Interlaboratory Validation of Toxicity Testing Using the Duckweed Lemna minor Root-Regrowth Test
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions
2.2. Toxicity Testing Procedure
2.2.1. Preparation of Medium
2.2.2. Preparation of Test Solution and Test Dilutions
2.2.3. Transfer of Test Organisms
2.2.4. Measurement Methods
2.3. Time Required for Each Step
2.4. Troubleshooting
2.5. Comparisons between ISO 20079 and the New Root-Regrowth Method
2.6. Inter-Laboratory Comparison Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Comparisons of the ISO 20079 Protocol vs. the Root-Regrowth Test
3.2. Interlaboratory Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.; Worm, B. How many species are there on Earth and in the ocean? PLoS Biol. 2011, 9, e1001127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO. ISO/TC147/SC5; Biological Methods. ISO: Geneva, Switzerland, 2019.
- OECD. OECD Guidelines for the Testing of Chemicals; Organization for Economic Cooperation and Development: Paris, France, 1994. [Google Scholar] [CrossRef]
- USEPA. Whole Effluent Toxicity Methods; USEPA: Washington, DC, USA, 2000. [Google Scholar]
- ASTM. Environmental Assessment Standards and Risk Management Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Hillman, W.S. The Lemnaceae, or duckweeds. Bot. Rev. 1961, 27, 221–287. [Google Scholar] [CrossRef]
- Mbagwu, I.; Adeniji, H. The nutritional content of duckweed (Lemna paucicostata Hegelm.) in the Kainji Lake area, Nigeria. Aquat. Bot. 1988, 29, 357–366. [Google Scholar] [CrossRef]
- Huebert, D.B.; Shay, J.M. The response of Lemna trisulca L. to cadmium. Environ. Pollut. 1993, 80, 247–253. [Google Scholar] [CrossRef]
