Pectins and Olive Pectins: From Biotechnology to Human Health
Abstract
Simple Summary
Abstract
1. Introduction
Pectins from Olives
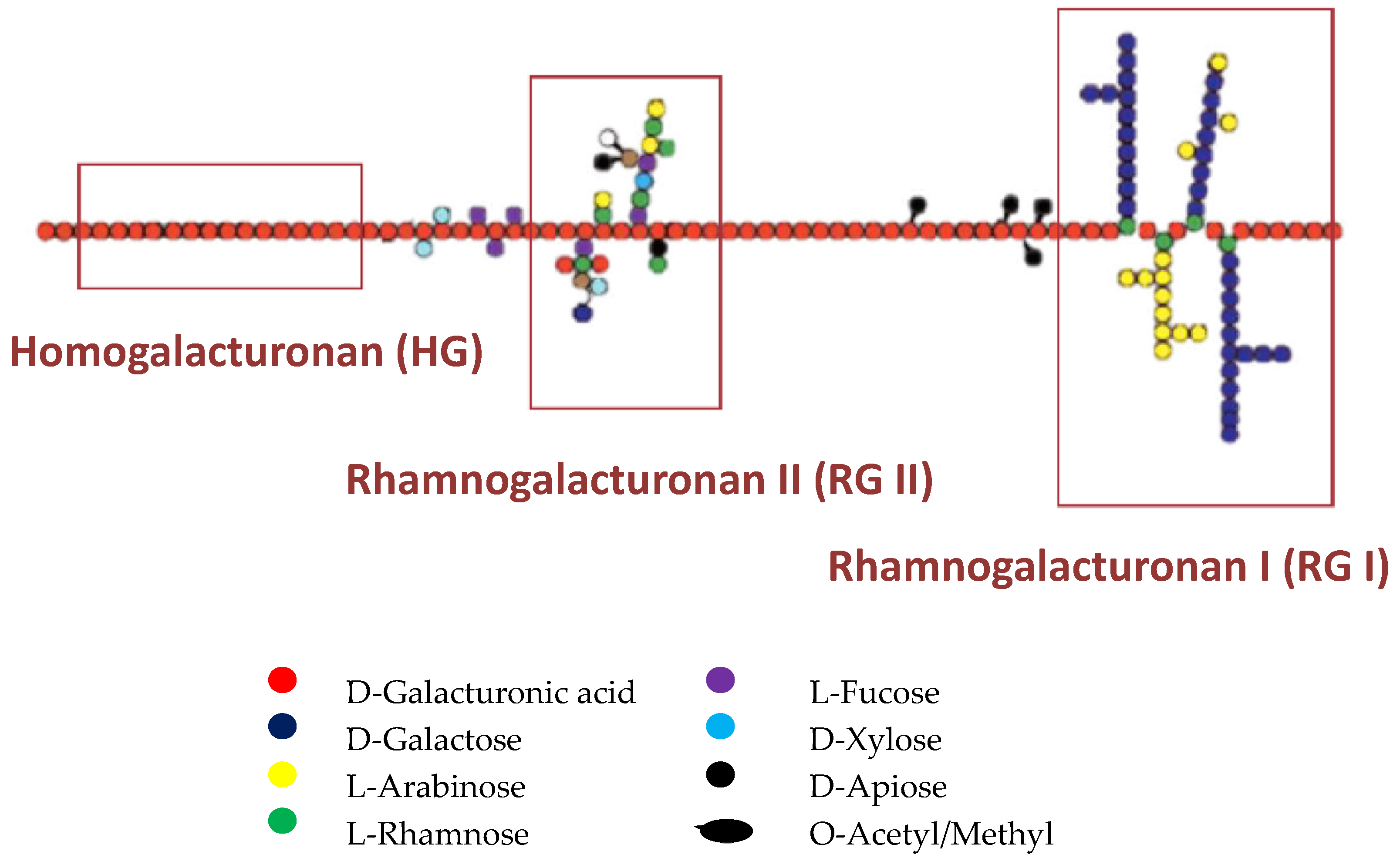
2. Structure, Quantification, and Qualification
2.1. Pectins
2.2. Olive Pectins
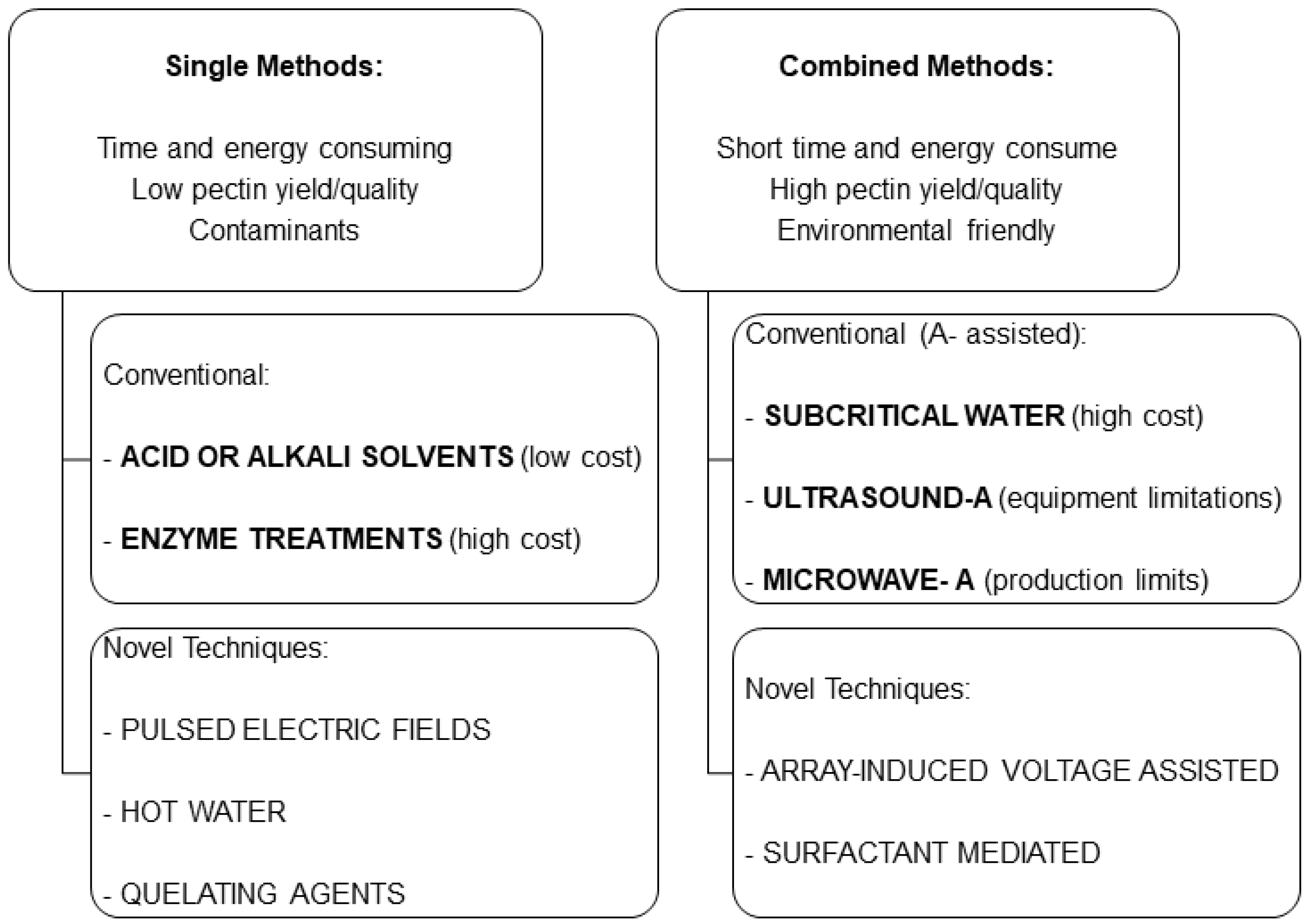
3. Extraction
3.1. Pectins
3.2. Olive Pectins
4. Industrial Applications
4.1. Pectins
4.2. Olive Pectins
5. Bioactivity
5.1. Pectins
5.2. Olive Pectins
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, J.; Zhao, C.; Feng, L.; Han, Y.; Du, H.; Xiao, H.; Zheng, J. Pectins from fruits: Relationships between extraction methods, structural characteristics, and functional properties. Trends Food Sci. Technol. 2021, 110, 39–54. [Google Scholar] [CrossRef]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The primary, secondary, and structures of higher levels of pectin polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef]
- Uluisik, S.; Seymour, G.B. Pectate lyases: Their role in plants and importance in fruit ripening. Food Chem. 2020, 309, 125559. [Google Scholar] [CrossRef]
- Voiniciuc, C.; Pauly, M.; Usadel, B. Monitoring polysaccharide dynamics in the plant cell wall. Plant Physiol. 2018, 176, 2590–2600. [Google Scholar] [CrossRef]
- Saffer, A.M. Expanding roles for pectins in plant development. J. Integr. Plant Biol. 2018, 60, 910–923. [Google Scholar] [CrossRef]
- Parra, R.; Paredes, M.A.; Labrador, J.; Nunes, C.; Coimbra, M.A.; Fernandez-Garcia, N.; Olmos, E.; Gallardo, M.; Gomez-Jimenez, M.C. Cellwall composition andultrastructural immunolocalization of pectin and arabinogalactan protein during olea europaea l. fruit abscission. Plant Cell Physiol. 2020, 61, 814–825. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Diffuse growth of plant cell walls. Plant Physiol. 2018, 176, 16–27. [Google Scholar] [CrossRef]
- Liu, J.; Bi, J.; McClements, D.J.; Liu, X.; Yi, J.; Lyu, J.; Zhou, M.; Verkerk, R.; Dekker, M.; Wu, X.; et al. Impacts of thermal and non-thermal processing on structure and functionality of pectin in fruit- and vegetable- based products: A review. Carbohydr. Polym. 2020, 250, 116890. [Google Scholar] [CrossRef]
- Fu, J.; Mort, A. Progress towards identifying a covalent cross-link between xyloglucan and rhamnogalacturonan in cotton cell walls. Plant Physiol. 1997, 114S, 83. [Google Scholar]
- Vidal, S.; Williams, P.; Doco, T.; Moutounet, M.; Pellerin, P. The polysaccharides of red wine: Total fractionation and characterization. Carbohydr. Polym. 2003, 54, 439–447. [Google Scholar] [CrossRef]
- Abdel-Massih, R.M.; Baydoun, E.A.-H.; Brett, C.T. In vitro biosynthesis of 1,4-b-galactan attached to a pectin–xyloglucan complex in pea. Planta 2003, 216, 502–511. [Google Scholar] [CrossRef]
- Popper, Z.A.; Fry, S.C. Xyloglucan–pectin linkages are formed intra-protoplasmically, contribute to wall-assembly, and remain stable in the cell wall. Planta 2008, 227, 781–794. [Google Scholar] [CrossRef]
- Femenia, A.; Rigby, N.M.; Selvendran, R.R.; Waldron, K.W. Investigation of the occurrence of pectic-xylan–xyloglucan complexes in cell walls of cauliflower stem tissues. Carbohydr. Res. 1999, 39, 151–164. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—A review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M. Olive Mill and Winery Wastes as Viable Sources of Bioactive Compounds: A Study on Polyphenols Recovery. Antioxidants 2020, 9, 1074. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.; Coelho, M.; Veiga, M.; Costa, E.M.; Silva, S.; Nunes, J.; Vicente, A.A.; Pintado, M. Are olive pomace powders a safe source of bioactives and nutrients? J. Sci. Food Agric. 2021, 101, 1963–1978. [Google Scholar] [CrossRef]
