Mapping the Energetic Costs of Free-Swimming Gilthead Sea Bream (Sparus aurata), a Key Species in European Marine Aquaculture
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
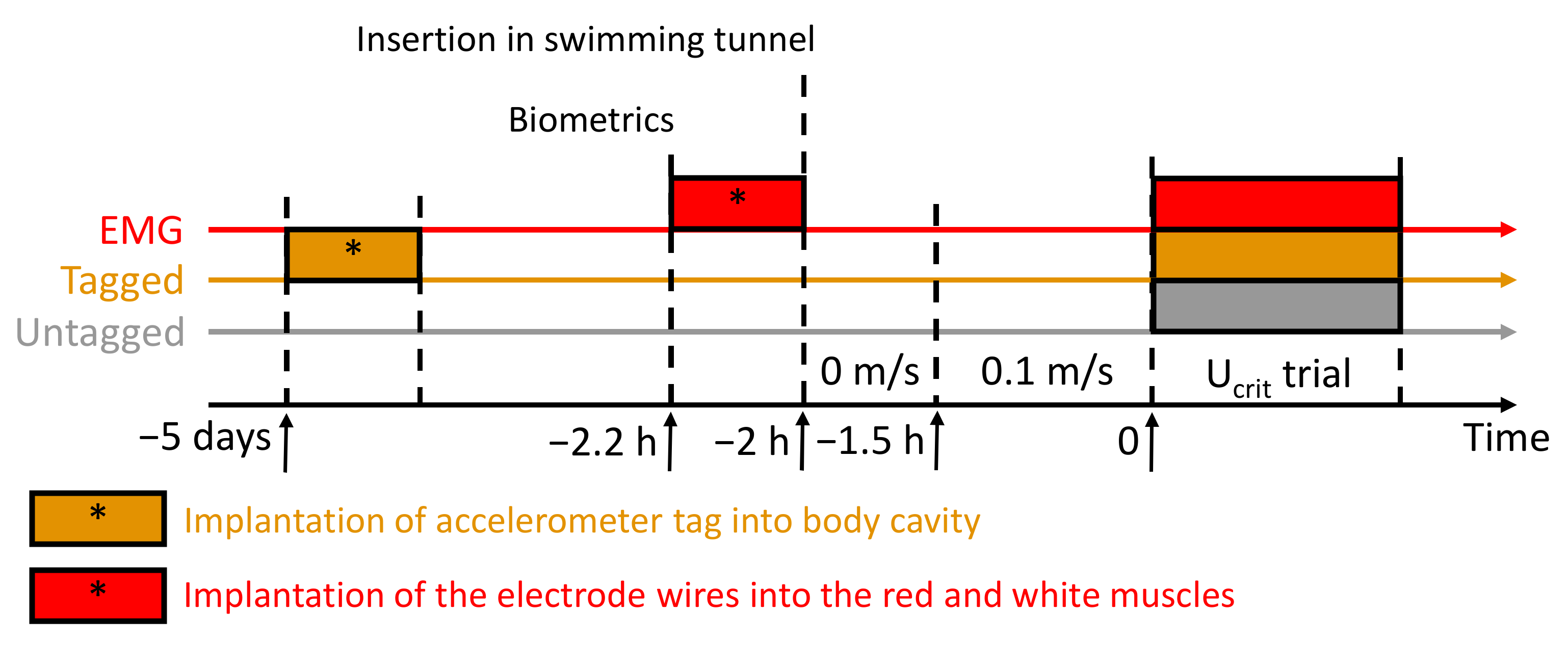
2.1. Fish-Holding Conditions
2.2. Critical Swimming Speed Tests (Ucrit), Estimation of Metabolic Traits, and Cost of Transport
2.3. EMG Analysis: Monitoring of the Red and White Muscle Activities during the Ucrit Trial
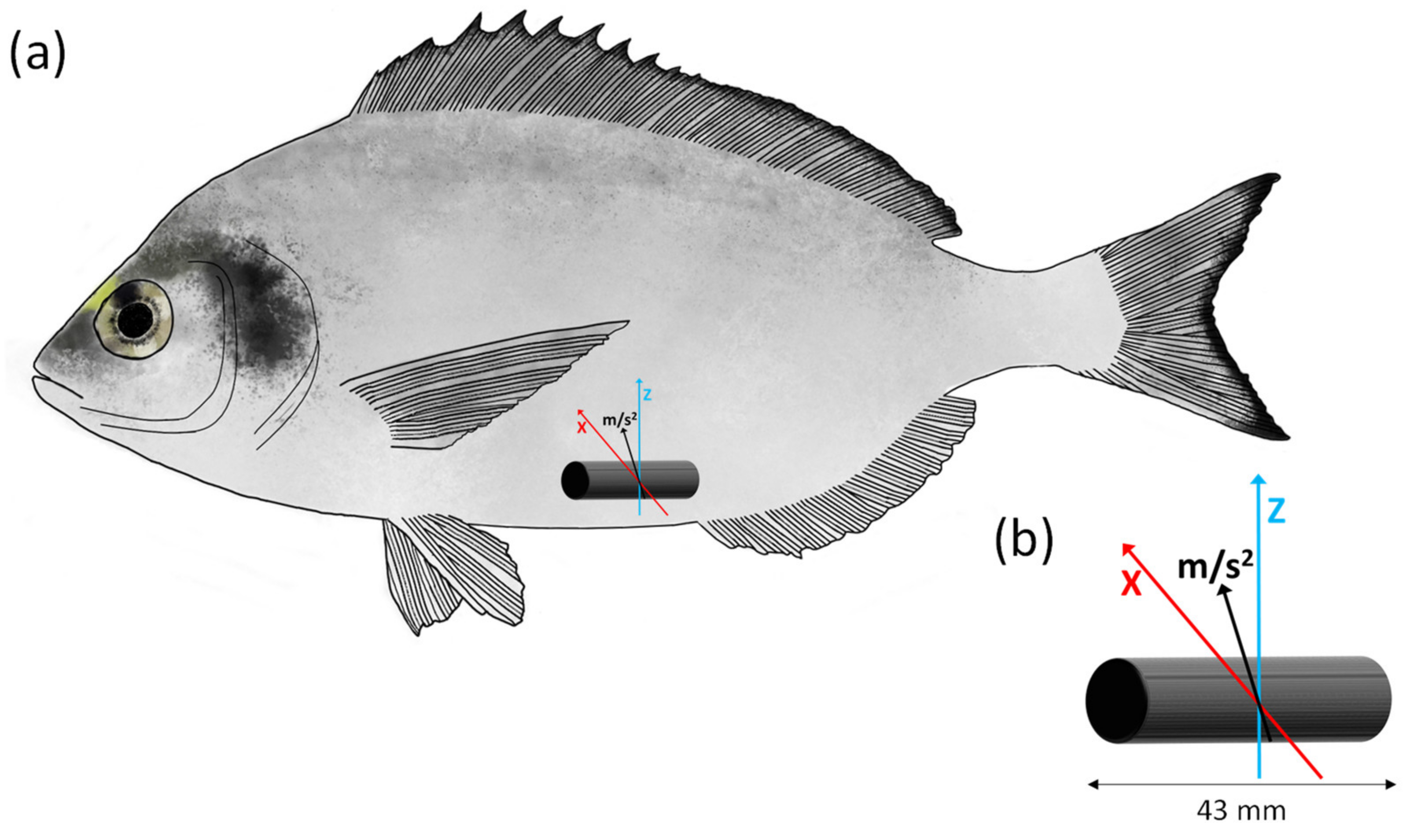
2.4. Implantation of Accelerometer Tags: Recording Fish Acceleration during the Ucrit Trial
2.5. Statistical Analyses
3. Results
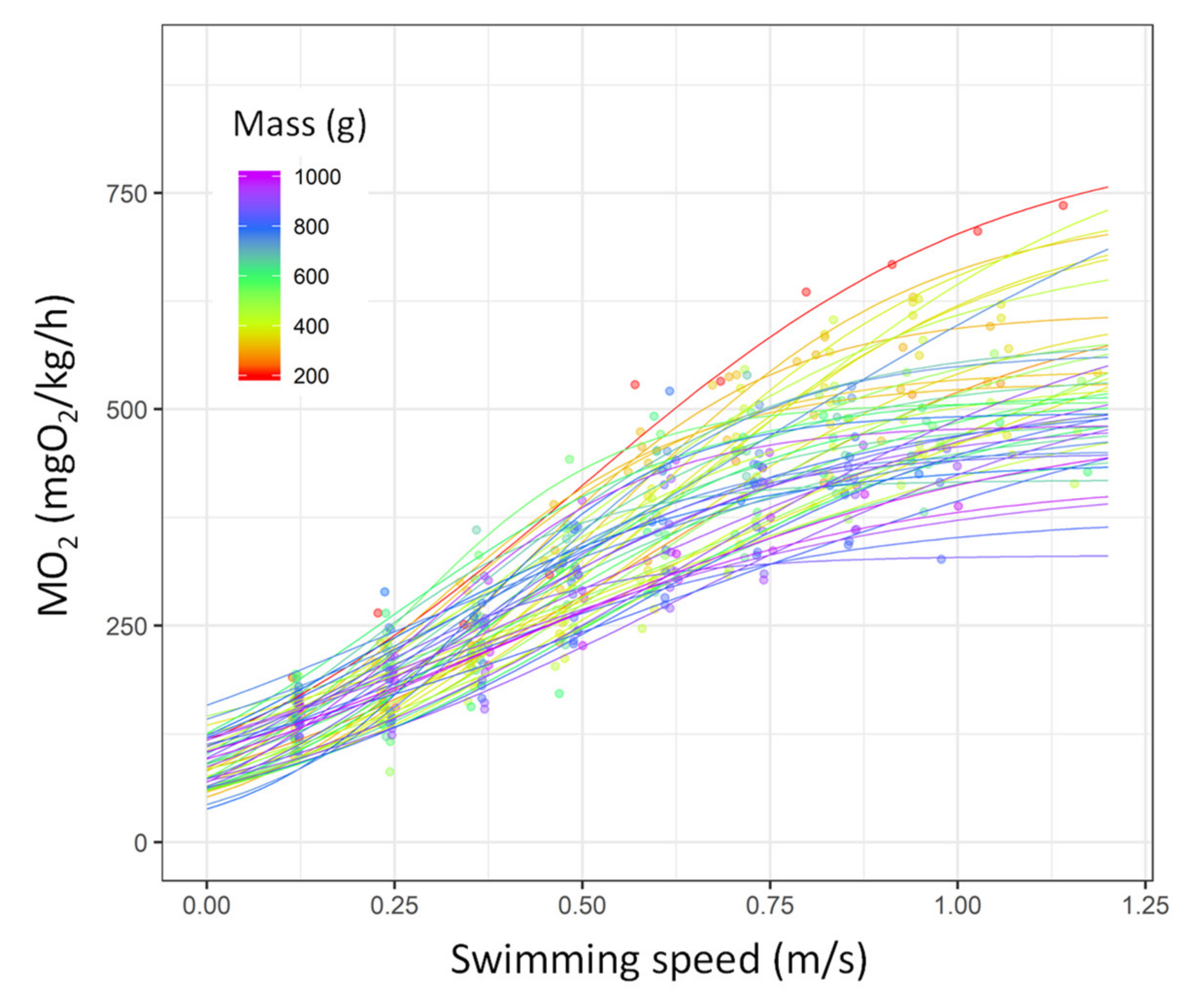
3.1. Critical Swimming Speed (Ucrit), Estimation of the Metabolic Traits, and COT
3.2. Red and White Muscle Activation Patterns during the Critical Swimming Test
3.3. Correlation of Acceleration Recorded by the Tags with the MO2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKenzie, D.J.; Axelsson, M.; Chabot, D.; Claireaux, G.; Cooke, S.J.; Corner, R.A.; De Boeck, G.; Domenici, P.; Guerreiro, P.M.; Hamer, B.; et al. Conservation physiology of marine fishes: State of the art and prospects for policy. Conserv. Physiol. 2016, 4, cow046. [Google Scholar] [CrossRef] [PubMed]
- Chabot, D.; McKenzie, D.J.; Craig, J.F. Metabolic rate in fishes: Definitions, methods and significance for conservation physiology. J. Fish Biol. 2016, 88, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Norin, T.; Clark, T.D. Measurement and relevance of maximum metabolic rate in fishes. J. Fish Biol. 2016, 88, 122–151. [Google Scholar] [CrossRef] [PubMed]
- Brett, J.R. The metabolic demand for oxygen in fish, particularly salmonids, and a comparison with other vertebrates. Respir. Physiol. 1972, 14, 151–170. [Google Scholar] [CrossRef]
- Fry, F.E.J. Effects of the environment on animal activity. Publ. Ont. Fish. Res. Lab. 1947, 68, 1–62. [Google Scholar]
- Nelson, J.A. Oxygen consumption rate v. rate of energy utilization of fishes: A comparison and brief history of the two measurements. J. Fish Biol. 2016, 88, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Mckenzie, D.J. Energetics of Fish Swimming; Elsevier Inc.: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Cooke, S.J.; Thorstad, E.B.; Hinch, S.G. Activity and energetics of free-swimming fish: Insights from electromyogram telemetry. Fish Fish. 2004, 5, 21–52. [Google Scholar] [CrossRef]
