer Patients. RadioGraphics 2003, 23, 315–340. [Google Scholar] [CrossRef]
- ten Hove, D.; Sinha, B.; Glaudemans, A.W.J.M.; Gomes, A.; Swart, L.E.; Tanis, W.; Budde, R.P.J.; Slart, R.H.J.A. 18F-FDG-Uptake in Mediastinal Lymph Nodes in Suspected Prosthetic Valve Endocarditis: Predictor or Confounder? Front. Cardiovasc. Med. 2021, 8, 717774. [Google Scholar] [CrossRef]
- Lyons, O.T.A.; Baguneid, M.; Barwick, T.D.; Bell, R.E.; Foster, N.; Homer-Vanniasinkam, S.; Hopkins, S.; Hussain, A.; Katsanos, K.; Modarai, B.; et al. Diagnosis of Aortic Graft Infection: A Case Definition by the Management of Aortic Graft Infection Collaboration (MAGIC). Eur. J. Vasc. Endovasc. Surg. 2016, 52, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Stoner, M.C.; Calligaro, K.D.; Chaer, R.A.; Dietzek, A.M.; Farber, A.; Guzman, R.J.; Hamdan, A.D.; Landry, G.J.; Yamaguchi, D.J. Reporting standards of the Society for Vascular Surgery for endovascular treatment of chronic lower extremity peripheral artery disease. J. Vasc. Surg. 2016, 64, e1–e21. [Google Scholar] [CrossRef] [PubMed]
- Lengelé, B.; Scalliet, P. Anatomical bases for the radiological delineation of lymph node areas. Part III: Pelvis and lower limbs. Radiother. Oncol. 2009, 92, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Lengelé, B.; Nyssen-behets, C.; Scalliet, P. Anatomical bases for the radiological delineation of lymph node areas. Upper limbs, chest and abdomen. Radiother. Oncol. 2007, 84, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Morón, F.E.; Szklaruk, J. Learning the nodal stations in the abdomen. Br. J. Radiol. 2007, 80, 841–848. [Google Scholar] [CrossRef]
- Casali, M.; Lauri, C.; Altini, C.; Bertagna, F.; Cassarino, G.; Cistaro, A.; Erba, A.P.; Ferrari, C.; Mainolfi, C.G.; Palucci, A.; et al. State of the art of 18F-FDG PET/CT application in inflammation and infection: A guide for image acquisition and interpretation. Clin. Transl. Imaging 2021, 9, 299–339. [Google Scholar] [CrossRef] [PubMed]
- Kaalep, A.; Sera, T.; Oyen, W.; Krause, B.J.; Chiti, A.; Liu, Y.; Boellaard, R. EANM/EARL FDG-PET/CT accreditation—Summary results from the first 200 accredited imaging systems. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 412–422. [Google Scholar] [CrossRef]
- Boellaard, R.; Oyen, W.J.G.; Hoekstra, C.J.; Hoekstra, O.S.; Visser, E.P.; Willemsen, A.T.; Arends, B.; Verzijlbergen, F.J.; Zijlstra, J.; Paans, A.M.; et al. The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-centre trials. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 2320–2333. [Google Scholar] [CrossRef] [PubMed]
- Rojoa, D.; Kontopodis, N.; Antoniou, S.A.; Ioannou, C.V.; Antoniou, G.A. 18F-FDG PET in the Diagnosis of Vascular Prosthetic Graft Infection: A Diagnostic Test Accuracy Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 292–301. [Google Scholar] [CrossRef]
