Effects of Green Light on Elongation Do Not Interact with Far-Red, Unless the Phytochrome Photostationary State (PSS) Changes in Tomato
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
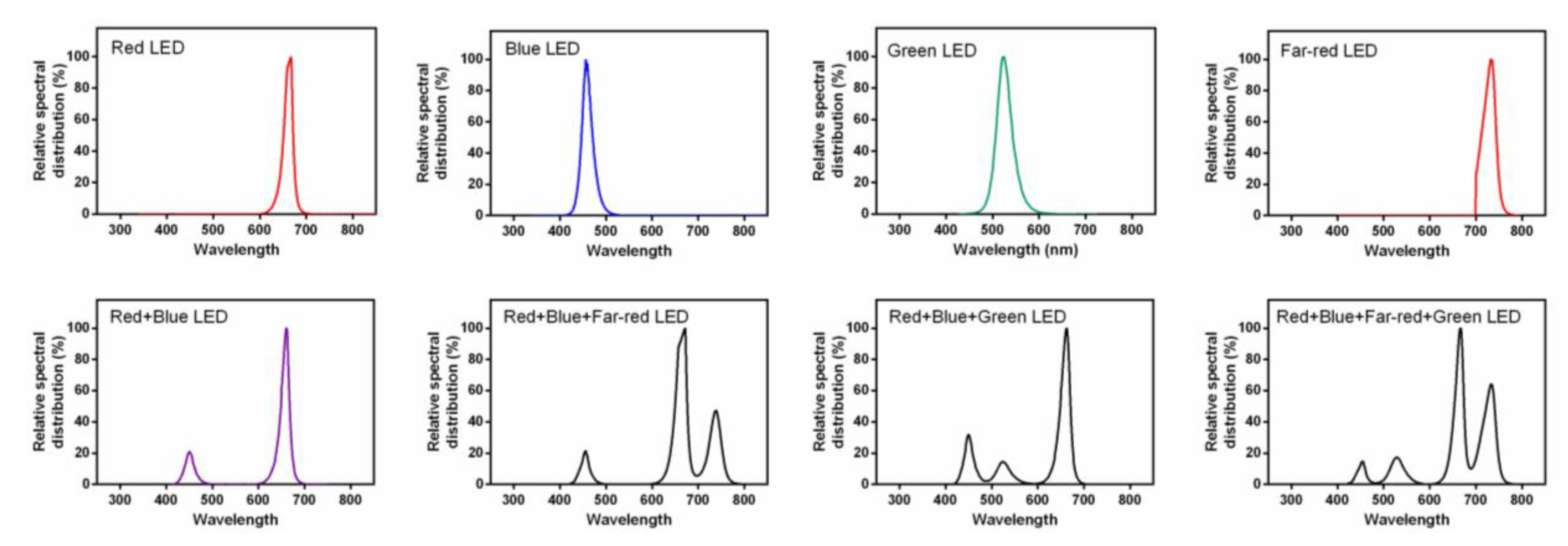
2.1. Plant Cultivation and Light Treatments
2.2. Measurements
2.3. Statistical Set-Up and Analysis
3. Results
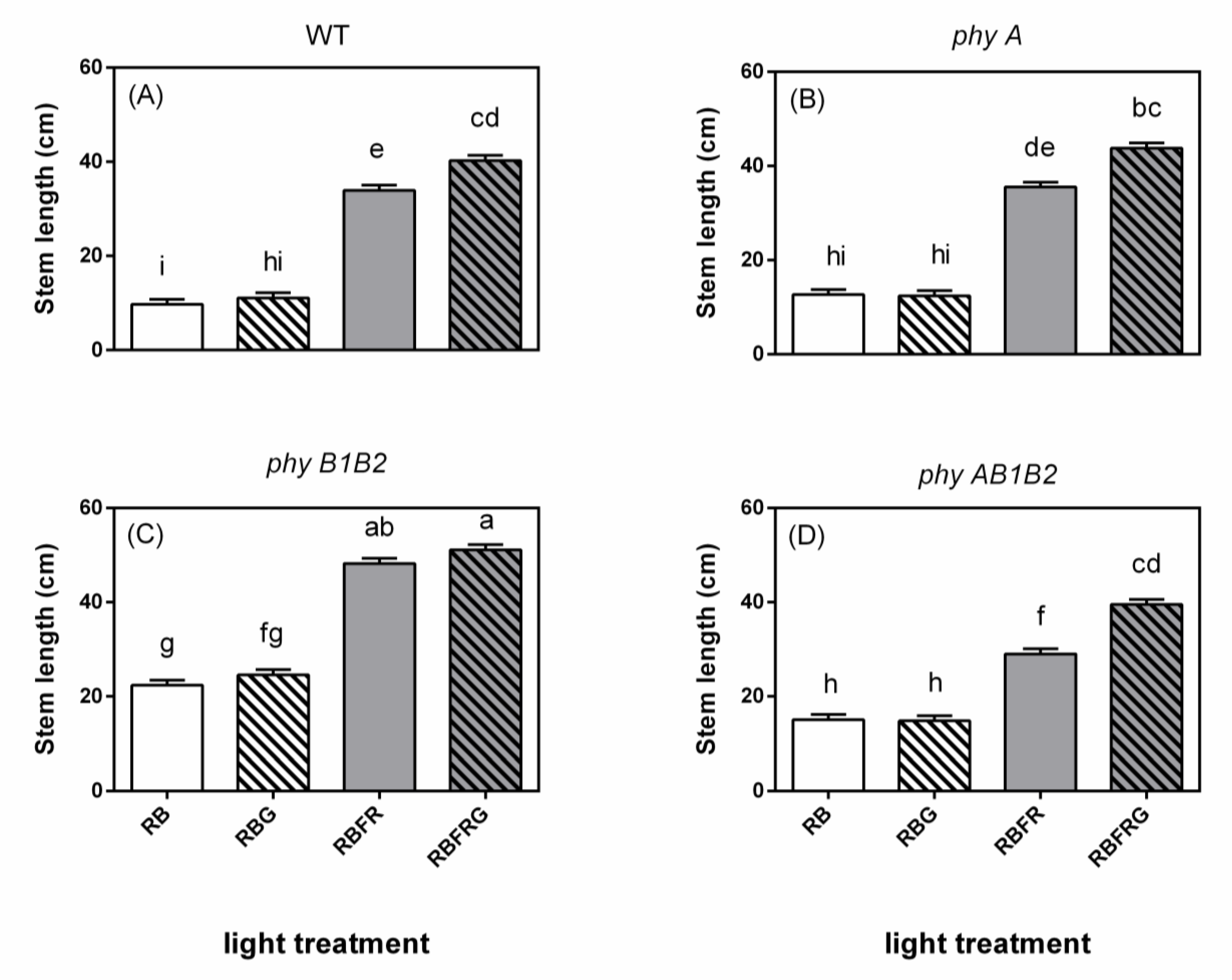
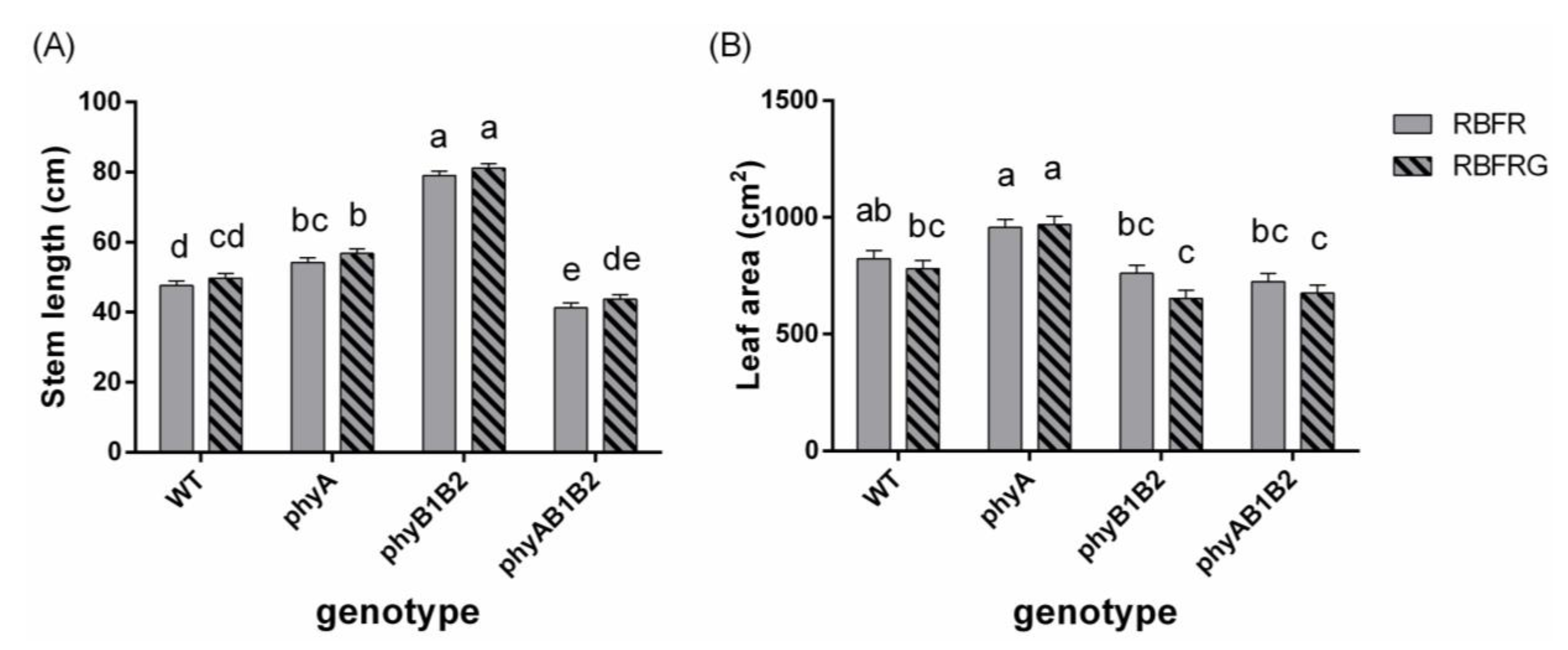
3.1. Effects of Green and Far-Red Light on Elongation
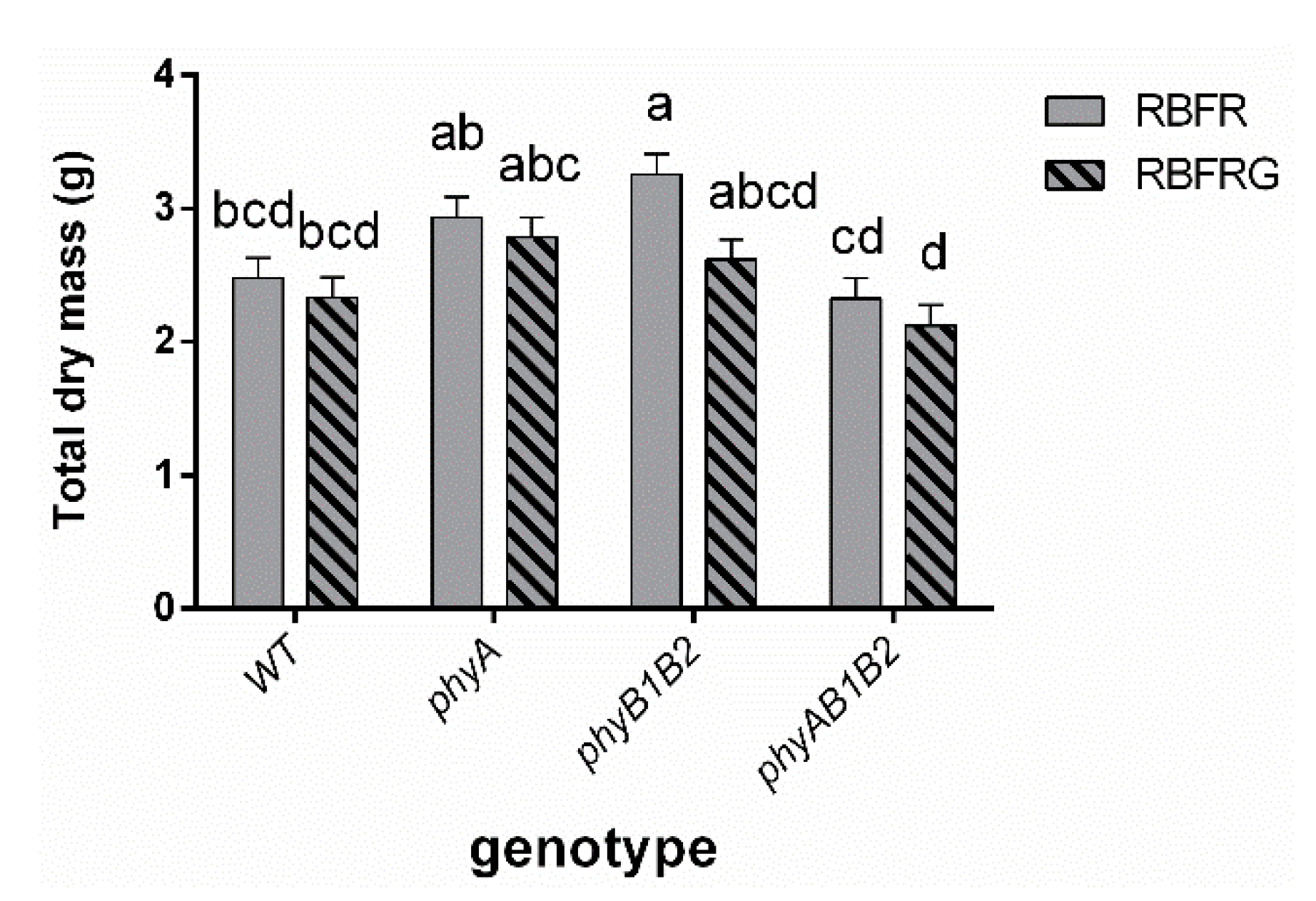
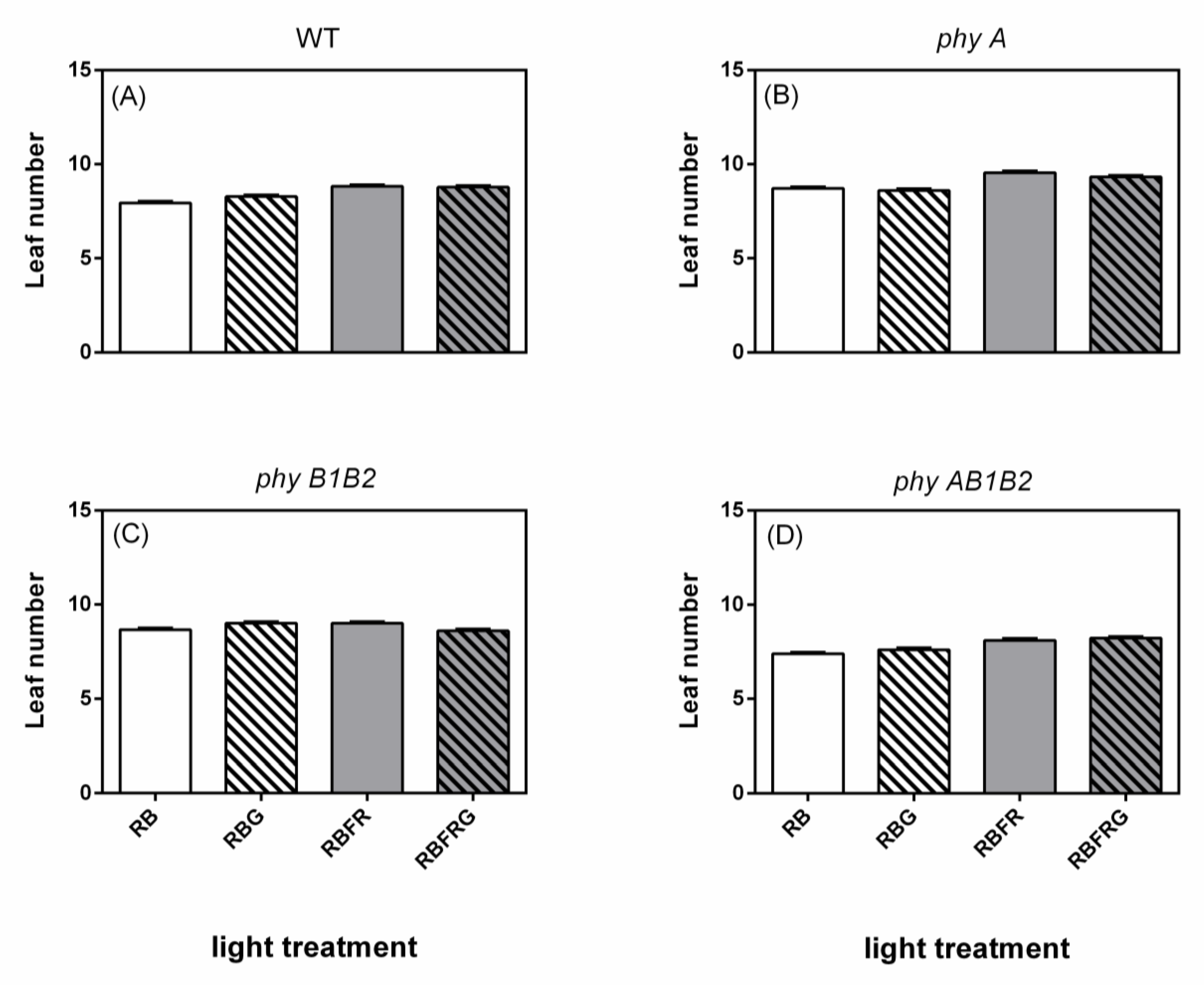
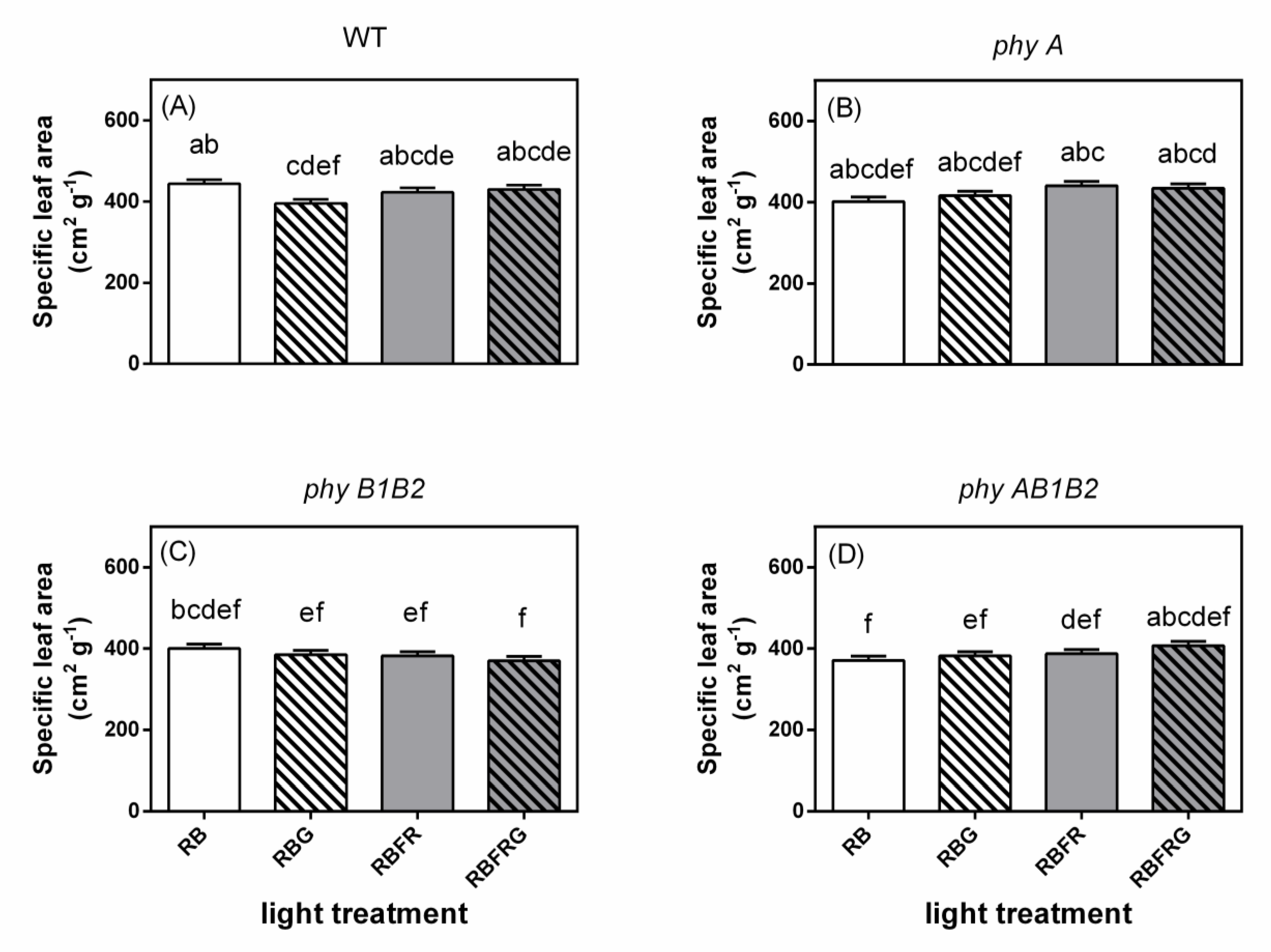
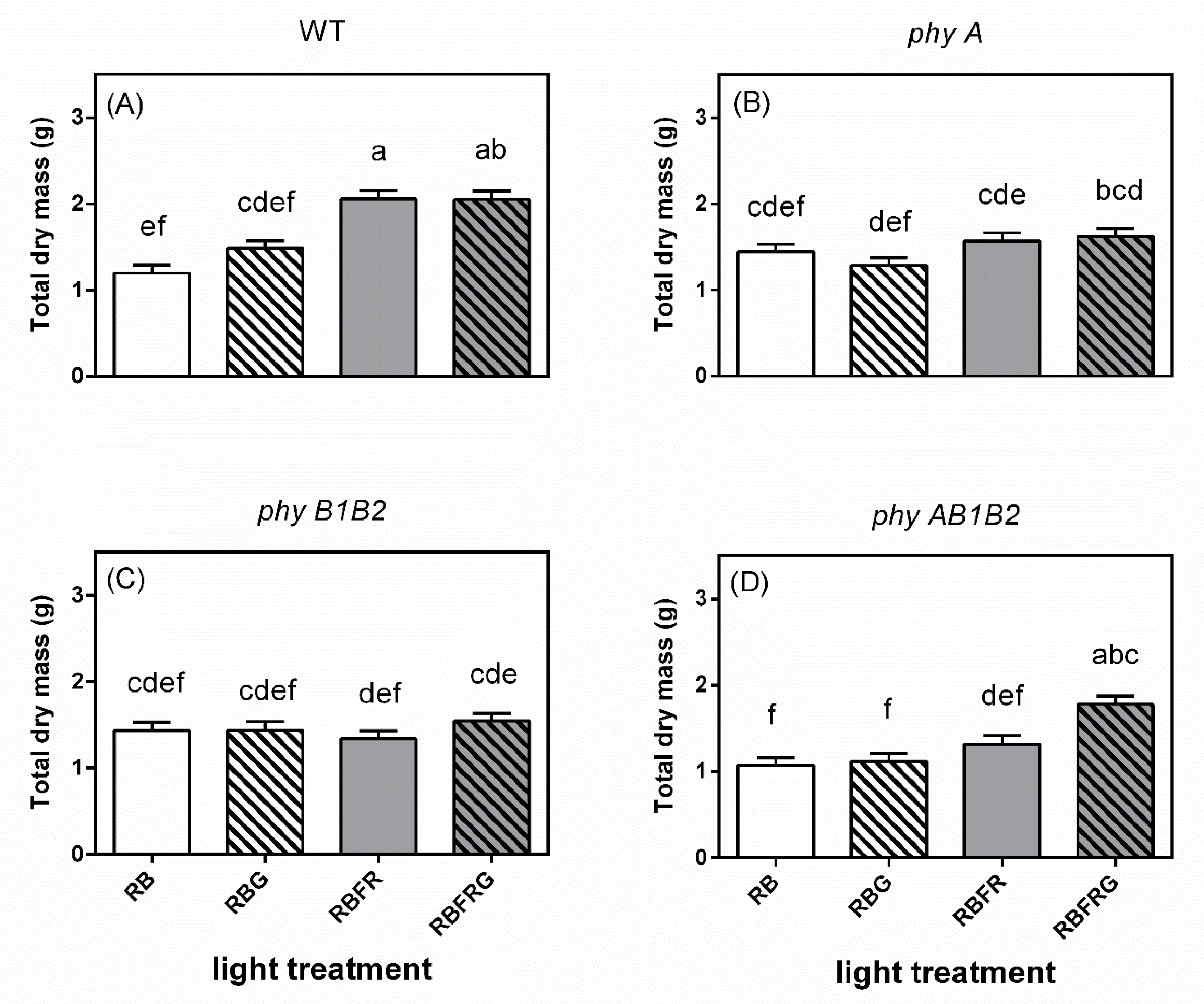
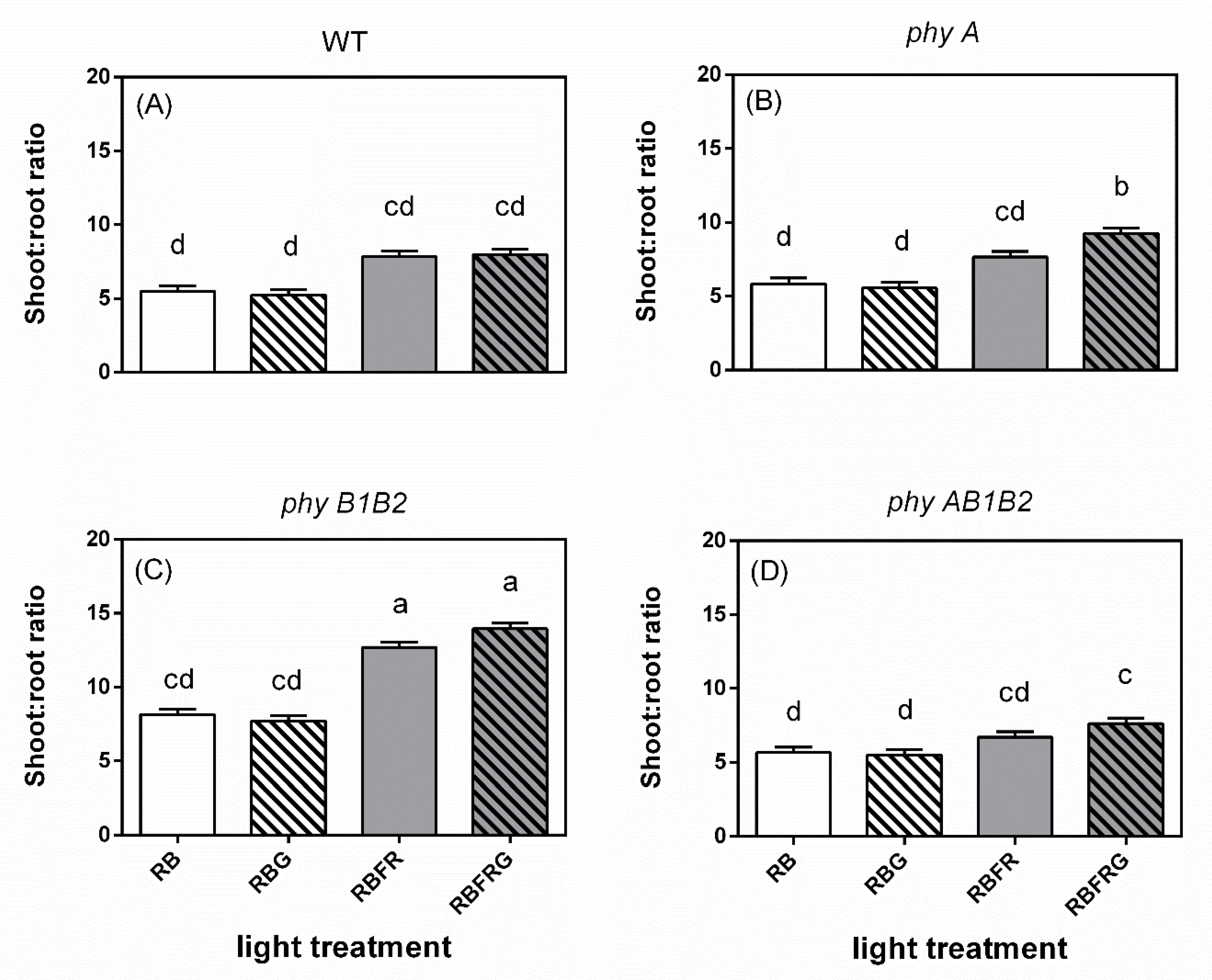
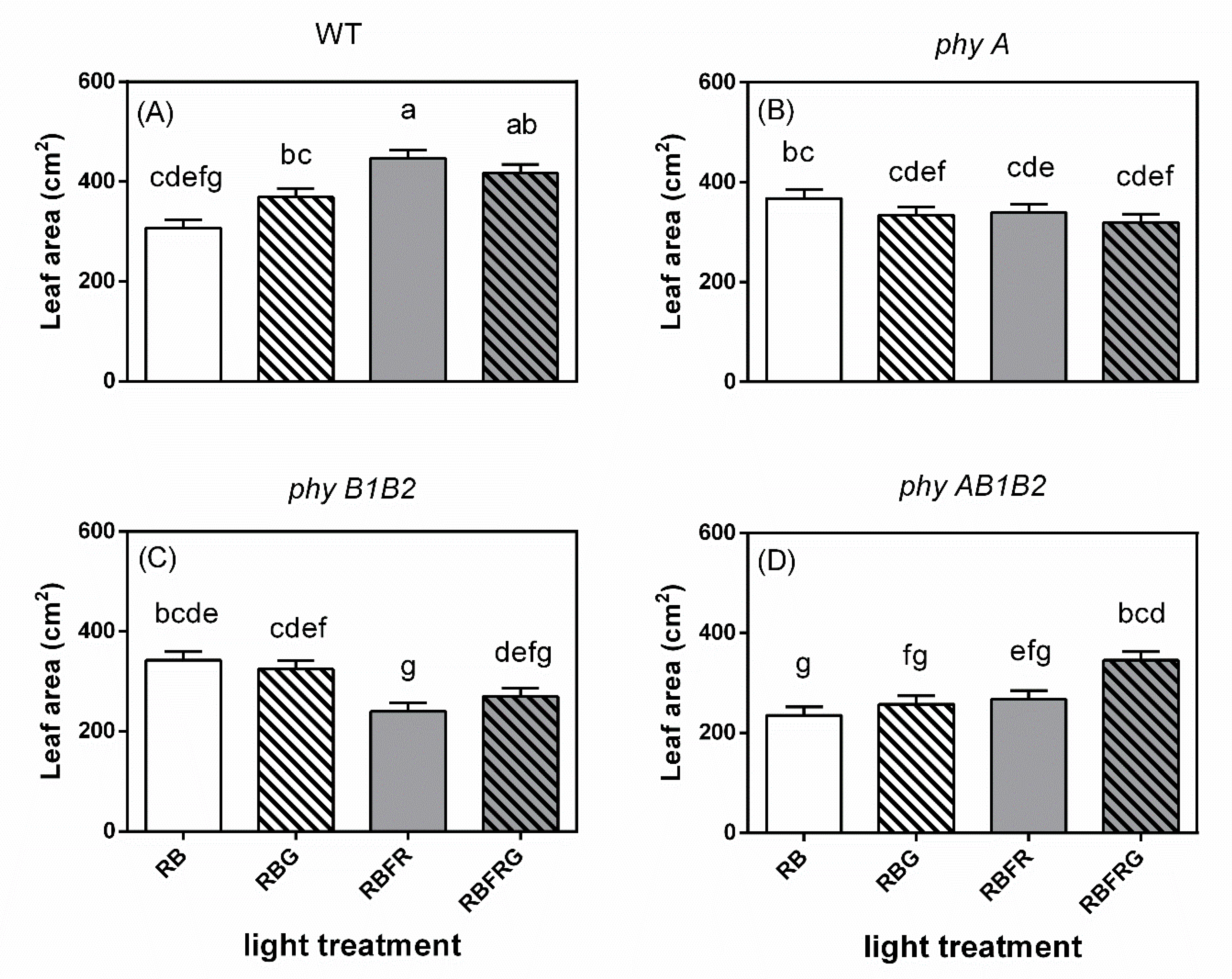
3.2. Effects of Green and Far-Red Light on Dry Mass, Shoot/Root Ratio, and Leaf Area
4. Discussion
4.1. No Interaction between Green and Far-Red Light on Elongation Unless the PSS Changes
4.2. Green Light Had No Effect on Total Dry Mass, Except in the phyAB1B2 Mutant When Interacting with FR
4.3. Far-Red Light Increased the Wild-Type Dry Mass Resulting from an Enhanced Leaf Area, Whereas It Decreased the phyB1B2 Mutant’s Leaf Area
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Spectral Treatment | Light Intensity (μmol m–2 s–1) | ||||||
|---|---|---|---|---|---|---|---|
| Block1 | Block2 | Block3 | Block4 | Block5 | Average | SEM | |