- Zupa, W.; Carbonara, P.; Spedicato, M.T.; Lembo, G. Modelling swimming activities and energetic costs in European sea bass (Dicentrarchus labrax L., 1758) during critical swimming tests. Mar. Freshw. Behav. Physiol. 2015, 48, 341–357. [Google Scholar] [CrossRef]
- Cooke, S.J.; Chandroo, K.P.; Beddow, T.A.; Moccia, R.D.; McKinley, R.S. Swimming activity and energetic expenditure of captive rainbow trout Oncorhynchus mykiss (Walbaum) estimated by electromyogram telemetry. Aquac. Res. 2000, 31, 495–505. [Google Scholar] [CrossRef]
- Beddow, T.A.; Mckinley, R.S. Effects of Thermal Environment on Electromyographical Signals Obtained from Atlantic Salmon (Salmo salar L.) during Forced Swimming. Hydrobiologia 1998, 371/372, 225–232. [Google Scholar] [CrossRef]
- Altringham, J.D.; Ellerby, D.J. Fish swimming: Patterns in muscle function. J. Exp. Biol. 1999, 202, 3397–3403. [Google Scholar] [CrossRef] [PubMed]
- Jayne, B.C.; Lauder, G.V. How swimming fish use slow and fast muscle fibers: Implications for models of vertebrate muscle recruitment. J. Comp. Physiol. A. 1994, 175, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Gesto, M.; Zupa, W.; Alfonso, S.; Spedicato, M.T.; Lembo, G.; Carbonara, P. Using acoustic telemetry to assess behavioral responses to acute hypoxia and ammonia exposure in farmed rainbow trout of different competitive ability. Appl. Anim. Behav. Sci. 2020, 230, 105084. [Google Scholar] [CrossRef]
- Carbonara, P.; Dioguardi, M.; Cammarata, M.; Zupa, W.; Vazzana, M.; Spedicato, M.T.; Lembo, G. Basic knowledge of social hierarchies and physiological profile of reared sea bass Dicentrarchus labrax (L.). PLoS ONE 2019, 14, 1–16. [Google Scholar] [CrossRef]
- Carbonara, P.; Alfonso, S.; Zupa, W.; Manfrin, A.; Fiocchi, E.; Pretto, T.; Spedicato, M.T.; Lembo, G. Behavioral and physiological responses to stocking density in sea bream (Sparus aurata): Do coping styles matter? Physiol. Behav. 2019, 212, 112698. [Google Scholar] [CrossRef]
- Metcalfe, J.D.; Wright, S.; Tudorache, C.; Wilson, R.P. Recent advances in telemetry for estimating the energy metabolism of wild fishes. J. Fish Biol. 2016, 88, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Lembo, G.; Carbonara, P.; Scolamacchia, M.; Spedicato, M.T.; McKinley, R.S. Use of muscle activity indices as a relative measure of well-being in cultured sea bass Dicentrarchus labrax (Linnaeus, 1758). Hydrobiologia 2007, 582, 271–280. [Google Scholar] [CrossRef]
- Lembo, G.; Carbonara, P.; Scolamacchia, M.; Spedicato, M.T.; Bjørnsen, J.E.; Holand, B.; McKinley, R.S. Introduction of a new physiological acoustic electromyogram transmitter. Fish. Manag. Ecol. 2008, 15, 333–338. [Google Scholar] [CrossRef]