- Christiaens, S.; Van Buggenhout, S.; Houben, K.; Jamsazzadeh Kermani, Z.; Moelants, K.R.N.; Ngouémazong, E.D.; Van Loey, A.; Hendrickx, M.E.G. Process–Structure–Function Relations of Pectin in Food. Crit. Rev. Food Sci. Nutr. 2016, 56, 1021–1042. [Google Scholar] [CrossRef]
- Levesque-Tremblay, G.; Pelloux, J.; Braybrook, S.A.; Müller, K. Tuning of pectin methylesterification: Consequences for cell wall biomechanics and development. Planta 2015, 242, 791–811. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.T.; Wightman, R.; Meyerowitz, E.M.; Peaucelle, A. Pectin homogalacturonan nanofilament expansion drives morphogenesis in plant epidermal cells. Science 2020, 367, 1003–1007. [Google Scholar] [CrossRef]
- Noreen, A.; Nazli, Z.I.H.; Akram, J.; Rasul, I.; Mansha, A.; Yaqoob, N.; Iqbal, R.; Tabasum, S.; Zuber, M.; Zia, K.M. Pectins functionalized biomaterials; a new viable approach for biomedical applications: A review. Int. J. Biol. Macromol. 2017, 101, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zheng, J.; Mao, G.; Hu, W.; Ye, X.; Linhardt, R.J.; Chen, S. Rethinking the impact of RG-I mainly from fruits and vegetables on dietary health. Crit. Rev. Food Sci. Nutr. 2020, 60, 2938–2960. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Extraction, purification and characterization of pectin from alternative sources with potential technological applications. Food Res. Int. 2018, 113, 327–350. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Dejonghe, W.; Gatti, M.; Sforza, S.; Elst, K. Pectic oligosaccharides from agricultural by-products: Production, characterization and health benefits. Crit. Rev. Biotechnol. 2016, 36, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Bokov, D.O.; Sharipova, R.I.; Potanina, O.G.; Nikulin, A.V.; Nasser, R.A.; Samylina, I.A.; Bessonov, V.V. Polysaccharides of crude herbal drugs as a group of biologically active compounds in the field of modern pharmacognosy: Physicochemical properties, classification, pharmacopoeial analysis. Proteins 2020, 2, 4–6. [Google Scholar]
- Coimbra, M.A.; Cardoso, S.M.; Lopes-Da-Silva, J.A. Olive pomace, a source for valuable Arabinan-rich pectic polysaccharides. In Carbohydrates in Sustainable Development I. Topics in Current Chemistry; Rauter, A., Vogel, P., Queneau, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 294, pp. 129–141. [Google Scholar] [CrossRef]
- Goulao, L.F.; Oliveira, C.M. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.; Heredia, A.; Vioque, B.; Castellano, J.M.; Guillén, R. Changes in cell-wall-degrading enzyme activities in stored olives in relation to respiration and ethylene production: Influence of exogenous ethylene. Z. Fur Leb. Unters. Und Forsch. 1997, 204, 293–299. [Google Scholar] [CrossRef]
- Vierhuis, E.; Schols, H.A.; Beldman, G.; Voragen, A.G.J. Isolation and characterization of cell wall material from olive fruit (Olea europaea cv koroneiki) at different ripening stages. Carbohydr. Polym. 2000, 43, 11–21. [Google Scholar] [CrossRef]
- Mafra, I.; Lanza, B.; Reis, A.; Marsilio, V.; Campestre, C.; De Angelis, M.; Coimbra, M.A. Effect of ripening on texture, microstructure and cell wall polysaccharide composition of olive fruit (Olea europaea). Physiol. Plant. 2001, 111, 439–447. [Google Scholar] [CrossRef]
- Parra, R.; Paredes, M.A.; Sanchez-Calle, I.M.; Gomez-Jimenez, M.C. Comparative transcriptional profiling analysis of olive ripe-fruit pericarp and abscission zone tissues shows expression differences and distinct patterns of transcriptional regulation. BMC Genom. 2013, 14, 866. [Google Scholar] [CrossRef]