| RB | 150.30 | 148.06 | 149.07 | 150.46 | 154.33 | 150.44 | 1.07 |
| RBG | 152.86 | 142.14 | 153.34 | 149.13 | 147.93 | 149.08 | 2.02 |
| RBFR | 142.12 | 145.08 | 146.63 | 150.40 | 149.77 | 146.80 | 1.53 |
| RBFRG | 151.81 | 151.46 | 148.40 | 151.50 | 149.96 | 150.63 | 0.64 |
| Spectral Treatment | Light Intensity (μmol m–2 s–1) | |||||
|---|---|---|---|---|---|---|
| Block1 | Block2 | Block3 | Block4 | Average | SEM | |
| RBFR | 142.69 | 144.14 | 145.68 | 143.75 | 144.06 | 0.62 |
| RBFRG | 139.17 | 139.94 | 142.96 | 142.04 | 141.03 | 0.88 |
References
- Smith, H. Light quality, photoperception, and plant strategy. Ann. Rev. Plant Physiol. 1982, 33, 481–518. [Google Scholar] [CrossRef]
- Pedmale, U.V.; Huang, S.S.; Zander, M.; Cole, B.J.; Hetzel, J.; Ljung, K.; Reis, P.A.; Sridevi, P.; Nito, K.; Nery, J.R.; et al. Cryptochromes Interact Directly with PIFs to Control Plant Growth in Limiting Blue Light. Cell 2016, 164, 233–245. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.M.; Jaillais, Y.; Pedmale, U.V.; Moreno, J.E.; Chory, J.; Ballare, C.L. Cryptochrome 1 and phytochrome B control shade-avoidance responses in Arabidopsis via partially independent hormonal cascades. Plant J. 2011, 67, 195–207. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Zhang, T.; Maruhnich, S.A.; Folta, K.M. Green Light Induces Shade Avoidance Symptoms. Plant Physiol. 2011, 157, 1528–1536. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, T.; Folta, K.M. Green light augments far-red-light-induced shade response. Plant Growth Regul. 2015, 77, 147–155. [Google Scholar] [CrossRef]