- Brijs, J.; Sandblom, E.; Axelsson, M.; Sundell, K.; Sundh, H.; Huyben, D.; Broström, R.; Kiessling, A.; Berg, C.; Gräns, A. The final countdown: Continuous physiological welfare evaluation of farmed fish during common aquaculture practices before and during harvest. Aquaculture 2018, 495, 903–911. [Google Scholar] [CrossRef]
- Brijs, J.; Sandblom, E.; Axelsson, M.; Sundell, K.; Sundh, H.; Kiessling, A.; Berg, C.; Gräns, A. Remote physiological monitoring provides unique insights on the cardiovascular performance and stress responses of freely swimming rainbow trout in aquaculture. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, P.; Zupa, W.; Bitetto, I.; Alfonso, S.; Dara, M.; Cammarata, M. Evaluation of the Effects of the Enriched-Organic Diets Composition on European Sea Bass Welfare through a Multi-Parametric Approach. J. Mar. Sci. Eng. 2020, 8, 934. [Google Scholar] [CrossRef]
- Svendsen, E.; Føre, M.; Økland, F.; Gräns, A.; Hedger, R.D.; Alfredsen, J.A.; Uglem, I.; Rosten, C.M.; Frank, K.; Erikson, U.; et al. Heart rate and swimming activity as stress indicators for Atlantic salmon (Salmo salar). Aquaculture 2021, 531, 735804. [Google Scholar] [CrossRef]
- Carbonara, P.; Alfonso, S.; Dioguardi, M.; Zupa, W.; Vazzana, M.; Dara, M.; Spedicato, M.T.; Lembo, G.; Cammarata, M. Calibrating accelerometer data, as a promising tool for health and welfare monitoring in aquaculture: Case study in European sea bass (Dicentrarchus labrax) in conventional or organic aquaculture. Aquac. Reports 2021, 21, 4–13. [Google Scholar] [CrossRef]
- Brijs, J.; Føre, M.; Gräns, A.; Clark, T.D.; Axelsson, M.; Johansen, J.L. Bio-sensing technologies in aquaculture: How remote monitoring can bring us closer to our farm animals. Philos. Trans. R. Soc. B 2021, 376, 20200218. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S. The role of sensors, big data and machine learning in modern animal farming. Sens. Bio-Sens. Res. 2020, 29, 100367. [Google Scholar] [CrossRef]
- Halachmi, I.; Guarino, M.; Bewley, J.; Pastell, M. Smart Animal Agriculture: Application of Real-Time Sensors to Improve Animal Well-Being and Production. Annu. Rev. Anim. Biosci. 2019, 7, 403–425. [Google Scholar] [CrossRef]
- Føre, M.; Frank, K.; Norton, T.; Svendsen, E.; Alfredsen, J.A.; Dempster, T.; Eguiraun, H.; Watson, W.; Stahl, A.; Sunde, L.M.; et al. Precision fish farming: A new framework to improve production in aquaculture. Biosyst. Eng. 2018, 173, 176–193. [Google Scholar] [CrossRef]
