A Newly Discovered Forest of the Whip Coral Viminella flagellum (Anthozoa, Alcyonacea) in the Mediterranean Sea: A Non-Invasive Method to Assess Its Population Structure
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
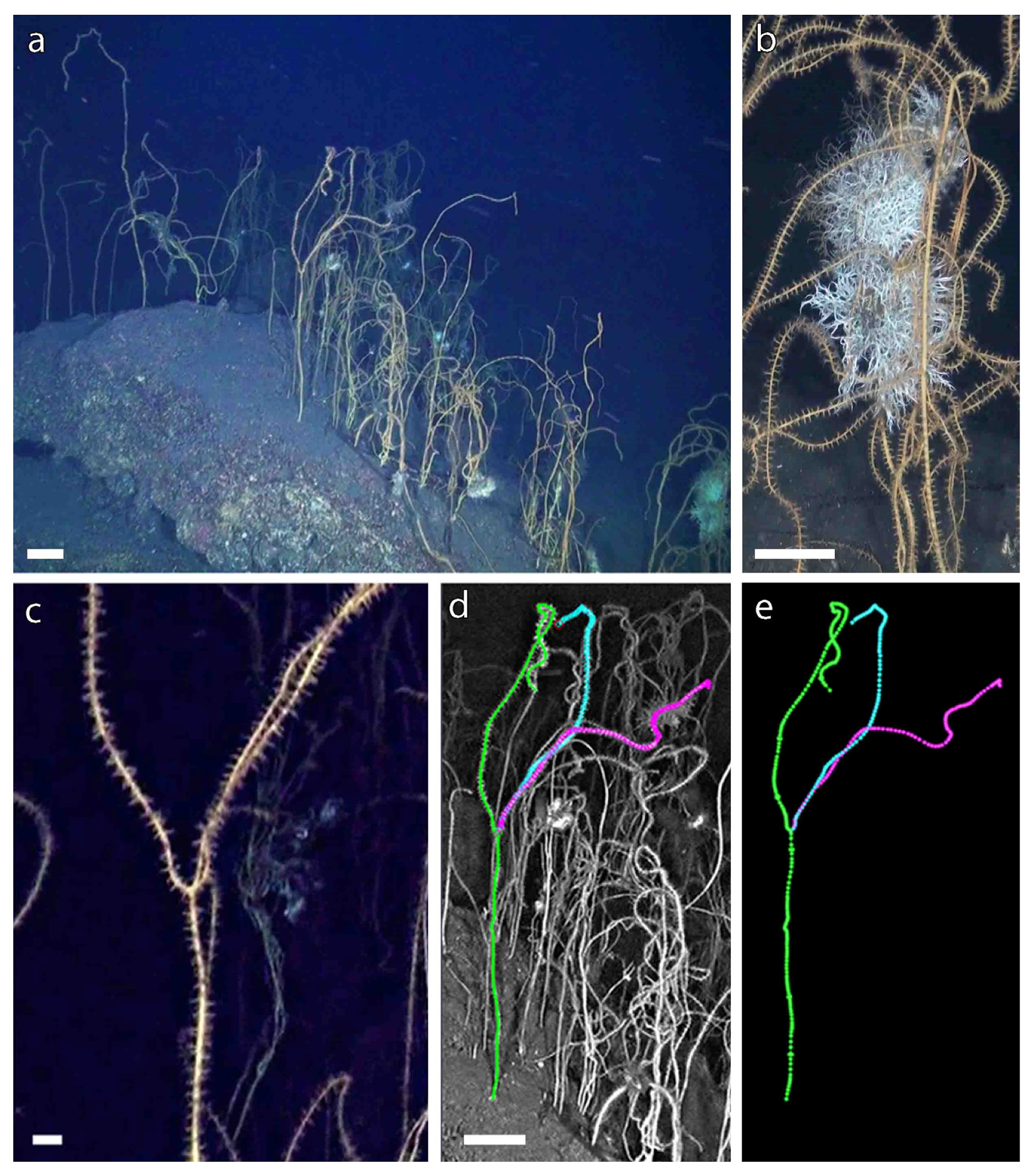
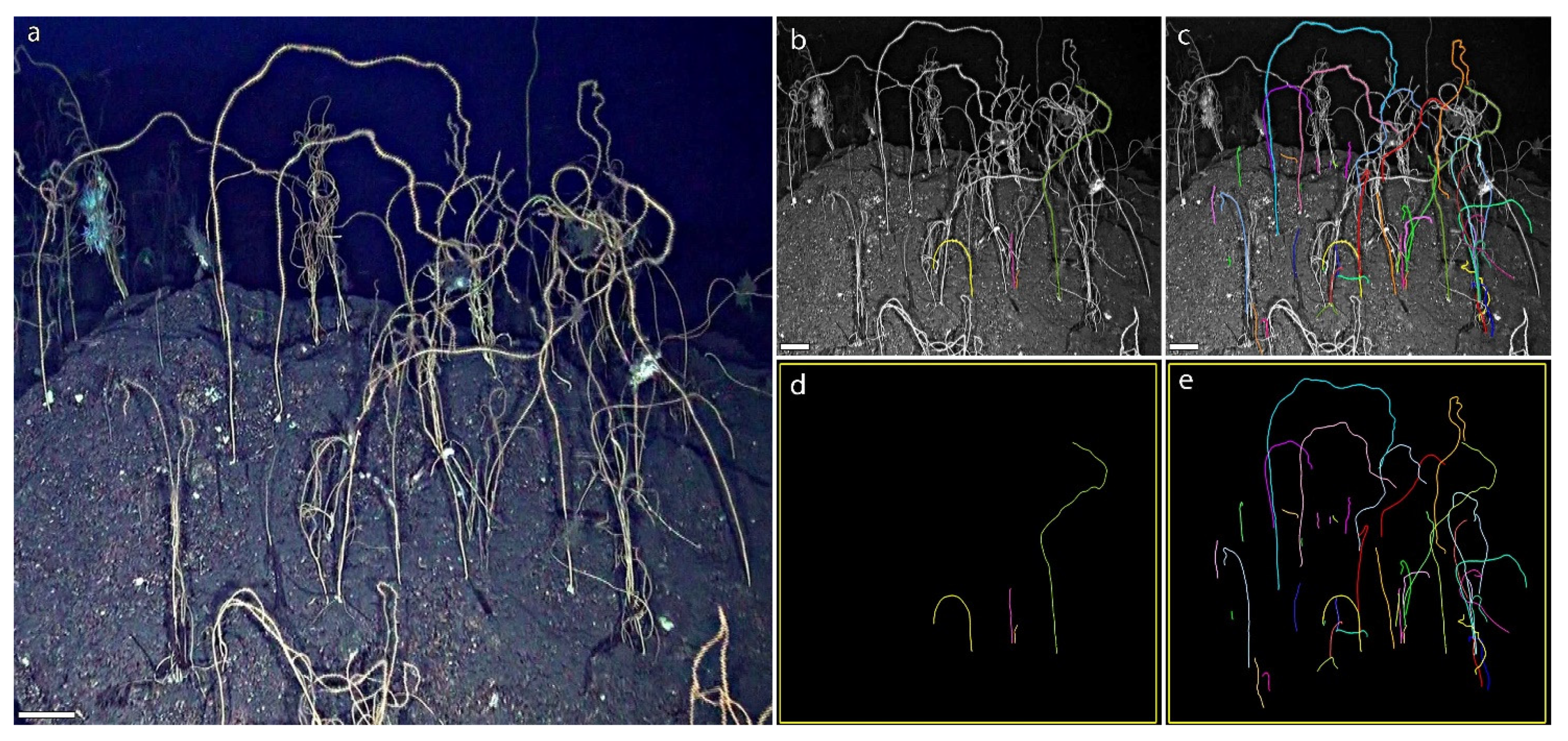
3.1. A Forest of Viminella flagellum
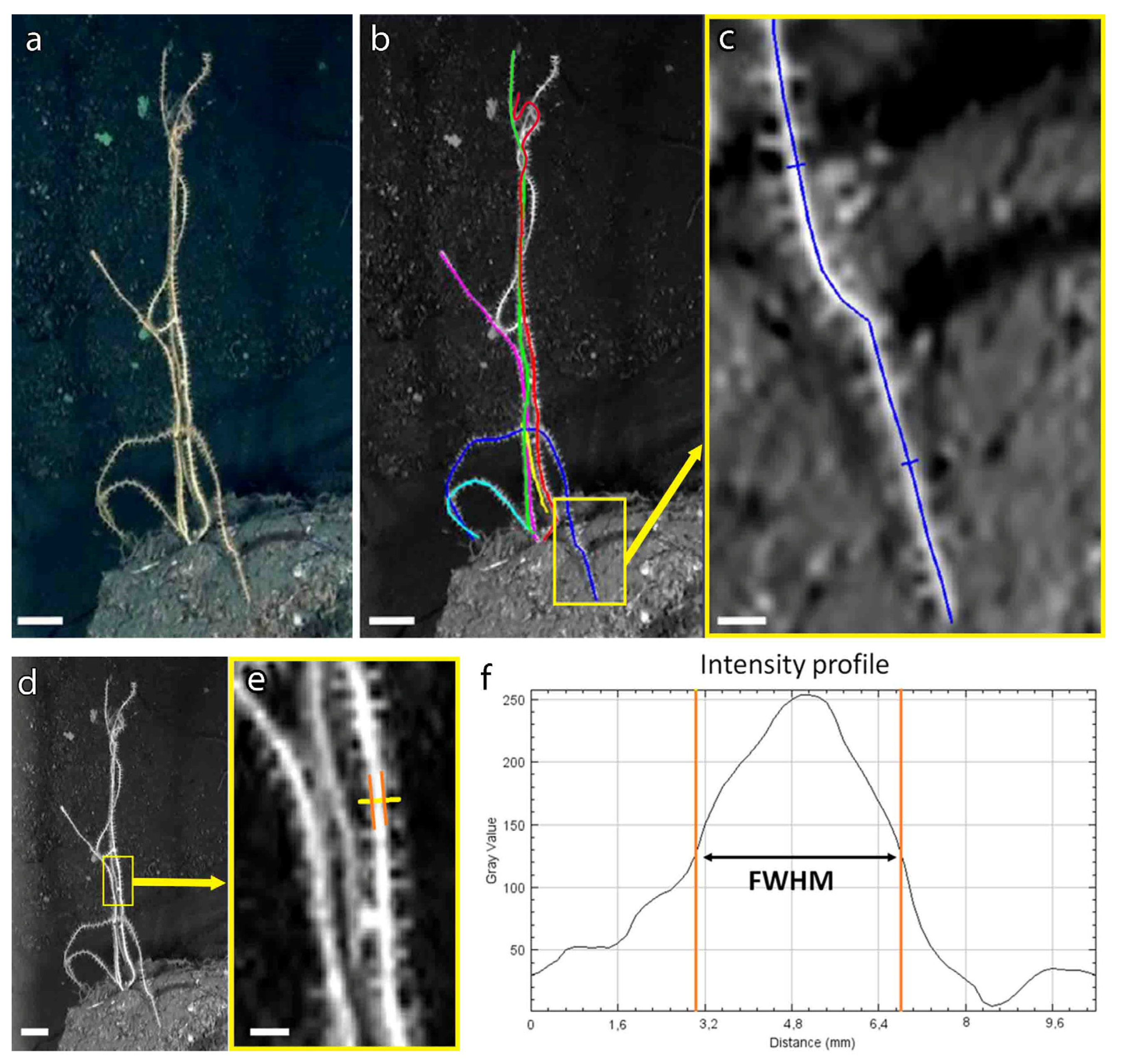
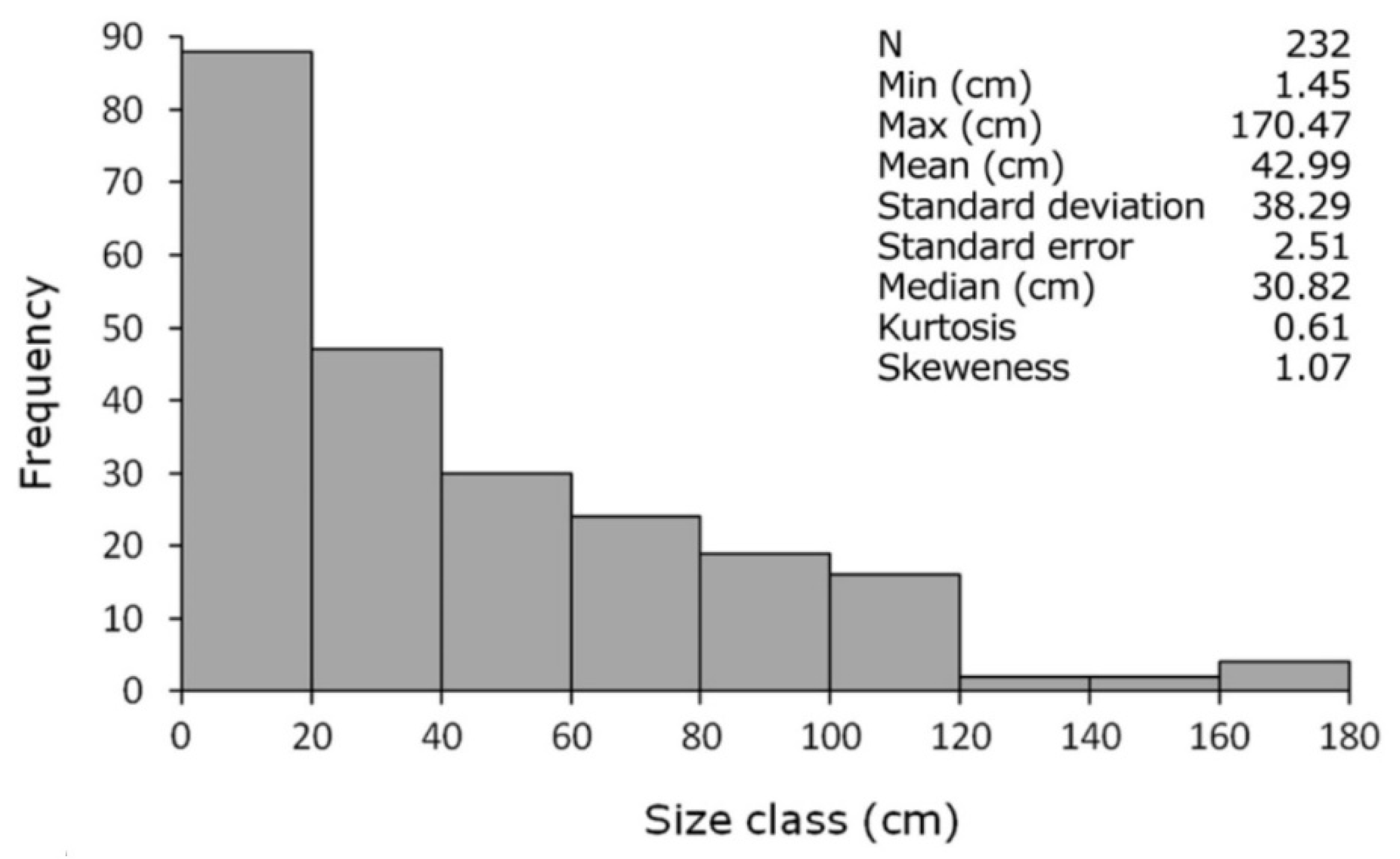
3.2. A New Method to Assess the Population Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpine, C.; Grasshoff, M. Les Gorgonaires de la Mediterranean. Bull. Inst. Océanogr. Monaco 1975, 71, 1–140. [Google Scholar]
- Chimienti, G.; Mastrototaro, F.; D’Onghia, G. Mesophotic and Deep-Sea Vulnerable Coral Habitats of the Mediterranean Sea: Overview and Conservation Perspectives. In Advances in the Studies of the Benthic Zone; Soto, L.A., Ed.; IntechOpen: London, UK, 2019; pp. 1–20. [Google Scholar]
- Grasshoff, M. Die Gorgonaria des östlichen Nordatlantik und des Mittelmeeres. I. Die Familie Ellisellidae (Cnidaria: Anthozoa). Meteor Forsch.-Ergeb. 1972, 10, 73–87. [Google Scholar]
- Fabricius, K.; Alderslade, P. Soft Corals and Sea Fans: A Comprehensive Guide to the Tropical Shallow Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea; Australian Institute of Marine Science: Townsville, Australia, 2001; pp. 1–272. [Google Scholar]
- Angiolillo, M.; Bavestrello, G.; Bo, M.; Cau, A.; Cau, A.; Giusti, M.; Salvati, E.; Tunesi, L.; Canese, S. Distribution of the deep-dwelling gorgonian Viminella flagellum in the Italian western Mediterranean Sea by means of multi-year ROV surveys. In Proceedings of the 1st Mediterranean Symposium on the conservation of Dark Habitat, Portorož, Slovenia, 27–31 October 2014; pp. 65–66. [Google Scholar]