- Wang, Y.; Folta, K.M. Contributions of green light to plant growth and development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef]
- Ruberti, I.; Sessa, G.; Ciolfi, A.; Possenti, M.; Carabelli, M.; Morelli, G. Plant adaptation to dynamically changing environment: The shade avoidance response. Biotechnol. Adv. 2012, 30, 1047–1058. [Google Scholar] [CrossRef]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef]
- Casal, J.J. Shade avoidance. Arab. Book 2012, 10, e0157. [Google Scholar] [CrossRef] [Green Version]
- Possart, A.; Fleck, C.; Hiltbrunner, A. Shedding (far-red) light on phytochrome mechanisms and responses in land plants. Plant Sci. 2014, 217–218, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Use of spectral data to determine photosynthetic efficiency and phytochrome photoequilibria. Am. Soc. Agric. Eng. 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Wang, H.; Wang Deng, X. Phytochrome signaling mechanisms. Arab. Book 2011, 9, e0148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, F.; Schafer, E. Phytochromes control photomorphogenesis by differentially regulated, interacting signaling pathways in higher plants. Annu. Rev. Plant Biol. 2002, 53, 329–355. [Google Scholar] [CrossRef]
- Franklin, K.A.; Quail, P.H. Phytochrome functions in Arabidopsis development. J. Exp. Bot. 2010, 61, 11–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Lorrain, S.; Allen, T.; Duek, P.D.; Whitelam, G.C.; Fankhauser, C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 2008, 53, 312–323. [Google Scholar] [CrossRef] [Green Version]
- Koini, M.A.; Alvey, L.; Allen, T.; Tilley, C.A.; Harberd, N.P.; Whitelam, G.C.; Franklin, K.A. High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr. Biol. 2009, 19, 408–413. [Google Scholar] [CrossRef] [Green Version]
- Goggin, D.E.; Steadman, K.J. Blue and green are frequently seen: Responses of seeds to short- and mid-wavelength light. Seed Sci. Res. 2012, 22, 27–35. [Google Scholar] [CrossRef]
- Bouly, J.P.; Schleicher, E.; Dionisio-Sese, M.; Vandenbussche, F.; van der Straeten, D.; Bakrim, N.; Meier, S.; Batschauer, A.; Galland, P.; Bittl, R.; et al. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J. Biol. Chem. 2007, 282, 9383–9391. [Google Scholar] [CrossRef] [Green Version]
- Sellaro, R.; Crepy, M.; Trupkin, S.A.; Karayekov, E.; Buchovsky, A.S.; Rossi, C.; Casal, J.J. Cryptochrome as a sensor of the blue/green ratio of natural radiation in Arabidopsis. Plant Physiol. 2010, 154, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Battle, M.W.; Vegliani, F.; Jones, M.A. Shades of green: Untying the knots of green photoperception. J. Exp. Bot. 2020, 71, 5764–5770. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Niinuma, K.; Yoshida, R. Day-neutral response of photoperiodic flowering in tomatoes: Possible implications based on recent molecular genetics of Arabidopsis and rice. Plant Biotechnol. 2007, 24, 83–86. [Google Scholar] [CrossRef] [Green Version]
- Schrager-Lavelle, A.; Herrera, L.A.; Maloof, J.N. Tomato phyE Is Required for Shade Avoidance in the Absence of phyB1 and phyB2. Front. Plant Sci. 2016, 7, 1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Bisbis, M.; Heuvelink, E.; Jiang, W.; Marcelis, L.F.M. Green light reduces elongation when partially replacing sole blue light independently from cryptochrome 1a. Physiol. Plant. 2021, 173, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Virsile, A.; Brazaityte, A.; Vastakaite-Kairiene, V.; Miliauskiene, J.; Jankauskiene, J.; Novickovas, A.; Lauzike, K.; Samuoliene, G. The distinct impact of multi-color LED light on nitrate, amino acid, soluble sugar and organic acid contents in red and green leaf lettuce cultivated in controlled environment. Food Chem. 2020, 310, 125799. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Praekelt, U.; Stoddart, W.M.; Billingham, O.E.; Halliday, K.J.; Whitelam, G.C. Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol. 2003, 131, 1340–1346. [Google Scholar] [CrossRef] [Green Version]