- Wilson, S.M.; Hinch, S.G.; Eliason, E.J.; Farrell, A.P.; Cooke, S.J. Calibrating acoustic acceleration transmitters for estimating energy use by wild adult Pacific salmon. Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 2013, 164, 491–498. [Google Scholar] [CrossRef]
- Thorstad, E.B.; Rikardsen, A.H.; Alp, A.; Økland, F. The use of eletronic tags in fish research—An overview of fish telemetry methods. Turk. J. Fish. Aquat. Sci. 2013, 13, 881–896. [Google Scholar] [CrossRef]
- Payne, N.L.; Gillanders, B.M.; Seymour, R.S.; Webber, D.M.; Snelling, E.P.; Semmens, J.M. Accelerometry estimates field metabolic rate in giant Australian cuttlefish Sepia apama during breeding. J. Anim. Ecol. 2011, 80, 422–430. [Google Scholar] [CrossRef]
- Zupa, W.; Alfonso, S.; Gai, F.; Gasco, L.; Spedicato, M.T.; Lembo, G.; Carbonara, P. Calibrating Accelerometer Tags with Oxygen Consumption Rate of Rainbow Trout (Oncorhynchus mykiss) and Their Use in Aquaculture Facility: A Case Study. Animals 2021, 11, 1496. [Google Scholar] [CrossRef] [PubMed]
- Halsey, L.G.; Shepard, E.L.C.; Wilson, R.P. Assessing the development and application of the accelerometry technique for estimating energy expenditure. Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 2011, 158, 305–314. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Basaran, F.; Ozbilgin, H.; Ozbilgin, Y.D. Comparison of the swimming performance of farmed and wild gilthead sea bream, Sparus aurata. Aquac. Res. 2007, 38, 452–456. [Google Scholar] [CrossRef]
- Remen, M.; Nederlof, M.A.J.; Folkedal, O.; Thorsheim, G.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J.; Oppedal, F.; Olsen, R.E. Effect of temperature on the metabolism, behaviour and oxygen requirements of Sparus aurata. Aquac. Environ. Interact. 2015, 7, 115–123. [Google Scholar] [CrossRef]
- Svendsen, J.C.; Tirsgaard, B.; Cordero, G.A.; Steffensen, J.F. Intraspecific variation in aerobic and anaerobic locomotion: Gilthead sea bream (Sparus aurata) and Trinidadian guppy (Poecilia reticulata) do not exhibit a trade-off between maximum sustained swimming speed and minimum cost of transport. Front. Physiol. 2015, 6, 43. [Google Scholar] [CrossRef]
- Arechavala-lopez, P.; Lankheet, M.J.; Díaz-gil, C.; Abbink, W. Swimming Activity of Gilthead Seabream (Sparus aurata) in Swim-Tunnels: Accelerations, Oxygen Consumption and Body Motion. Front. Anim. Sci. 2021, 2, 1–14. [Google Scholar] [CrossRef]