- Yu, C.; Sun, C.; Yu, L.; Zhu, M.; Xu, H.; Zhao, J.; Ma, Y.; Zhou, G. Comparative analysis of duckweed cultivation with sewage water and SH media for production of fuel ethanol. PLoS ONE 2014, 9, e115023. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ma, S.; Huang, M.; Peng, M.; Bog, M.; Sree, K.S.; Appenroth, K.-J.; Zhang, J. Species distribution, genetic diversity and barcoding in the duckweed family (Lemnaceae). Hydrobiologia 2015, 743, 75–87. [Google Scholar] [CrossRef]
- Wang, W.; Williams, J.M. The use of phytotoxicity tests (common duckweed, cabbage, and millet) for determining effluent toxicity. Environ. Monit. Assess. 1990, 14, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Ekperusi, A.O.; Sikoki, F.D.; Nwachukwu, E.O. Application of common duckweed (Lemna minor) in phytoremediation of chemicals in the environment: State and future perspective. Chemosphere 2019, 223, 285–309. [Google Scholar] [CrossRef]
- Baudo, R.; Foudoulakis, M.; Arapis, G.; Perdaen, K.; Lanneau, W.; Paxinou, A.-C.; Kouvdou, S.; Persoone, G. History and sensitivity comparison of the Spirodela polyrhiza microbiotest and Lemna toxicity tests. Knowl. Manag. Aquat. Ecosyst. 2015, 416, 23. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.S.; Han, T. Physiological response of Lemna species to herbicides and its probable use in toxicity testing. Toxicol. Environ. Health Sci. 2010, 2, 39–49. [Google Scholar] [CrossRef]
- Acosta, K.; Appenroth, K.J.; Borisjuk, L.; Edelman, M.; Heinig, U.; Jansen, M.A.; Oyama, T.; Pasaribu, B.; Schubert, I.; Sorrels, S. Return of the Lemnaceae: Duckweed as a model plant system in the genomics and postgenomics era. Plant Cell 2021, 33, 3207–3234. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, P.; Sree, K.; Appenroth, K.-J. Duckweeds for water remediation and toxicity testing. Toxicol. Environ. Chem. 2016, 98, 1127–1154. [Google Scholar] [CrossRef]
- ASTM. Standard Guide for Conducting Static Toxicity Tests with Lemna gibba G3; American Society for Testing Materials: West Conshohocken, PA, USA, 1991; pp. 13–20. [Google Scholar]