- Diarte, C.; Iglesias, A.; Romero, A.; Casero, T.; Ninot, A.; Gatius, F.; Graell, J.; Lara, I. Ripening-related cell wall modifications in olive (Olea europaea L.) fruit: A survey of nine genotypes. Food Chem. 2021, 338, 127754. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, F.; Wu, J.; Wang, Z.; Liao, X.; Hu, X. Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. J. Food Eng. 2007, 78, 693–700. [Google Scholar] [CrossRef]
- Pasandide, B.; Khodaiyan, F.; Mousavi, Z.E.; Hosseini, S.S. Optimization of aqueous pectin extraction from Citrus medica peel. Carbohydr. Polym. 2017, 178, 27–33. [Google Scholar] [CrossRef]
- Guo, X.; Han, D.; Xi, H.; Rao, L.; Liao, X.; Hu, X.; Wu, J. Extraction of pectin from navel orange peel assisted by ultra-high pressure, microwave or traditional heating: A comparison. Carbohydr. Polym. 2012, 88, 441–448. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Huang, X.; Li, D.; Wang, L. jun Characterization of pectin extracted from sugar beet pulp under different drying conditions. J. Food Eng. 2017, 211, 1–6. [Google Scholar] [CrossRef]
- Seixas, F.L.; Fukuda, D.L.; Turbiani, F.R.B.; Garcia, P.S.; Petkowicz, C.L.D.O.; Jagadevan, S.; Gimenes, M.L. Extraction of pectin from passion fruit peel (Passiflora edulis f.flavicarpa) by microwave-induced heating. Food Hydrocoll. 2014, 38, 186–192. [Google Scholar] [CrossRef]
- Swamy, G.J.; Muthukumarappan, K. Optimization of continuous and intermittent microwave extraction of pectin from banana peels. Food Chem. 2017, 220, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Methacanon, P.; Krongsin, J.; Gamonpilas, C. Pomelo (Citrus maxima) pectin: Effects of extraction parameters andits properties. Food Hydrocoll. 2014, 35, 383–391. [Google Scholar] [CrossRef]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Shakhmatov, E.G.; Toukach, P.V.; Makarova, E.N. Structural studies of the pectic polysaccharide from fruits of Punica granatum. Carbohydr. Polym. 2020, 235, 115978. [Google Scholar] [CrossRef]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, Characterisation, and Application of Pectin from Tropical and Sub-Tropical Fruits: A Review. Food Rev. Int. 2020. [Google Scholar] [CrossRef]
- Nowacka, M.; Tappi, S.; Wiktor, A.; Rybak, K.; Miszczykowska, A.; Czyzewski, J.; Drozdzal, K.; Witrowa-Rajchert, D.; Tylewicz, U. The Impact of Pulsed Electric Field on the Extraction of Bioactive Compounds from Beetroot. Foods 2019, 7, 244. [Google Scholar] [CrossRef]
- Khedmat, L.; Izadi, A.; Mofid, V.; Mojtahedi, S.Y. Recent advances in extracting pectin by single and combined ultrasound techniques: A review of techno-functional and bioactive health-promoting aspects. Carbohydr. Polym. 2020, 229, 115474. [Google Scholar] [CrossRef]
- Jiménez, A.; Rodríguez, R.; Ferriáhdez-Caro, I.; Guillén, R.; Fernández-Bolaños, J.; Heredia, A. Olive fruit cell wall: Degradation of peptic polysaccharides during ripening. J. Agric. Food Chem. 2001, 49, 409–415. [Google Scholar] [CrossRef]
- Moustakime, Y.; Hazzoumi, Z.; Joutei, K.A. Effect of proteolytic activities in combination with the pectolytic activities on extractability of the fat and phenolic compounds from olives. SpringerPlus 2016, 5, 739. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sánchez-Romero, C.; Guillén, R.; Heredia, A.; Jiménez, A.; Fernández-Bolaños, J. Degradation of pectic polysaccharides in pickled green olives. J. Food Prot. 1998, 61, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Vierhuis, E.; Korver, M.; Schols, H.A.; Voragen, A.G.J. Structural characteristics of pectic polysaccharides from olive fruit (Olea europaea cv moraiolo) in relation to processing for oil extraction. Carbohydr. Polym. 