- Palstra, A.P.; Kals, J.; Böhm, T.; Bastiaansen, J.W.M.; Komen, H. Swimming Performance and Oxygen Consumption as Non-lethal Indicators of Production Traits in Atlantic Salmon and Gilthead Seabream. Front. Physiol. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hachim, M.; Rouyer, T.; Dutto, G.; Kerzerho, V.; Bernard, S.; Bourjea, J.; McKenzie, D.J. Oxygen uptake, heart rate and activities of locomotor muscles during a critical swimming speed protocol in the gilthead sea bream Sparus aurata. J. Fish Biol. 2021, 98, 886–890. [Google Scholar] [CrossRef]
- McFarlane, W.J.; Cubitt, K.F.; Williams, H.; Rowsell, D.; Moccia, R.; Gosine, R.; McKinley, R.S. Can feeding status and stress level be assessed by analyzing patterns of muscle activity in free swimming rainbow trout (Oncorhynchus mykiss Walbaum)? Aquaculture 2004, 239, 467–484. [Google Scholar] [CrossRef]
- Coyle, S.D.; Durborow, R.M.; Tidwell, J.H. Anesthetics in Aquaculture. South. Reg. Aquac. Cent. 2004, 3900, 1–6. [Google Scholar]
- Martos-Sitcha, J.A.; Simó-Mirabet, P.; Piazzon, M.C.; de las Heras, V.; Calduch-Giner, J.A.; Puyalto, M.; Tinsley, J.; Makol, A.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Dietary sodium heptanoate helps to improve feed efficiency, growth hormone status and swimming performance in gilthead sea bream (Sparus aurata). Aquac. Nutr. 2018, 24, 1638–1651. [Google Scholar] [CrossRef]
- Carbonara, P.; Corsi, I.; Focardi, S.; Lembo, G.; Rochira, S.; Scolamacchia, M.; Spedicato, M.T.; Mckinley, R.S. The effects of stress induced by cortisol administration on the repeatability of swimming performance tests in the European sea bass (Dicentrarchus labrax L.). Mar. Freshw. Behav. Physiol. 2010, 43, 283–296. [Google Scholar] [CrossRef]
- Brett, J.R. The Respiratory Metabolism and Swimming Performance of Young Sockeye Salmon. J. Fish. Res. Board Can. 1964, 21, 1183–1226. [Google Scholar] [CrossRef]
- Smit, H.; Amelink-Koutstaal, J.M.; Vijverberg, J.; Von Vaupel-Klein, J.C. Oxygen consumption and efficiency of swimming goldfish. Comp. Biochem. Physiol.-Part A Physiol. 1971, 39, 1–28. [Google Scholar] [CrossRef]
- Kline, R.J.; Parkyn, D.C.; Murie, D.J. Empirical Modelling of Solid-blocking Effect in a Blazka Respirometer for Gag, a Large Demersal Reef Fish. Adv. Zool. Bot. 2015, 3, 193–202. [Google Scholar] [CrossRef]
- Claireaux, G.; Couturier, C.; Groison, A.-L. Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). J. Exp. Biol. 2006, 209, 3420–3428. [Google Scholar] [CrossRef]