- Weller, J.L.; Schreuder, M.E.L.; Smith, H.; Koornneef, M.; Kendrick, R.E. Physiological interactions of phytochromes A, B1 and B2 in the control of development in tomato. Plant J. 2000, 24, 345–356. [Google Scholar] [CrossRef]
- Li, Q.; Wu, G.; Zhao, Y.; Wang, B.; Zhao, B.; Kong, D.; Wei, H.; Chen, C.; Wang, H. CRISPR/Cas9-Mediated Knockout and Overexpression Studies Reveal a Role of Maize Phytochrome C in Regulating Flowering Time and Plant Height. Plant Biotechnol. J. 2020, 18, 2520–2532. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Duchovskis, P.; Urbonavičiūtė, A. The effect of light-emitting diodes lighting on cucumber transplants and after-effect on yield. Zemdirbyste 2009, 96, 102–118. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Duchovskis, P.; Urbonavičiūtė, A. The effect of light-emitting diodes lighting on the growth of tomato transplants. Zemdirbyste 2010, 97, 89–98. [Google Scholar] [CrossRef]
- Kalaitzoglou, P.; van Ieperen, W.; Harbinson, J.; van der Meer, M.; Martinakos, S.; Weerheim, K.; Nicole, C.C.S.; Marcelis, L.F.M. Effects of Continuous or End-of-Day Far-Red Light on Tomato Plant Growth, Morphology, Light Absorption, and Fruit Production. Front. Plant Sci. 2019, 10, 322. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, Y.; Yang, Q.; Li, T. Overhead supplemental far-red light stimulates tomato growth under intra-canopy lighting with LEDs. J. Integr. Agr. 2019, 18, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Kozuka, T.; Horiguchi, G.; Kim, G.T.; Ohgishi, M.; Sakai, T.; Tsukaya, H. The different growth responses of the Arabidopsis thaliana leaf blade and the petiole during shade avoidance are regulated by photoreceptors and sugar. Plant Cell Physiol. 2005, 46, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Reed, J.W.; Nagpal, P.; Poole, D.S.; Furuya, M.; Chory, J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 1993, 5, 147–157. [Google Scholar] [PubMed] [Green Version]
- Devlin, P.F.; Patel, S.R.; Whitelam, G.C. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell 1998, 10, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Casal, J.J.; Aphalo, P.J.; Sanchez, R.A. Phytochrome effects on leaf growth and chlorophyll content in Petunia axilaris. Plant Cell Environ. 1987, 10, 509–514. [Google Scholar] [CrossRef]

| Spectral Treatment | Light Intensity (μmol m−2 s−1) | |||||
|---|---|---|---|---|---|---|
| Total PAR * | Red (R) | Blue (B) | Green (G) | Far-Red (FR) | PSS ** | |
| RB | 150.44 ± 1.07 | 112.5 | 37.5 | 0.88 | ||
| RBG | 149.08 ± 2.02 | 90 | 30 | 30 | 0.88 | |
| RBFR | 146.80 ± 1.53 | 112.5 | 37.5 | 100 | 0.73 | |
| RBFRG | 150.63 ± 0.64 | 90 | 30 | 30 | 100 | 0.68 |
| Spectral Treatment | Light Intensity (μmol m–2 s–1) | |||||
|---|---|---|---|---|---|---|