2003, 51, 135–148. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Fernández-Bolaños, J. Production, characterization and isolation of neutral and pectic oligosaccharides with low molecular weights from olive by-products thermally treated. Food Hydrocoll. 2012, 28, 92–104. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. Pectin extracted from thermally treated olive oil by-products: Characterization, physico-chemical properties, invitro bile acid andglucose binding. Food Hydrocoll. 2015, 43, 311–321. [Google Scholar] [CrossRef]
- Gullón, B.; Gómez, B.; Martínez-Sabajanes, M.; Yáñez, R.; Parajó, J.C.; Alonso, J.L. Pectic oligosaccharides: Manufacture and functional properties. Trends Food Sci. Technol. 2013, 30, 153–161. [Google Scholar] [CrossRef]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging concepts in the nutraceutical and functional properties of pectin—A Review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef]
- Holck, J.; Hotchkiss, A.T.; Meyer, A.S.; Mikkelsen, J.D.; Rastall, R.A. 5 Production and Bioactivity of Pectic Oligosaccharides from Fruit and Vegetable Biomass. Prod. Anal. Bioactivity 2014, 76–87. [Google Scholar]
- Katav, T.; Liu, L.S.; Traitel, T.; Goldbart, R.; Wolfson, M.; Kost, J. Modified pectin-based carrier for gene delivery: Cellular barriers in gene delivery course. J. Control. Release 2008, 130, 183–191. [Google Scholar] [CrossRef]
- Smistad, G.; Bøyum, S.; Alund, S.J.; Samuelsen, A.B.C.; Hiorth, M. The potential of pectin as a stabilizer for liposomal drug delivery systems. Carbohydr. Polym. 2012, 90, 1337–1344. [Google Scholar] [CrossRef]
- Rehman, A.; Ahmad, T.; Aadil, R.M.; Spotti, M.J.; Bakry, A.M.; Khan, I.M.; Zhao, L.; Riaz, T.; Tong, Q. Pectin polymers as wall materials for the nano-encapsulation of bioactive compounds. Trends Food Sci. Technol. 2019, 90, 35–46. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Nešić, A.; Onjia, A.; Davidović, S.; Dimitrijević, S.; Errico, M.E.; Santagata, G.; Malinconico, M. Design of pectin-sodium alginate based films for potential healthcare application: Study of chemico-physical interactions between the components of films and assessment of their antimicrobial activity. Carbohydr. Polym. 2017, 157, 981–990. [Google Scholar] [CrossRef]
- Wong, T.W.; Colombo, G.; Sonvico, F. Pectin matrix as oral drug delivery vehicle for colon cancer treatment. AAPS PharmSciTech 2011, 12, 201–214. [Google Scholar] [CrossRef]
- Krivorotova, T.; Staneviciene, R.; Luksa, J.; Serviene, E.; Sereikaite, J. Preparation and characterization of nisin-loaded pectin-inulin particles as antimicrobials. LWT Food Sci. Technol. 2016, 72, 518–524. [Google Scholar] [CrossRef]
- Zhang, T.; Lan, Y.; Zheng, Y.; Liu, F.; Zhao, D.; Mayo, K.H.; Zhou, Y.; Tai, G. Identification of the bioactive components from pH-modified citrus pectin and their inhibitory effects on galectin-3 function. Food Hydrocoll. 2016, 58, 113–119. [Google Scholar] [CrossRef]
- Dash, K.K.; Ali, N.A.; Das, D.; Mohanta, D. Thorough evaluation of sweet potato starch and lemon-waste pectin based-edible films with nano-titania inclusions for food packaging applications. Int. J. Biol. Macromol. 2019, 139, 449–458. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Fraqueza, M.J.; Fernandes, M.H.; Moldão-Martins, M.; Alves, V.D. Application of edible alginate films with pineapple peel active compounds on beef meat preservation. Antioxidants 2020, 9, 667. [Google Scholar] [CrossRef]