- Alfonso, S.; Zupa, W.; Manfrin, A.; Fiocchi, E.; Dioguardi, M.; Dara, M.; Lembo, G.; Carbonara, P.; Cammarata, M. Surgical implantation of electronic tags does not induce medium—Term effect: Insights from growth and stress physiological profile in two marine fish species. Anim. Biotelemetry 2020, 8, 21. [Google Scholar] [CrossRef]
- Brownscombe, J.W.; Lennox, R.J.; Danylchuk, A.J.; Cooke, S.J. Estimating fish swimming metrics and metabolic rates with accelerometers: The influence of sampling frequency. J. Fish Biol. 2018, 93, 207–214. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team R Software. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Chabot, D.; Steffensen, J.F.; Farrell, A.P. The determination of standard metabolic rate in fishes. J. Fish Biol. 2016, 88, 81–121. [Google Scholar] [CrossRef]
- Geist, D.R.; Brown, R.S.; Cullinan, V.I.; Mesa, M.G.; Vanderkooi, S.P.; McKinstry, C.A. Relationships between metabolic rate, muscle electromyograms and swim performance of adult chinook salmon. J. Fish Biol. 2003, 63, 970–989. [Google Scholar] [CrossRef]
- Sfakiotakis, M.; Lane, D.M.; Davies, J.B.C. Review of fish swimming modes for aquatic locomotion. IEEE J. Ocean. Eng. 1999, 24, 237–252. [Google Scholar] [CrossRef]
- Jayne, B.; Lauder, G. Are muscle fibers within fish myotomes activated synchronously? Patterns of recruitment within deep myomeric musculature during swimming in largemouth bass. J. Exp. Biol. 1995, 198, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T. The dynamics of carangiform swimming motions. J. Fluid Mech. 1978, 87, 533–560. [Google Scholar] [CrossRef]
- Perera, E.; Rosell-Moll, E.; Martos-Sitcha, J.A.; Naya-Català, F.; Simó-Mirabet, P.; Calduch-Giner, J.; Manchado, M.; Afonso, J.M.; Pérez-Sánchez, J. Physiological trade-offs associated with fasting weight loss, resistance to exercise and behavioral traits in farmed gilthead sea bream (Sparus aurata) selected by growth. Aquac. Reports 2021, 20, 100645. [Google Scholar] [CrossRef]
- Føre, M.; Svendsen, E.; Økland, F.; Gräns, A.; Alfredsen, J.A.; Finstad, B.; Hedger, R.D.; Uglem, I. Heart rate and swimming activity as indicators of post-surgical recovery time of Atlantic salmon (Salmo salar). Anim. Biotelemetry 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Wright, S.; Metcalfe, J.D.; Hetherington, S.; Wilson, R. Estimating activity-specific energy expenditure in a teleost fish, using accelerometer loggers. Mar. Ecol. Prog. Ser. 2014, 496, 19–32. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Cataldi, E.; Romano, P.; Owen, S.F.; Taylor, E.W.; Bronzi, P. Effects of acclimation to brackish water on the growth, respiratory metabolism, and swimming performance of young-of-the-year Adriatic sturgeon (Acipenser naccarii). Can. J. Fish. Aquat. Sci. 2001, 58, 1104–1112. [Google Scholar] [CrossRef]