- USEPA. Aquatic Plant Toxicity Test Using Lemna spp., Tiers I and II “Public Draft”, Ecological Effects Test Guidelines OPPTS 850.4400; USEPA: Washington, DC, USA, 1996; p. 5. [Google Scholar]
- EC. Biological Test Method: Test for Measuring the Inhibition of Growth Using the Freshwater Macrophyte, Lemna Minor; EC: Ottawa, ON, Canada, 1999; p. 98. [Google Scholar]
- AFNOR. Determination of the Inhibitory Effect on the Growth of L. Minor; Association Française de Normalisation: Saint-Denis, France, 1996; p. 10. [Google Scholar]
- SIS. Water Quality—Determination of Growth Inhibition (7-d) L. Minor, Duckweed; Swedish Standards Institute: Stockholm, Sweden, 1995; p. 15. [Google Scholar]
- ISO. Water Quality—Determination of the Toxic Effect of Water Constituents and Wastewater to Duckweed Lemna Minor; International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- OECD. Test No. 221: Lemna sp. Growth Inhibition Test; OECD: Paris, France, 2006. [Google Scholar]
- Wang, W. Literature review on duckweed toxicity testing. Environ. Res. 1990, 52, 7–22. [Google Scholar] [CrossRef]
- Edelman, M. The state of duckweed affairs in the literature. Int. Steer. Comm. Duckweed Res. Appl. 2015, 3, 1–33. [Google Scholar]
- Park, J.; Brown, M.T.; Depuydt, S.; Kim, J.K.; Won, D.-S.; Han, T. Comparing the acute sensitivity of growth and photosynthetic endpoints in three Lemna species exposed to four herbicides. Environ. Pollut. 2017, 220, 818–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalapillai, Y.; Vigneault, B.; Hale, B.A. Root length of aquatic plant, Lemna minor L., as an optimal toxicity endpoint for biomonitoring of mining effluents. Integr. Environ. Assess. Manag. 2014, 10, 493–497. [Google Scholar] [CrossRef] [PubMed]
- ISO. Water Quality—Aquatic Toxicity Test Based on Root Re-Growth in Lemna Minor. (ISO/AWI 4979); International Organization for Standardization: Geneva, Switzerland, 2020.