- Grassino, A.N.; Halambek, J.; Djaković, S.; Rimac Brnčić, S.; Dent, M.; Grabarić, Z. Utilization of tomato peel waste from canning factory as a potential source for pectin production and application as tin corrosion inhibitor. Food Hydrocoll. 2016, 52, 265–274. [Google Scholar] [CrossRef]
- Zhang, W.; Song, J.; He, Q.; Wang, H.; Lyu, W.; Feng, H.; Xiong, W.; Guo, W.; Wu, J.; Chen, L. Novel pectin based composite hydrogel derived from grapefruit peel for enhanced Cu(II) removal. J. Hazard. Mater. 2020, 384, 121445. [Google Scholar] [CrossRef]
- Khan, A.A.; Randhawa, M.A.; Karim, R.; Ahmed, W. Extraction and characterization of pectin from grapefruit (Duncan cultivar) and its utilization as gelling agent. Int. Food Res. J. 2014, 21, 2195. [Google Scholar]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Ultrasound-microwave assisted extraction of pectin from fig (Ficus carica L.) skin: Optimization, characterization and bioactivity. Carbohydr. Polym. 2019, 222, 114992. [Google Scholar] [CrossRef]
- Liu, L.S.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef]
- Kusrini, E.; Wicaksono, W.; Gunawan, C.; Daud, N.Z.A.; Usman, A. Kinetics, mechanism, and thermodynamics of lanthanum adsorption on pectin extracted from durian rind. J. Environ. Chem. Eng. 2018, 6, 6580–6588. [Google Scholar] [CrossRef]
- Xu, S.Y.; Liu, J.P.; Huang, X.; Du, L.P.; Shi, F.L.; Dong, R.; Huang, X.T.; Zheng, K.; Liu, Y.; Cheong, K.L. Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. LWT Food Sci. Technol. 2018, 90, 577–582. [Google Scholar] [CrossRef]
- Hua, X.; Wang, K.; Yang, R.; Kang, J.; Yang, H. Edible coatings from sunflower head pectin to reduce lipid uptake in fried potato chips. LWT Food Sci. Technol. 2015, 62, 1220–1225. [Google Scholar] [CrossRef]
- Encalada, A.M.I.; Pérez, C.D.; Flores, S.K.; Rossetti, L.; Fissore, E.N.; Rojas, A.M. Antioxidant pectin enriched fractions obtained from discarded carrots (Daucus carota L.) by ultrasound-enzyme assisted extraction. Food Chem. 2019, 289, 453–460. [Google Scholar] [CrossRef]
- Idrovo Encalada, A.M.; Pérez, C.D.; Calderón, P.A.; Zukowski, E.; Gerschenson, L.N.; Rojas, A.M.; Fissore, E.N. High-power ultrasound pretreatment for efficient extraction of fractions enriched in pectins and antioxidants from discarded carrots (Daucus carota L.). J. Food Eng. 2019, 256, 28–36. [Google Scholar] [CrossRef]
- Nguyen, B.M.N.; Pirak, T. Physicochemical properties and antioxidant activities of white dragon fruit peel pectin extracted with conventional and ultrasound-assisted extraction. Cogent Food Agric. 2019, 5, 1633076. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, P.; Ferreira, P.; de Sousa, H.C.; Batista, P.; Rodrigues, M.A.; Correia, I.J.; Gil, M.H. Preparation and chemical and biological characterization of a pectin/chitosan polyelectrolyte complex scaffold for possible bone tissue engineering applications. Int. J. Biol. Macromol. 2011, 48, 112–118. [Google Scholar] [CrossRef]
- Munarin, F.; Guerreiro, S.G.; Grellier, M.A.; Tanzi, M.C.; Barbosa, M.A.; Petrini, P.; Granja, P.L. Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar] [CrossRef]
- Markov, P.A.; Popov, S.V.; Nikitina, I.R.; Ovodova, R.G.; Ovodov, Y.S. Anti-inflammatory activity of pectins and their galacturonan backbone. Russ. J. Bioorganic Chem. 2011, 37, 817–821. [Google Scholar] [CrossRef]
- Singh, V.; Yeoh, B.S.; Walker, R.E.; Xiao, X.; Saha, P.; Golonka, R.M.; Cai, J.; Bretin, A.C.A.; Cheng, X.; Liu, Q.; et al. Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut 2019, 68, 1801–1812. [Google Scholar] [CrossRef]