- Cau, A.; Follesa, M.C.; Moccia, D.; Alvito, A.; Bo, M.; Angiolillo, M.; Canese, S.; Paliaga, E.M.; Orrù, P.E.; Sacco, F.; et al. Deepwater corals biodiversity along roche du large ecosystems with different habitat complexity along the south Sardinia continental margin (CW Mediterranean Sea). Mar. Biol. 2015, 162, 1865–1878. [Google Scholar] [CrossRef]
- Giusti, M.; Bo, M.; Angiolillo, M.; Cannas, R.; Cau, A.; Follesa, M.C.; Canese, S. Habitat preference of Viminella flagellum (Alcyonacea: Ellisellidae) in relation to bathymetric variables in southeastern Sardinian waters. Cont. Shelf Res. 2017, 138, 41–50. [Google Scholar] [CrossRef]
- Ocaña, O.; de Matos, V.; Aguilar, R.; García, S.; Brito, A. Illustrated catalogue of cold water corals (Cnidaria: Anthozoa) from Alboran basin and North Eastern Atlantic submarine mountains, collected in Oceana campaigns. Rev. Acad. Canar. Cienc. 2017, 29, 221–256. [Google Scholar]
- Chimienti, G.; Bo, M.; Taviani, M.; Mastrototaro, F. Occurrence and Biogeography of Mediterranean Cold-Water Corals. In Mediterranean Cold-Water Corals: Past, Present and Future; Orejas, C., Jimennez, C., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; Volume 9, pp. 213–243. [Google Scholar]
- Genin, A.; Dayton, P.K.; Lonsdale, P.F.; Spiess, F.N. Corals on seamount peaks provide evidence of current acceleration over deep-sea topography. Nature 1986, 322, 59–61. [Google Scholar] [CrossRef]