- Steinberg, R.A. Mineral requirements of Lemna minor. Plant Physiol. 1946, 21, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J. Evaluation of Freshwater Lemna spp. and Spirodela Polyrhiza, Higher Plant Lactuca Sativa and Marine Macroalga Ulva Pertusa as Potential Phytotoxicity Test Model Organisms; Incheon National University: Incheon, Korea, 2017. [Google Scholar]
- Lumpkin, T.A.; Plucknett, D.L. Azolla as a Green Manure: Use and Management in Crop Production; Westview Press, Inc.: Boulder, CO, USA, 1982. [Google Scholar]

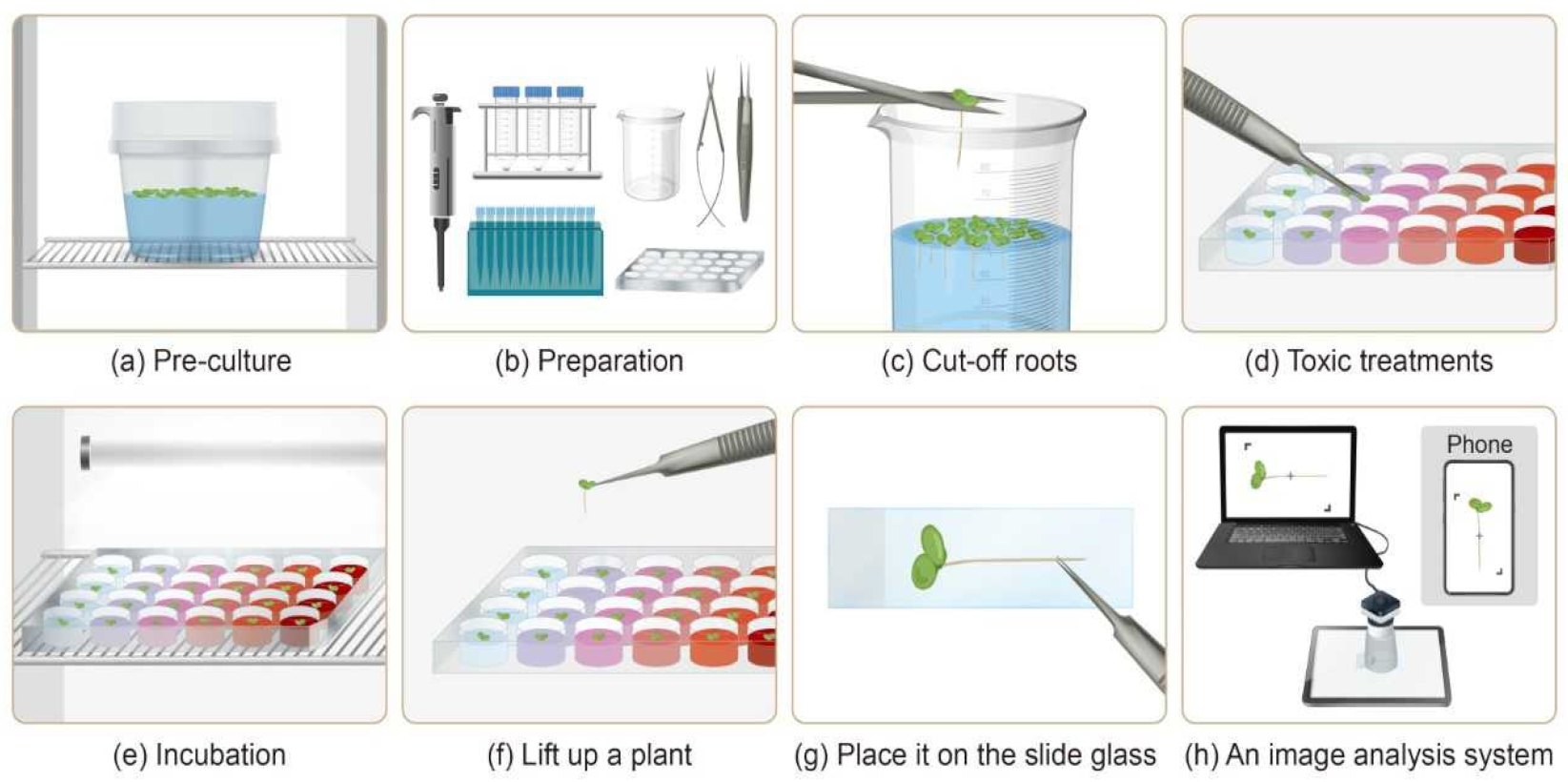
| Characteristic | This Study | ISO 20079 | OECD 221 | USEPA |
|---|---|---|---|---|
| Test species | Lemna minor 5631 | Lemna minor 9441 | Lemna gibba, Lemna minor | Lemna gibba G3, Lemna minor |
| Test duration | 72 h | 168 h | 168 h | 168 h |
| Temperature | 25 ± 1 °C | 24 ± 2 °C | 24 ± 2 °C | 25 ± 2 |
| Photon Flux Density | 90–100 μmol photons m–2 s–1 | 85–135 μmol photons m–2 s–1 | 6500–10,000 lux | 4200–6700 lux |
| Photoperiod | Continuous light | Continuous light | Continuous light | Continuous light |
| Test vessel type | 24-well plates | Beaker | Flask, Petri dish | Beaker, flask |
| Medium | Steinberg medium | Steinberg medium | Swedish Standard (SIS) Lemna medium (for L. minor) or 20× AAP growth medium (for L. gibba) | M-Hoagland’s medium or 20×-AAP nutrient medium |
| Test solution volume | 3.0 mL | 100 mL (minimum) | 100 mL (minimum) | 150 mL |
| Test solution pH | 6.9 ± 0.2 | 5.5 | 6.5 ± 0.2 | 7.5 ± 0.1 |
| Test organism size | One colony per test vessel (two or three fronds per colony) | 10–16 fronds per test vessel (two or three fronds per colony) | 9–12 fronds per test vessel | 12–16 fronds per test vessel |
| Endpoint | Root regrowth length | Growth rate (frond number, frond area, dry weight, chlorophyll contents) | Average specific growth rate, final biomass, area under the growth curve | Total frond number, growth rate (number of fronds per day), mortality (% of dead fronds to total number of fronds) and dry weight, chlorophyll and pheophytin pigment analyses |