- Licht, T.R.; Hansen, M.; Bergström, A.; Poulsen, M.; Krath, B.N.; Markowski, J.; Dragsted, L.O.; Wilcks, A. Effects of apples and specific apple components on the cecal environment of conventional rats: Role of apple pectin. BMC Microbiol. 2010, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, C.; Huang, R.; Song, J.; Li, D.; Xia, M. Butyrate from pectin fermentation inhibits intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E-deficient mice. J. Nutr. Biochem. 2018, 56, 175–182. [Google Scholar] [CrossRef]
- Viebke, C.; Al-Assaf, S.; Phillips, G.O. Food hydrocolloids and health claims. Bioact. Carbohydr. Diet. Fibre 2014, 4, 101–114. [Google Scholar] [CrossRef]
- Jiang, T.; Gao, X.; Wu, C.; Tian, F.; Lei, Q.; Bi, J.; Xie, B.; Wang, H.; Chen, S.; Wang, X. Apple-Derived Pectin Modulates Gut Microbiota, Improves Gut Barrier Function, and Attenuates Metabolic Endotoxemia in Rats with Diet-Induced Obesity. Nutrients 2016, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Hogan, V.; Honjo, Y.; Baccarini, S.; Tait, L.; Bresalier, R.; Raz, A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J. Natl. Cancer Inst. 2002, 94, 1854–1862. [Google Scholar] [CrossRef]
- Dutta, R.K.; Sahu, S. Development of oxaliplatin encapsulated in magnetic nanocarriers of pectin as a potential targeted drug delivery for cancer therapy. Results Pharma Sci. 2012, 2, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Leclere, L.; Van Cutsem, P.; Michiels, C. Anti-cancer activities of pH- or heat-modified pectin. Front. Pharmacol. 2013, 4, 128. [Google Scholar] [CrossRef]
- Delphi, L.; Sepehri, H.; Khorramizadeh, M.R.; Mansoori, F. Pectic-oligoshaccharides from apples induce apoptosis and cell cycle arrest in MDA-MB-231 cells, a model of human breast cancer. Asian Pac. J. Cancer Prev. 2015, 16, 5265–5271. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Alaiz, M.; Vioque, J.; Girón-Calle, J.; Fernández-Bolaños, J. Pectin-rich extracts from olives inhibit proliferation of Caco-2 and THP-1 cells. Food Funct. 2019, 10, 4844. [Google Scholar] [CrossRef]
- Mao, G.; Wu, D.; Wei, C.; Tao, W.; Ye, X.; Linhardt, R.J.; Orfila, C.; Chen, S. Reconsidering conventional and innovative methods for pectin extraction from fruit and vegetable waste: Targeting rhamnogalacturonan I. Trends Food Sci. Technol. 2019, 94, 65–78. [Google Scholar] [CrossRef]
- Gómez, B.; Gullón, B.; Remoroza, C.; Schols, H.A.; Parajó, J.C.; Alonso, J.L. Purification, characterization, and prebiotic properties of pectic oligosaccharides from orange peel wastes. J. Agric. Food Chem. 2014, 62, 9769–9782. [Google Scholar] [CrossRef] [PubMed]
- Manderson, K.; Pinart, M.; Tuohy, K.M.; Grace, W.E.; Hotchkiss, A.T.; Widmer, W.; Yadhav, M.P.; Gibson, G.R.; Rastall, R.A. In vitro determination of prebiotic properties of oligosaccharides derived from an orange juice manufacturing by-product stream. Appl. Environ. Microbiol. 2005, 71, 8383–8389. [Google Scholar] [CrossRef] [PubMed]
- Gómez, B.; Gullón, B.; Yáñez, R.; Schols, H.; Alonso, J.L. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods 2016, 20, 108–121. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93, 127. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, M.A.H.M.; Palframan, R.J.; Cooper, J.M.; Gibson, G.R.; Rastall, R.A. In vitro fermentation of sugar beet arabinan and arabino-oligosaccharides by the human gut microflora. J. Appl. Microbiol. 2006, 100, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Modified sugar beet pectin induces apoptosis of colon cancer cells via an interaction with the neutral sugar side-chains. Carbohydr. Polym. 