| Total PAR * | Red (R) | Blue (B) | Green (G) | Far-Red (FR) | PSS ** | |
| RBFR | 144.06 ± 0.62 | 105 | 35 | 40 | 0.80 | |
| RBFRG | 141.03 ± 0.88 | 90 | 30 | 30 | 34 | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Heuvelink, E.; Melegkou, M.; Yuan, X.; Jiang, W.; Marcelis, L.F.M. Effects of Green Light on Elongation Do Not Interact with Far-Red, Unless the Phytochrome Photostationary State (PSS) Changes in Tomato. Biology 2022, 11, 151. https://doi.org/10.3390/biology11010151
Zhang X, Heuvelink E, Melegkou M, Yuan X, Jiang W, Marcelis LFM. Effects of Green Light on Elongation Do Not Interact with Far-Red, Unless the Phytochrome Photostationary State (PSS) Changes in Tomato. Biology. 2022; 11(1):151. https://doi.org/10.3390/biology11010151
Chicago/Turabian StyleZhang, Xue, Ep Heuvelink, Michaela Melegkou, Xin Yuan, Weijie Jiang, and Leo F. M. Marcelis. 2022. "Effects of Green Light on Elongation Do Not Interact with Far-Red, Unless the Phytochrome Photostationary State (PSS) Changes in Tomato" Biology 11, no. 1: 151. https://doi.org/10.3390/biology11010151
APA StyleZhang, X., Heuvelink, E., Melegkou, M., Yuan, X., Jiang, W., & Marcelis, L. F. M. (2022). Effects of Green Light on Elongation Do Not Interact with Far-Red, Unless the Phytochrome Photostationary State (PSS) Changes in Tomato. Biology, 11(1), 151. https://doi.org/10.3390/biology11010151