- Aguilar, R.; Pastor, X.; García, S.; Marín, P. Importance of seamount-like features for conserving Mediterranean marine habitats and threatened species. In Proceedings of the 40th CIESM Congress, Marseille, France, 28 October–1 November 2013. [Google Scholar]
- Maldonado, M.; López-Acosta, M.; Sánchez-Tocino, L.; Sitjà, C. The rare, giant gorgonian Ellisella paraplexauroides: Demographics and conservation concerns. Mar. Ecol. Progr. Ser. 2013, 479, 127–141. [Google Scholar] [CrossRef]
- Fourt, M.; Goujard, A. Rapport Final de la Campagne MEDSEACAN (Têtes des Canyons Méditerranéens Continentaux) Novembre 2008–Avril 2010; Partenariat Agence des aires marines protégées–GIS Posidonie; GIS Posidonie Publ.: Marseille, France, 2012; pp. 1–218. [Google Scholar]
- Cau, A.; Moccia, D.; Follesa, M.C.; Alvito, A.; Canese, S.; Angiolillo, M.; Coccu, D.; Bo, M.; Cannas, R. Coral forests diversity in the outer shelf of the South Sardinian continental margin. Deep-Sea Res. Part 1 Oceanogr. Res. Pap. 2017, 112, 60–70. [Google Scholar] [CrossRef]
- Bo, M.; Cerrano, C.; Canese, S.; Salvati, E.; Angiolillo, M.; Santangelo, G.; Bavestrello, G. The coral assemblages of an off-shore deep Mediterranean rocky bank (NW Sicily, Italy). Mar. Ecol. 2014, 35, 332–342. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). International Guidelines for the Management of Deep-Sea Fisheries in the High Seas; FAO: Rome, Italy, 2009; pp. 1–21. [Google Scholar]