2016, 136, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Islamova, Z.I.; Ogai, D.K.; Abramenko, O.I.; Lim, A.L.; Abduazimov, B.B.; Malikova, M.K.; Rakhmanberdyeva, R.K.; Khushbaktova, Z.A.; Syrov, V.N. Comparative Assessment of the Prebiotic Activity of Some Pectin Polysaccharides. Pharm. Chem. J. 2017, 51, 288–291. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, M.; Yang, Z.; Pan, S. Anti-diabetic effect of citrus pectin in diabetic rats and potential mechanism via PI3K/Akt signaling pathway. Int. J. Biol. Macromol. 2016, 89, 484–488. [Google Scholar] [CrossRef]
- Espinal-Ruiz, M.; Restrepo-Sánchez, L.P.; Narváez-Cuenca, C.E.; McClements, D.J. Impact of pectin properties on lipid digestion under simulated gastrointestinal conditions: Comparison of citrus and banana passion fruit (Passiflora tripartita var. mollissima) pectins. Food Hydrocoll. 2016, 52, 329–342. [Google Scholar] [CrossRef]
- Guess, B.W.; Scholz, M.C.; Strum, S.B.; Lam, R.Y.; Johnson, H.J.; Jennrich, R.I. Modified citrus pectin (MCP) increases the prostate-specific antigen doubling time in men with prostate cancer: A phase II pilot study. Prostate Cancer Prostatic Dis. 2003, 6, 301–304. [Google Scholar] [CrossRef]
- Bergman, M.; Djaldetti, M.; Salman, H.; Bessler, H. Effect of citrus pectin on malignant cell proliferation. Biomed. Pharmacother. 2010, 64, 44–47. [Google Scholar] [CrossRef]
- Liu, H.Y.; Huang, Z.L.; Yang, G.H.; Lu, W.Q.; Yu, N.R. Inhibitory effect of modified citrus pectin on liver metastases in a mouse colon cancer model. World J. Gastroenterol. 2008, 14, 7386–7391. [Google Scholar] [CrossRef] [PubMed]
- Glinsky, V.V.; Raz, A. Modified citrus pectin anti-metastatic properties: One bullet, multiple targets. Carbohydr. Res. 2009, 344, 1788–1791. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Rhamnogalacturonan i containing homogalacturonan inhibits colon cancer cell proliferation by decreasing ICAM1 expression. Carbohydr. Polym. 2015, 132, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Azémar, M.; Hildenbrand, B.; Haering, B.; Heim, M.E.; Unger, C. Clinical Benefit in Patients with Advanced Solid Tumors Treated with Modified Citrus Pectin: A Prospective Pilot Study. Clin. Med. Oncol. 2007, 1, CMO.S285. [Google Scholar] [CrossRef]
- Huang, P.H.; Fu, L.C.; Huang, C.S.; Wang, Y.T.; Wu, M.C. The uptake of oligogalacturonide and its effect on growth inhibition, lactate dehydrogenase activity and galactin-3 release of human cancer cells. Food Chem. 2012, 132, 1987–1995. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, F.; Liu, X.; St. Ange, K.; Zhang, A.; Li, Q.; Linhardt, R.J. Isolation of a lectin binding rhamnogalacturonan-I containing pectic polysaccharide from pumpkin. Carbohydr. Polym. 2017, 163, 330–336. [Google Scholar] [CrossRef]
- Mandalari, G.; Nueno Palop, C.; Tuohy, K.; Gibson, G.R.; Bennett, R.N.; Waldron, K.W.; Bisignano, G.; Narbad, A.; Faulds, C.B. In vitro evaluation of the prebiotic activity of a pectic oligosaccharide-rich extract enzymatically derived from bergamot peel. Appl. Microbiol. Biotechnol. 2007, 73, 1173–1179. [Google Scholar] [CrossRef]
- Wathoni, N.; Yuan Shan, C.; Yi Shan, W.; Rostinawati, T.; Indradi, R.B.; Pratiwi, R.; Muchtaridi, M. Characterization and antioxidant activity of pectin from Indonesian mangosteen (Garcinia mangostana L.) rind. Heliyon 2019, 5, e02299. [Google Scholar] [CrossRef]
- Amaral, S.d.C.; Barbieri, S.F.; Ruthes, A.C.; Bark, J.M.; Brochado Winnischofer, S.M.; Silveira, J.L.M. Cytotoxic effect of crude and purified pectins from Campomanesia xanthocarpa Berg on human glioblastoma cells. Carbohydr. Polym. 2019, 224, 115140. [Google Scholar] [CrossRef]