| Test type | Static non-renewal | Static non-renewal | Static none-renewal | Static none-renewal |
| Condition | Axenic or non-axenic culture | Axenic culture | Axenic culture | Axenic culture |
| Methods | Endpoints | EC50 (95% CI) | CV (%) |
|---|---|---|---|
| Conventional method | Frond number | 3.514 (2.986–3.670) | 3.22 |
| Dry weight | 2.250 (0.586–3.187) | 20.10 | |
| Chlorophyll a | 3.349 (3.141–3.520) | 1.88 | |
| Chlorophyll b | 3.425 (3.042–3.639) | 2.75 | |
| Carotenoids | 3.338 (2.988–3.594) | 2.83 | |
| This study | Root regrowth length | 2.441 (1.239–2.992) | 17.31 |
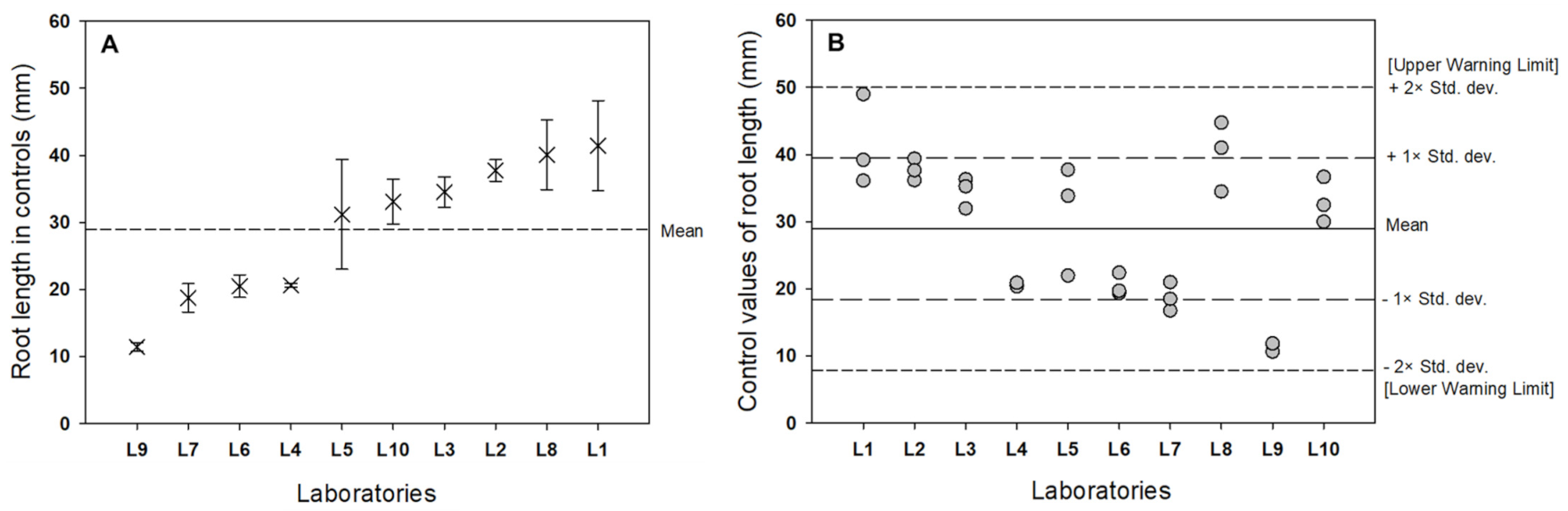
| Laboratory | Control Root Length (mm) (95% CI) | CV (%) |
|---|---|---|
| Lab 1 | 41.442 ± 7.596 | 16.20 |
| Lab 2 | 37.745 ± 1.815 | 4.26 |
| Lab 3 | 34.525 ± 2.568 | 6.57 |
| Lab 4 | 20.605 ± 0.302 | 1.30 |
| Lab 5 | 31.192 ± 9.291 | 26.32 |
| Lab 6 | 20.483 ± 1.889 | 8.15 |
| Lab 7 | 18.742 ± 2.402 | 11.33 |
| Lab 8 | 40.083 ± 5.869 | 12.94 |
| Lab 9 | 11.410 ± 0.760 | 5.89 |
| Lab 10 | 33.056 ± 3.811 | 10.19 |
| 95% PI * | 28.928 ± 20.280 |
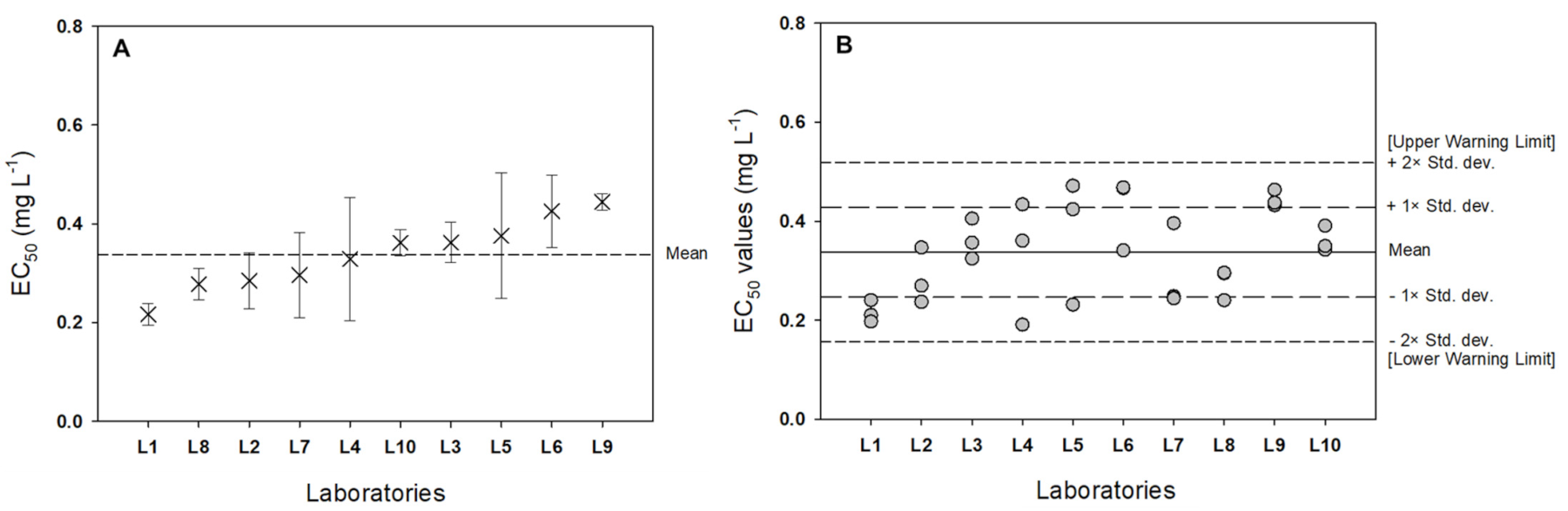
| Laboratory | EC50 (95% CI) | CV (%) |
|---|---|---|
| Lab 1 | 0.216 ± 0.025 | 10.23 |
| Lab 2 | 0.285 ± 0.064 | 19.80 |
| Lab 3 | 0.362 ± 0.046 | 11.25 |
| Lab 4 | 0.329 ± 0.141 | 37.88 |
| Lab 5 | 0.376 ± 0.144 | 33.82 |
| Lab 6 | 0.426 ± 0.083 | 17.20 |
| Lab 7 | 0.296 ± 0.098 | 29.14 |
| Lab 8 | 0.277 ± 0.036 | 11.46 |
| Lab 9 | 0.455 ± 0.019 | 3.78 |
| Lab 10 | 0.361 ± 0.030 | 7.26 |
| 95% PI * | 0.337 ± 0.138 |
| Sample | l | n | ο% | X | R(SR) | CV-R% | r(Sr) | CV-r% |
|---|---|---|---|---|---|---|---|---|
| Control | 10 | 10 | 0 | 28.928 | 30.127 (10.869) | 37.573 | 11.302 (4.077) | 14.095 |
| Sample | l | n | ο% | X | R(SR) | CV-R% | r(Sr) | CV-r% |