- Chimienti, G.; D’Onghia, G.; Mastrototaro, F. Macrobenthic invertebrates. In Incidental Catch of Vulnerable Species in Mediterranean and Black Sea Fisheries—A Review; General Fisheries Commission for the Mediterranean; Studies and Reviews; Carpentieri, P., Nastasi, A., Sessa, M., Srour, A., Eds.; FAO: Rome, Italy, 2021; Volume 101, pp. 261–317. [Google Scholar]
- Angiolillo, M.; Lorenzo, B.D.; Farcomeni, A.; Bo, M.; Bavestrello, G.; Santangelo, G.; Cau, A.; Mastascusa, V.; Cau, A.; Sacco, F.; et al. Distribution and assessment of marine debris in the deep Tyrrhenian Sea (NW Mediterranean Sea, Italy). Mar. Pollut. Bull. 2015, 92, 149–159. [Google Scholar] [CrossRef]
- Otero, M.M.; Numa, C.; Bo, M.; Orejas, C.; Garrabou, J.; Cerrano, C.; Kruži´c, P.; Antoniadou, C.; Aguilar, R.; Kipson, S.; et al. Overview of the Conservation Status of Mediterranean Anthozoans; IUCN: Málaga, Spain, 2017; pp. 1–73. [Google Scholar]
- European Parliament. Council of the European Union Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for Community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off. J. Eur. Union 2008, L164, 19. [Google Scholar]
- Chimienti, G.; Bo, M.; Mastrototaro, F. Know the distribution to assess the changes: Mediterranean cold-water coral bioconstructions. Rend. Lincei Sci. Fis. Nat. 2018, 29, 583–588. [Google Scholar] [CrossRef]
- Morato, T.; González-Irusta, J.M.; Dominguez-Carrió, C.; Wei, C.L.; Davies, A.; Sweetman, A.K.; Taranto, G.H.; Beazley, L.; García-Alegre, A.; Grehan, A.; et al. Climate-induced changes in the suitable habitat of cold-water corals and commercially important deep-sea fishes in the North Atlantic. Glob. Chang. Biol. 2020, 26, 2181–2202. [Google Scholar] [CrossRef] [PubMed]