- Pijl, J.P.; Nienhuis, P.H.; Kwee, T.C.; Glaudemans, A.W.J.M.; Slart, R.H.J.A.; Gormsen, L.C. Limitations and Pitfalls of FDG-PET/CT in Infection and Inflammation. Semin. Nucl. Med. 2021, 51, 633–645. [Google Scholar] [CrossRef]
- Zeman, M.N.; Green, C.; Akin, E.A. Spectrum of [18F]FDG-PET/CT Findings in Benign Lymph Node Pathology. Mol. Imaging Biol. 2021, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Auletta, S.; Varani, M.; Horvat, R.; Galli, F.; Signore, A.; Hess, S. PET Radiopharmaceuticals for Specific Bacteria Imaging: A Systematic Review. J. Clin. Med. 2019, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, S.; Moskal, P.; Karp, J.S. State of the art in total body PET. EJNMMI Phys. 2020, 7, 35. [Google Scholar] [CrossRef] [PubMed]
| Suspected Infection (n = 59) | ||||||
|---|---|---|---|---|---|---|
| Proven Infection | Without Infection | Other Referral | Total | p | ||
| General characteristics | ||||||
| Number of patients (%) | 54 (68.4) | 5 (6.6) | 20 (25.0) | 79 (100) | ||
| Age—mean (SD) [years] | 68.6 (8.7) | 73.6 (7.4) | 67.9 (7.4) | 68.7 (8.4) | 0.383 | |
| Gender—n male (%) | 43 (79.6) | 5 (100) | 17 (85.0) | 65 (82.3) | 0.694 | |
| Body mass index—mean (SD) | 26.3 (4.3) | 24.5 (2.7) | 25.2 (3.9) | 25.9 (4.1) | 0.448 | |
| Medical history | ||||||
| Tobacco usage—n (%) * | 0.858 | |||||
| Non-smoker | 22 (44.0) | 1 (20.0) | 7 (41.2) | 30 (41.7) | ||
| Former smoker | 4 (8.0) | 1 (20.0) | 2 (11.8) | 7 (9.7) | ||
| Current smoker | 24 (48.0) | 3 (60.0) | 8 (47.1) | 35 (48.6) | ||
| Diabetes—n (%) | 18 (33.3) | 1 (20.0) | 2 (10.0) | 21 (26.6) | 0.106 | |
| Hypertension—n (%) | 25 (46.3) | 3 (60.0) | 16 (80.0) | 44 (55.7) | 0.027 | |
| Hyperlipidemia—n (%) | 13 (24.1) | 4 (80.0) | 12 (60.0) | 29 (36.7) | 0.002 | |
| Cardiac status—n (%) | 32 (59.3) | 4 (80.0) | 10 (50.0) | 46 (58.2) | 0.507 | |
| Pulmonary status—n (%) | 16 (29.6) | 2 (40.0) | 6 (30.0) | 24 (30.4) | 0.920 | |
| Renal status—n (%) | 15 (27.8) | 0 (0) | 9 (45.0) | 24 (30.4) | 0.118 | |
| Surgical characteristics | ||||||
| Type of surgical procedure—n (%) | 0.011 | |||||
| Open | 43 (79.6) | 3 (60.0) | 9 (45.0) | 55 (69.6) | ||
| Endovascular | 11 (20.4) | 2 (40.0) | 11 (55.0) | 24 (30.4) | ||
| Graft location—n (%) | ** | 0.315 | ||||
| Aortoiliac | 18 (33.3) | 3 (60.0) | 11 (57.9) | 32 (40.5) | ||
| Iliofemoral | 19 (35.2) | 1 (20.0) | 3 (15.8) | 23 (29.1) | ||
| Other | 17 (31.5) | 1 (20.0) | 5 (26.3) | 23 (29.1) | ||
| Time from last surgery to PET imaging in months – mean (SD) | 47.2 (54.0) | 43.0 (31.4) | 55.2 (50.9) | 48.9 (51.8) | 0.814 | |
| Other clinical characteristics | ||||||
| Use of antibiotics before PET/CT scan—n (%) | 44 (83.0) ** | 3 (75.0) ** | 5 (26.3) ** | 52 (68.4) | <0.001 | |