|---|---|---|---|---|---|---|---|---|
| Cu (mg L−1) | 10 | 10 | 0 | 0.337 | 0.255 (0.0918) | 27.2 | 0.200 (0.0720) | 21.3 |
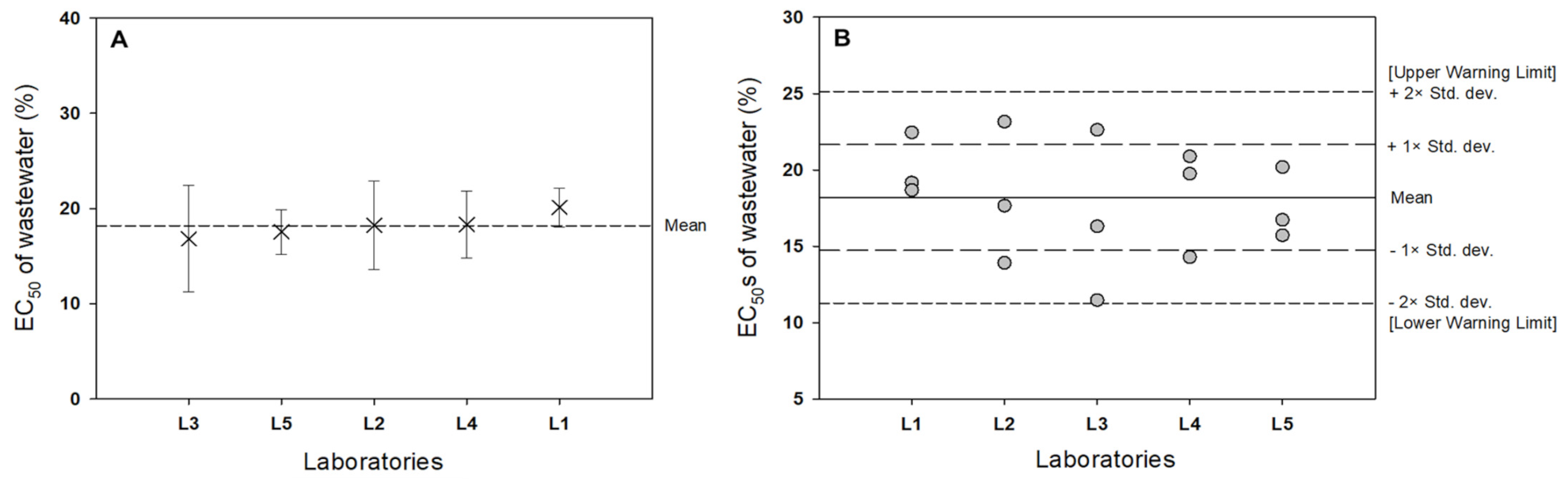
| Laboratory | EC50 ± 95% CI | CV (%) |
|---|---|---|
| Lab 1 | 20.109 ± 2.318 | 10.19 |
| Lab 2 | 18.253 ± 5.260 | 25.47 |
| Lab 3 | 16.812 ± 6.332 | 33.28 |
| Lab 4 | 18.321 ± 3.995 | 19.27 |
| Lab 5 | 17.550 ± 2.652 | 13.36 |
| 95% PI * | 18.209 ± 2.402 |
| Sample | l | n | ο% | X | R(SR) | CV-R% | r(Sr) | CV-r% |
|---|---|---|---|---|---|---|---|---|
| Wastewater | 5 | 5 | 0 | 18.209 | 9.405 (3.393) | 18.634 | 10.741 (3.875) | 21.280 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Yoo, E.-J.; Shin, K.; Depuydt, S.; Li, W.; Appenroth, K.-J.; Lillicrap, A.D.; Xie, L.; Lee, H.; Kim, G.; et al. Interlaboratory Validation of Toxicity Testing Using the Duckweed Lemna minor Root-Regrowth Test. Biology 2022, 11, 37. https://doi.org/10.3390/biology11010037
Park J, Yoo E-J, Shin K, Depuydt S, Li W, Appenroth K-J, Lillicrap AD, Xie L, Lee H, Kim G, et al. Interlaboratory Validation of Toxicity Testing Using the Duckweed Lemna minor Root-Regrowth Test. Biology. 2022; 11(1):37. https://doi.org/10.3390/biology11010037
Chicago/Turabian StylePark, Jihae, Eun-Jin Yoo, Kisik Shin, Stephen Depuydt, Wei Li, Klaus-J. Appenroth, Adam D. Lillicrap, Li Xie, Hojun Lee, Geehyoung Kim, and et al. 2022. "Interlaboratory Validation of Toxicity Testing Using the Duckweed Lemna minor Root-Regrowth Test" Biology 11, no. 1: 37. https://doi.org/10.3390/biology11010037
APA StylePark, J., Yoo, E.-J., Shin, K., Depuydt, S., Li, W., Appenroth, K.-J., Lillicrap, A. D., Xie, L., Lee, H., Kim, G., Saeger, J. D., Choi, S., Kim, G., Brown, M. T., & Han, T. (2022). Interlaboratory Validation of Toxicity Testing Using the Duckweed Lemna minor Root-Regrowth Test. Biology, 11(1), 37. https://doi.org/10.3390/biology11010037