- Morato, T.; Pham, C.K.; Fauconnet, L.; Taranto, G.H.; Chimienti, G.; Cordes, E.; Dominguez-Carrió, C.; Durán Muñoz, P.; Egilsdottir, H.; González-Irusta, J.-M.; et al. North Atlantic Basin-Scale Multi-Criteria Assessment Database to Inform Effective Management and Protection of Vulnerable Marine Ecosystems. Front. Mar. Sci. 2021, 8, 637078. [Google Scholar] [CrossRef]
- Chimienti, G.; Di Nisio, A.; Lanzolla, A.M.L.; Andria, G.; Tursi, A.; Mastrototaro, F. Towards non-invasive methods to assess population structure and biomass in vulnerable sea pen fields. Sensors 2019, 19, 2255. [Google Scholar] [CrossRef]
- Chimienti, G.; De Padova, D.; Mossa, M.; Mastrototaro, F. A mesophotic black coral forest in the Adriatic Sea. Sci. Rep. 2020, 10, 8504. [Google Scholar] [CrossRef]
- Linares, C.; Coma, R.; Garrabou, J.; Díaz, D.; Zabala, M. Size distribution, density and disturbance in two Mediterranean gorgonians: Paramuricea clavata and Eunicella singularis. J. Appl. Ecol. 2008, 45, 688–699. [Google Scholar] [CrossRef]
- Grinyó, J.; Gori, A.; Ambroso, S.; Purroy, A.; Calatayud, C.; Dominguez-Carrió, C.; Coppari, M.; Iacono, C.L.; López-González, P.J.; Gili, J.M. Diversity, distribution and population size structure of deep Mediterranean gorgonian assemblages (Menorca Channel, Western Mediterranean Sea). Prog. Oceanogr. 2016, 145, 42–56. [Google Scholar] [CrossRef]
- Chimienti, G.; Di Nisio, A.; Lanzolla, A.M.L. Size/age models for monitoring of the pink sea fan Eunicella verrucosa (Cnidaria: Alcyonacea) and a case study application. J. Mar. Sci. Eng. 2020, 8, 951. [Google Scholar] [CrossRef]