| Blood glucose level at time of 18F-FDG injection – mean (SD) [mmol/L] | 5.7 (1.0) ** | 5.5 (0.9) | 6.2 (1.9) | 5.8 (1.3) | 0.298 | |
| Vascular Graft Infection | ||||||
|---|---|---|---|---|---|---|
| Yes (n = 54) | No (n = 25) | Total (n = 79) | p | |||
| Vascular graft | ||||||
| 18F-FDG uptake—n (%) | < 0.001 | |||||
| Positive | 49 (90.7) | 8 (32.0) | 57 (72.2) | |||
| Negative | 5 (9.3) | 17 (68.0) | 22 (27.8) | |||
| Uptake pattern—n (%) | < 0.001 | |||||
| Heterogeneous | 44 (81.5) | 3 (12.0) | 47 (59.5) | |||
| Homogeneous | 10 (18.5) | 22 (88.0) | 32 (40.5) | |||
| Lymph nodes | ||||||
| 18F-FDG uptake—n (%) | 0.014 | |||||
| Positive | 11 (20.4) | 0 (0) | 11 (13.9) | |||
| Negative | 43 (79.6) | 25 (100) | 68 (86.1) | |||
| Enlarged (>10 mm)—n (%) | 0.016 | |||||
| Yes | 15 (27.8) | 1 (4.0) | 16 (20.3) | |||
| No | 39 (72.2) | 24 (96.0) | 63 (79.7) | |||
| Combined FDG uptake and enlarged—n (%) | 0.002 | |||||
| Yes | 19 (35.2) | 1 (4.0) | 20 (25.3) | |||
| No | 35 (64.8) | 24 (96.0) | 59 (74.7) | |||
| Vascular Graft Infection | p | |||
|---|---|---|---|---|
| Yes (n = 49) | No (n = 25) | |||
| Vascular graft—mean (SD) | ||||
| SUVmax | 9.48 (4.03) | 5.37 (2.50) | <0.001 | |
| SUVpeak | 7.27 (3.24) | 4.37 (2.05) | <0.001 | |
| Lymph nodes (n = 20)—mean (SD) | ||||
| SUVmax | 4.16 (1.61) | 2.46 * | 0.321 | |
| SUVmean—mean (SD) | ||||
| Liver | 2.50 (0.64) | 2.73 (1.01) | 0.318 | |
| Spleen | 2.47 (0.58) | 2.40 (0.84) | 0.710 | |
| Bone marrow | 2.51 (0.90) | 2.11 (0.81) | 0.062 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Rijsewijk, N.D.; Helthuis, J.H.G.; Glaudemans, A.W.J.M.; Wouthuyzen-Bakker, M.; Prakken, N.H.J.; Liesker, D.J.; Saleem, B.R.; Slart, R.H.J.A. Added Value of Abnormal Lymph Nodes Detected with FDG-PET/CT in Suspected Vascular Graft Infection. Biology 2023, 12, 251. https://doi.org/10.3390/biology12020251
van Rijsewijk ND, Helthuis JHG, Glaudemans AWJM, Wouthuyzen-Bakker M, Prakken NHJ, Liesker DJ, Saleem BR, Slart RHJA. Added Value of Abnormal Lymph Nodes Detected with FDG-PET/CT in Suspected Vascular Graft Infection. Biology. 2023; 12(2):251. https://doi.org/10.3390/biology12020251
Chicago/Turabian Stylevan Rijsewijk, Nick D., Jasper H. G. Helthuis, Andor W. J. M. Glaudemans, Marjan Wouthuyzen-Bakker, Niek H. J. Prakken, David J. Liesker, Ben R. Saleem, and Riemer H. J. A. Slart. 2023. "Added Value of Abnormal Lymph Nodes Detected with FDG-PET/CT in Suspected Vascular Graft Infection" Biology 12, no. 2: 251. https://doi.org/10.3390/biology12020251
APA Stylevan Rijsewijk, N. D., Helthuis, J. H. G., Glaudemans, A. W. J. M., Wouthuyzen-Bakker, M., Prakken, N. H. J., Liesker, D. J., Saleem, B. R., & Slart, R. H. J. A. (2023). Added Value of Abnormal Lymph Nodes Detected with FDG-PET/CT in Suspected Vascular Graft Infection. Biology, 12(2), 251. https://doi.org/10.3390/biology12020251