- Farrell, A.P.; Lee, C.G.; Tierney, K.; Hodaly, A.; Clutterham, S.; Healey, M.; Hinch, S.; Lotto, A. Field-based measurements ofoxygen uptake and swimming performance with adult Pacific salmon using a mobile respirometer swim tunnel. J. Fish Biol. 2003, 62, 64–84. [Google Scholar] [CrossRef]
- Steinhausen, M.F.; Steffensen, J.F.; Andersen, N.G. The effects of swimming pattern on the energy use of gilthead seabream (Sparus aurata L.). Mar. Freshw. Behav. Physiol. 2010, 43, 227–241. [Google Scholar] [CrossRef]
- Kır, M. Thermal tolerance and standard metabolic rate of juvenile gilthead seabream (Sparus aurata) acclimated to four temperatures. J. Therm. Biol. 2020, 93. [Google Scholar] [CrossRef]
- Ibarz, A.; Fernández-Borràs, J.; Blasco, J.; Gallardo, M.A.; Sánchez, J. Oxygen consumption and feeding rates of gilthead sea bream (Sparus aurata) reveal lack of acclimation to cold. Fish Physiol. Biochem. 2003, 29, 313–321. [Google Scholar] [CrossRef]
- Burton, T.; Killen, S.S.; Armstrong, J.D.; Metcalfe, N.B. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc. R. Soc. B Biol. Sci. 2011, 278, 3465–3473. [Google Scholar] [CrossRef]
- Jepsen, N.; Davis, L.E.; Schreck, C.B.; Siddens, B. The Physiological Response of Chinook Salmon Smolts to Two Methods of Radio-Tagging. Trans. Am. Fish. Soc. 2011, 130, 495–500. [Google Scholar] [CrossRef]
- Cruz-Font, L.; Shuter, B.J.; Blanchfield, P.J. Energetic costs of activity in wild lake trout: A calibration study using acceleration transmitters and positional telemetry. Can. J. Fish. Aquat. Sci. 2016, 73, 1237–1250. [Google Scholar] [CrossRef]
- Martos-Sitcha, J.A.; Simó-Mirabet, P.; de las Heras, V.; Calduch-Giner, J.À.; Pérez-Sánchez, J. Tissue-Specific Orchestration of Gilthead Sea Bream Resilience to Hypoxia and High Stocking Density. Front. Physiol. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Elliott, J.M.; Davison, W. Energy equivalents of oxygen consumption in animal energetics. Oecologia 1975, 19, 195–201. [Google Scholar] [CrossRef]
- Cooke, S.J.; Brownscombe, J.W.; Raby, G.D.; Broell, F.; Hinch, S.G.; Clark, T.D.; Semmens, J.M. Remote bioenergetics measurements in wild fish: Opportunities and challenges. Comp. Biochem. Physiol.-Part A Mol. Integr. Physiol. 2016, 202, 23–37. [Google Scholar] [CrossRef]