- Murillo, F.J.; MacDonald, B.W.; Kenchington, E.; Campana, S.E.; Sainte-Marie, B.; Sacau, M. Morphometry and growth of sea pen species from dense habitats in the Gulf of St. Lawrence, eastern Canada. Mar. Biol. Res. 2018, 14, 366–382. [Google Scholar] [CrossRef]
- Mastrototaro, F.; Chimienti, G.; Capezzuto, F.; Carlucci, R.; Williams, G. First record of Protoptilum carpenteri (Cnidaria: Octocorallia: Pennatulacea) in the Mediterranean Sea. Ital. J. Zool. 2015, 82, 61–68. [Google Scholar] [CrossRef]
- Lupton, J.; de Ronde, C.; Sprovieri, M.; Baker, E.T.; Bruno, P.P.; Italiano, F.; Walker, S.; Faure, K.; Leybourne, M.; Britten, K.; et al. Active hydrothermal discharge on the submarine Aeolian Arc. J. Geophys. Res. 2011, 116, B02102. [Google Scholar]
- Chimienti, G.; Aguilar, R.; Gebruk, A.V.; Mastrototaro, F. Distribution and swimming ability of the deep-sea holothuroid Penilpidia ludwigi (Holothuroidea: Elasipodida: Elpidiidae). Mar. Biodiv. 2019, 49, 2369–2380. [Google Scholar] [CrossRef]
- Mastrototaro, F.; Chimienti, G.; Montesanto, F.; Perry, A.L.; García, S.H.; Alvarez, H.; Blanco, J.; Aguilar, R. Finding of the macrophagous deep-sea ascidian Dicopia antirrhinum Monniot, 1972 (Chordata: Tunicata) in the Tyrrhenian Sea and updating of its distribution. Eur. Zool. J. 2019, 86, 181–188. [Google Scholar] [CrossRef]
- Mastrototaro, F.; Aguilar, R.; Alvarez, H.; Blanco, J.; García, S.; Montesanto, F.; Perry, A.L.; Chimienti, G. Mesophotic rocks dominated by Diazona violacea: A Mediterranean codified habitat. Eur. Zool. J. 2021, 87, 688–695. [Google Scholar] [CrossRef]
- Weinberg, S. The minimal area problem in invertebrate communities of Mediterranean rocky substrata. Mar. Biol. 1978, 49, 33–40. [Google Scholar] [CrossRef]
- Chimienti, G.; Angeletti, L.; Furfaro, C.; Taviani, M. Habitat, morphology and trophism of Tritonia callogorgiae sp. nov., a large nudibranch inhabiting Callogorgia verticillata forests in the Mediterranean Sea. Deep Sea Res. I Oceanogr. Res. Pap. 2020, 165, 103364. [Google Scholar] [CrossRef]
- Chimienti, G. Vulnerable forests of the pink sea fan Eunicella verrucosa in the Mediterranean Sea. Diversity 2020, 12, 176. [Google Scholar] [CrossRef]
- Pan, J.S.; Bingham, G.P. With an eye to low vision: Optic flow enables perception despite image blur. Optom. Vis. Sci. 2013, 90, 1119–1127. [Google Scholar] [CrossRef]
- De Haan, G.; Bellers, E.B. Deinterlacing—An overview. Proc. IEEE 1998, 86, 1839–1857. [Google Scholar] [CrossRef]
- Kremer, J.R.; Mastronarde, D.N.; McIntosh, J.R. Computer Visualization of Three-Dimensional Image Data Using IMOD. J. Struct. Biol. 1996, 116, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Mastrototaro, F.; Montesanto, F.; Chimienti, G. Monitoring the seagrass Posidonia oceanica to understand the effects of local disturbances in a marine protected area. In Proceedings of the 2021 IEEE International Workshop on Metrology for the Sea (MetroSea), Virtual Conference, 4–6 October 2021. [Google Scholar]
- Komsta, L.; Novomestky, F. Moments, Cumulants, Skewness, Kurtosis and Related Tests. R Package Version 0.13. 2012. Available online: http://CRAN.R-project.org/package=moments (accessed on 10 October 2021).