- Nyack, A.C.; Locke, B.R.; Valencia, A.; Dillaman, R.M.; Kinsey, S.T. Scaling of postcontractile phosphocreatine recovery in fish white muscle: Effect of intracellular diffusion. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R2077–R2088. [Google Scholar] [CrossRef][Green Version]
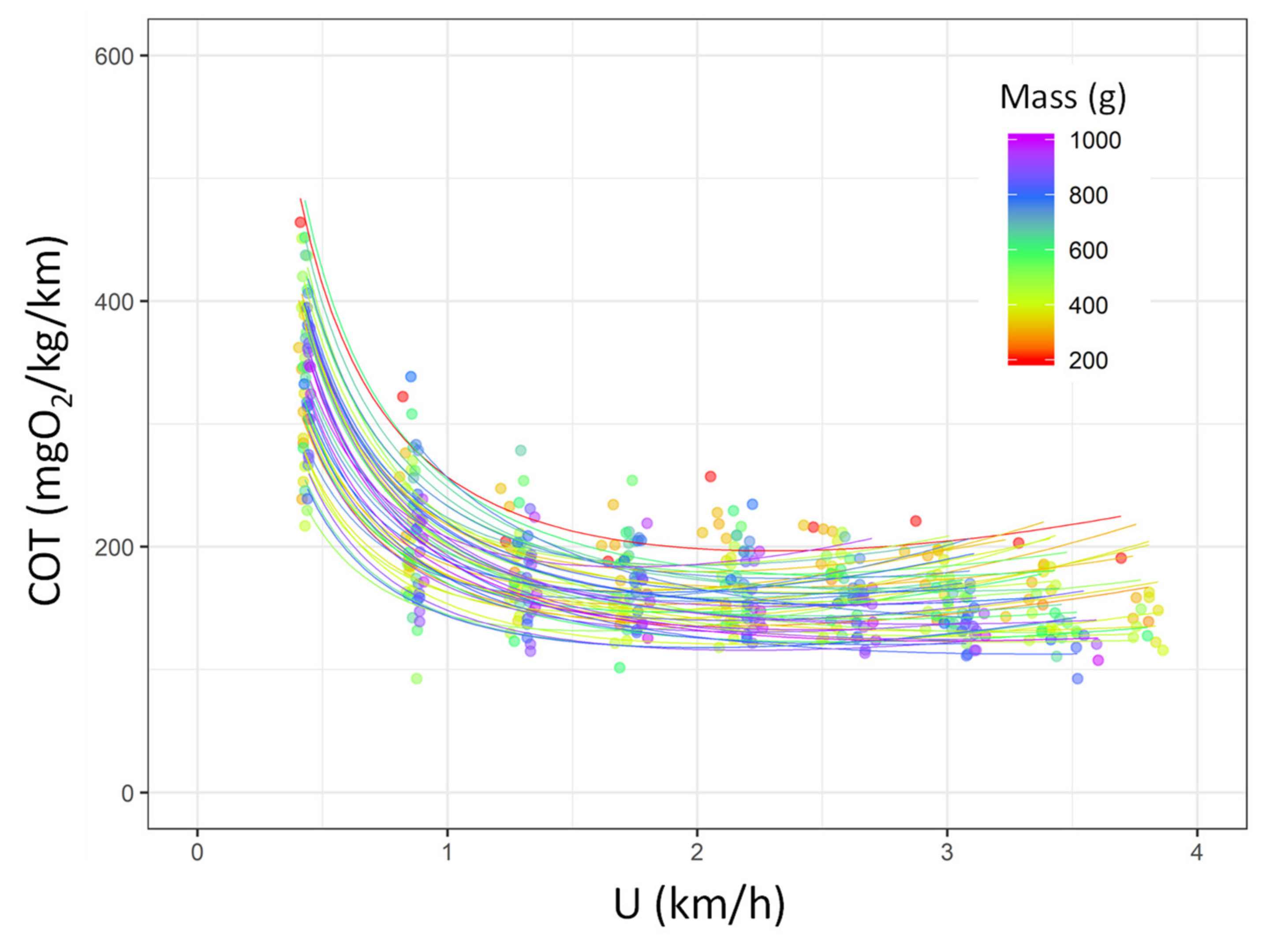

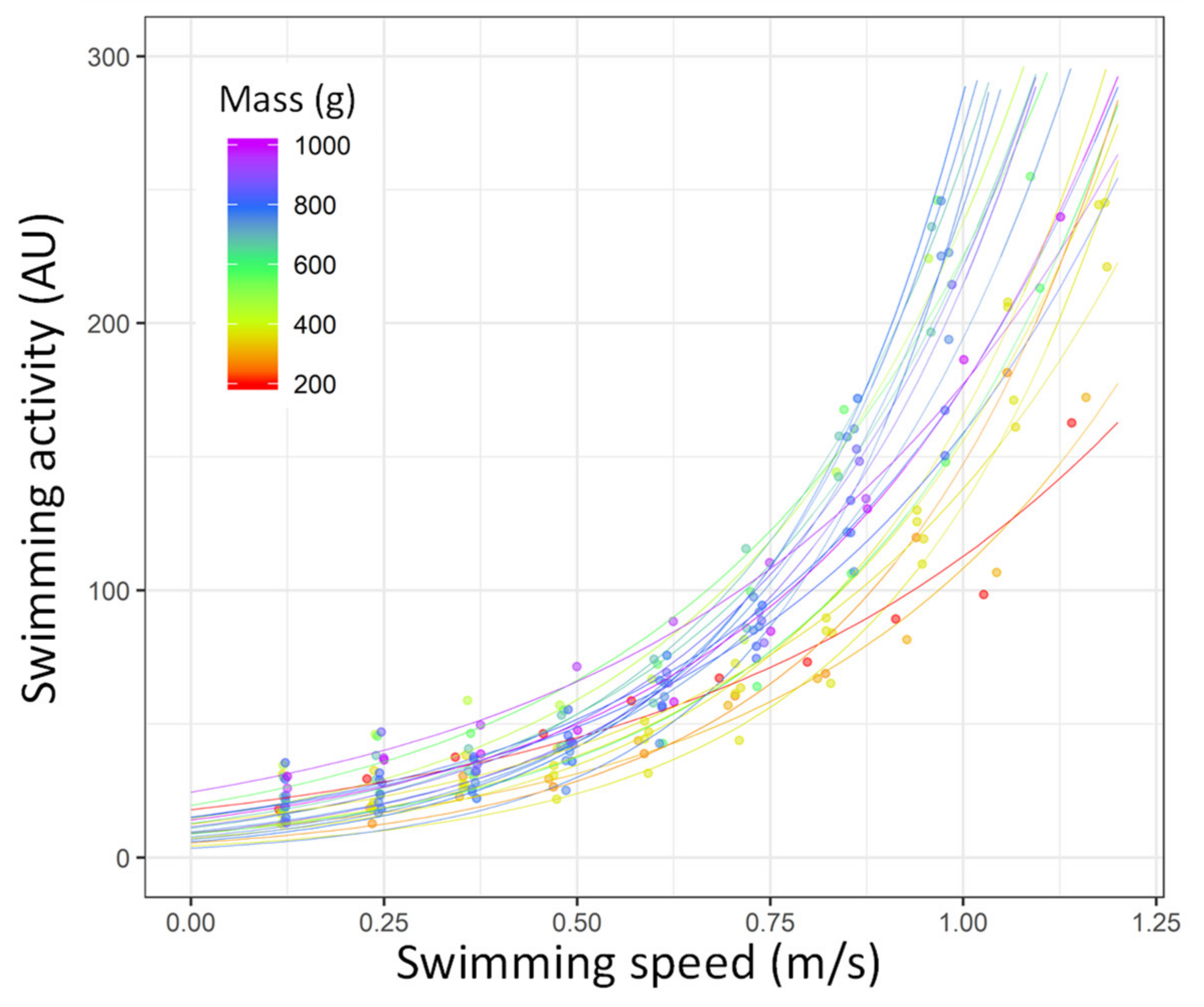
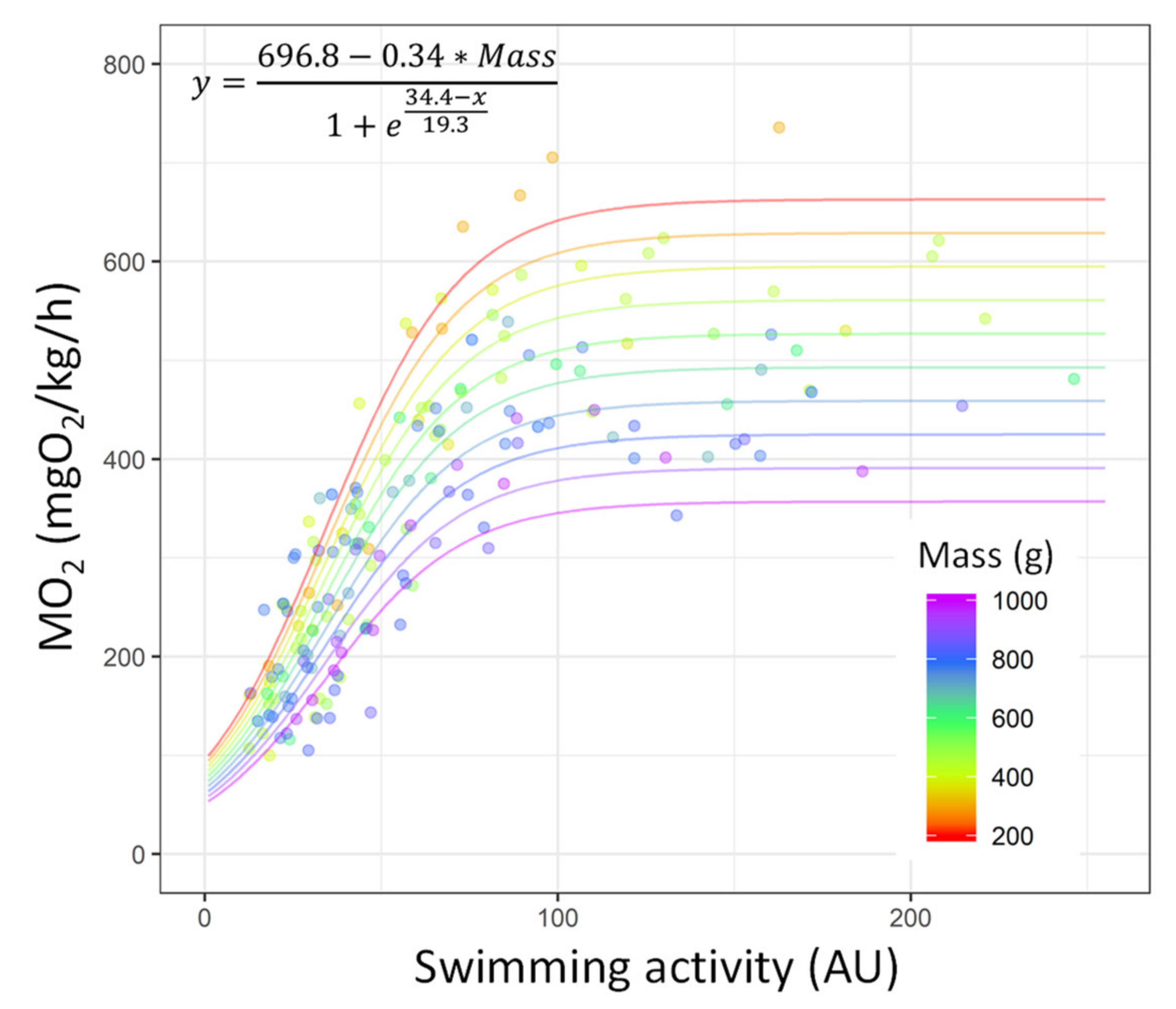
| Condition | n | Mass (g) | Total Length (mm) |
|---|---|---|---|
| Untagged | 25 | 590.39 ± 195.05 | 328.36 ± 41.78 |
| Tagged | 27 | 647.27 ± 224.85 | 342.93 ± 47.5 |
| EMG | 27 | 631.07 ± 205.67 | 340.77 ± 44.88 |
| Total | 79 | 623.73 ± 207.91 | 337.58 ± 44.74 |
| EMG Signal—Red Muscle | ||||
| Parameter | Estimate | Std. error | t Value | p Value |
| Asym | 101.203 | 4.600 | 22.001 | <0.001 |
| xmid | 41.604 | 2.893 | 14.381 | <0.001 |
| scal | 21.853 | 2.556 | 8.549 | <0.001 |
| EMG Signal—White Muscle | ||||
| Parameter | Estimate | Std. error | t Value | p Value |
| α | 3.938 | 0.731 | 5.383 | <0.001 |
| β | 0.031 | 0.002 | 15.467 | <0.001 |
| Asym—Linear Regression | ||||
| Parameter | Estimate | Std. error | t Value | p Value |
| (intercept) | 696.79 | 38.28 | 18.20 | <0.001 |
| Mass | −0.34 | 0.06 | −5.74 | <0.001 |
| Calibration model—SSlogis | ||||
| Parameter | Estimate | Std. error | t value | p value |
| xmid | 34.43 | 1.01 | 33.9 | <0.001 |
| scal | 19.36 | 1.33 | 14.51 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfonso, S.; Zupa, W.; Spedicato, M.T.; Lembo, G.; Carbonara, P. Mapping the Energetic Costs of Free-Swimming Gilthead Sea Bream (Sparus aurata), a Key Species in European Marine Aquaculture. Biology 2021, 10, 1357. https://doi.org/10.3390/biology10121357
Alfonso S, Zupa W, Spedicato MT, Lembo G, Carbonara P. Mapping the Energetic Costs of Free-Swimming Gilthead Sea Bream (Sparus aurata), a Key Species in European Marine Aquaculture. Biology. 2021; 10(12):1357. https://doi.org/10.3390/biology10121357
Chicago/Turabian StyleAlfonso, Sébastien, Walter Zupa, Maria Teresa Spedicato, Giuseppe Lembo, and Pierluigi Carbonara. 2021. "Mapping the Energetic Costs of Free-Swimming Gilthead Sea Bream (Sparus aurata), a Key Species in European Marine Aquaculture" Biology 10, no. 12: 1357. https://doi.org/10.3390/biology10121357
APA StyleAlfonso, S., Zupa, W., Spedicato, M. T., Lembo, G., & Carbonara, P. (2021). Mapping the Energetic Costs of Free-Swimming Gilthead Sea Bream (Sparus aurata), a Key Species in European Marine Aquaculture. Biology, 10(12), 1357. https://doi.org/10.3390/biology10121357