- Anscombe, F.J.; Gynn, W.J. Distribution of the kurtosis statistic b2 for normal samples. Biometrika 1983, 70, 227–234. [Google Scholar] [CrossRef]
- Grasshoff, M.; Bargibant, G. Coral Reef Gorgonians of New Caledonia; IRD Editions: Paris, France, 2001. [Google Scholar]
- Walker, T.A.; Bull, G.D. A newly discovered method of reproduction in gorgonian coral. Mar. Ecol. Prog. Ser. 1983, 12, 137–143. [Google Scholar] [CrossRef]
- Bak, R.P.M.; Meesters, E.H. Coral population structure: The hidden information of colony size-frequency distributions. Mar. Ecol. Prog. Ser. 1998, 162, 301–306. [Google Scholar] [CrossRef]
- Meesters, E.H.; Hilterman, M.; Kardinaal, E.; Keetman, M.; deVries, M.; Bak, R.P.M. Colony size-frequency distributions of scleractinian coral populations: Spatial and interspecific variation. Mar. Ecol. Prog. Ser. 2001, 209, 43–54. [Google Scholar] [CrossRef]
- Rakka, M.; Sampaio, I.; Colaço, A.; Carreiro-Silva, M. Reproductive Features of the Deep-Sea Octocorals Dentomuricea aff. Meteor and Viminella flagellum in the Azores; PANGAEA: Chicago, IL, USA, 2020. [Google Scholar]
- Giusti, M.; Bo, M.; Bavestrello, G.; Angiolillo, M.; Salvati, E.; Canese, S. Record of Viminella flagellum (Alcyonacea: Ellisellidae) in Italian waters (Mediterranean Sea). Mar. Biodivers. Rec. 2012, 5, E34. [Google Scholar] [CrossRef][Green Version]
- Chimienti, G.; De Padova, D.; Adamo, M.; Mossa, M.; Bottalico, A.; Lisco, A.; Ungaro, N.; Mastrototaro, F. Effects of global warming on Mediterranean coral forests. Sci. Rep. 2021, 11, 20703. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chimienti, G.; Aguilar, R.; Maiorca, M.; Mastrototaro, F. A Newly Discovered Forest of the Whip Coral Viminella flagellum (Anthozoa, Alcyonacea) in the Mediterranean Sea: A Non-Invasive Method to Assess Its Population Structure. Biology 2022, 11, 39. https://doi.org/10.3390/biology11010039
Chimienti G, Aguilar R, Maiorca M, Mastrototaro F. A Newly Discovered Forest of the Whip Coral Viminella flagellum (Anthozoa, Alcyonacea) in the Mediterranean Sea: A Non-Invasive Method to Assess Its Population Structure. Biology. 2022; 11(1):39. https://doi.org/10.3390/biology11010039
Chicago/Turabian StyleChimienti, Giovanni, Ricardo Aguilar, Michela Maiorca, and Francesco Mastrototaro. 2022. "A Newly Discovered Forest of the Whip Coral Viminella flagellum (Anthozoa, Alcyonacea) in the Mediterranean Sea: A Non-Invasive Method to Assess Its Population Structure" Biology 11, no. 1: 39. https://doi.org/10.3390/biology11010039
APA StyleChimienti, G., Aguilar, R., Maiorca, M., & Mastrototaro, F. (2022). A Newly Discovered Forest of the Whip Coral Viminella flagellum (Anthozoa, Alcyonacea) in the Mediterranean Sea: A Non-Invasive Method to Assess Its Population Structure. Biology, 11(1), 39. https://doi.org/10.3390/biology11010039