- Zaid, R.M.; Mishra, P.; Wahid, Z.A.; Sakinah, A.M.M. Hylocereus polyrhizus peel’s high-methoxyl pectin: A potential source of hypolipidemic agent. Int. J. Biol. Macromol. 2019, 134, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. Chemical characterization and properties of a polymeric phenolic fraction obtained from olive oil waste. Food Res. Int. 2013, 54, 2122–2129. [Google Scholar] [CrossRef]
| Source | Total Production (Tonnes) | By-Product (% Fruit Weight) | % Pectin in By-Product |
|---|---|---|---|
| Apple waste | 3.8 × 105 pulp | 5–10% pomace | 15–21% |
| Lemon peel | 8 × 104 | NA | 30% |
| Grapefruit pomace | NA | 5–10% | NA |
| Pomelo peel | NA | 10–15% | NA |
| Sugar beet pulp | 9.1 × 107 | NA | 15–30% |
| Potato pulp | 1.3 × 105 | NA | 15% |
| Watermelon rind | NA | 50–60% | 13–30% |
| Mango peel | NA | 15–20% | 10–15% |
| Passion fruit peel | NA | 50–60% | 15–20% |
| Banana peel | NA | 20–30% | 4–6% |
| Olive pomace | 1.6 × 106 | NA | 34% |
| Source | Yield of Extracted Pectin | Galacturonic Acid Content | Degree of Esterification |
|---|---|---|---|
| Apple pomace | 10–20% | 58–67% | 52–76% |
| Lime peel | 13–26% | 91% | 82% |
| Orange peel | 24% | 68% | 37% |
| Grapefruit waste | 25–30% | NA | NA |
| Pomelo peel | 6–37% | NA | NA |
| Sugar beet pulp | 24% | 72% | 28–52% |
| Pumpkin waste | 7% | 63–73% | 3–18% |
| Carrot pomace | 5–15% | 62–69% | 53–77% |
| Carrot peel | 9% | ||
| Tomato pomace | 7% | 78% | 76–88% |
| Tomato peel | 32% | ||
| Watermelon rind | 3–28% | 68–74% | 61–63% |
| Mango peel | 5–17% | 29–53% | 85–88% |
| Passion fruit peel | 8–12% | 66–68% | 45–60% |
| Banana peel | 2–9% | 40–71% | 1–80% |
| Source | Solvent Extraction | Enzyme Extraction | SWE | UAE | MAE | UMAE |
|---|---|---|---|---|---|---|
| Apple pomace [1,14] | 3–23% | 3–14% | 10–16% | 9% | 23% | |
| Lime peel [44] | 23% | |||||
| Orange peel [1,14,44,46] | 3–23% | 11% | 28% | 5–26% | ||
| Grapefruit waste [1,14,46] | 17–24% | 3–32% | ||||
| Pomelo peel [1,14,44,46] | 3% | 3–19% | 3–38% | 0.05–29% | 36% | |
| Sugar beet pulp [14,46] | 26% | 5–32% | ||||
| Pumpkin waste [14,46] | 22–23% | 3–7% | ||||
| Carrot waste [14,46] | 5–15% | 27–35% | ||||
| Tomato waste [14,46] | 9–19% | 15–36% | ||||
| Watermelon rind [14,44] | 13–24% | |||||
| Mango peel [44,46] | 5% | 8–17% | ||||
| Passion fruit peel [14,44,46] | 5–14% | 3–26% | 7–13% | 30% | ||
| Banana peel [14,44,46] | 5–12% | 21% | 1–2% |
| Cultivar | Maturity Stage | Oil Content (g/100 g) DW | AIR (g/100 g) | Degree of Esterification |
|---|---|---|---|---|
| ‘Arbequina’ | Green | 39.3 | 7.8 | 56.9% |
| Turning | 43.3 | 7.6 | 49.8% | |
| Ripe | 52.2 | 3.6 | 64.8% | |
| ‘Argudell’ | Green | 39.8 | 12.3 | 77.9% |
| Turning | 48.0 | 11.4 | 93.1% | |
| Ripe | 50.1 | 8.4 | 78.4% | |
| ‘Empeltre’ | Green | 45.9 | 5.1 | 88.5% |
| Turning | 45.5 | 3.8 | 48.9% | |
| Ripe | 56.1 | 6.4 | 31.9% | |
| ‘Farga’ | Green | 36.4 | 8.6 | 52.1% |
| Turning | 40.9 | 5.0 | 58.2% | |
| Ripe | 51.2 | 4.0 | 71.2% | |
| ‘Manzanilla’ | Green | 45.0 | 4.0 | 62.4% |
| Ripe | 50.6 | 3.0 | 67.5% | |
| ‘Marfil’ | Green | 46.1 | 7.93 | 49.8% |
| Ripe | 34.2 | 3.5 | 50.9% | |
| ‘Morrut’ | Green | 27.0 | 15.2 | 70.0% |
| Turning | 37.2 | 12.7 | 76.6% | |
| Ripe | 45.0 | 6.2 | 74.3% | |
| ‘Picual’ | Green | 35.6 | 8.4 | 58.3% |
| Turning | 48.6 | 11.6 | 68.1% | |
| Ripe | 55.4 | 7.3 | 76.5% | |
| ‘Sevillenca’ | Green | 43.8 | 10.1 | 62.7% |
| Ripe | 57.0 | 9.7 | 67.5% |
| Use in Food Industry | Use in Non-Food Industry | |
|---|---|---|
| Citrus [56,60,61,62,63] | Antimicrobial, gelling, and thickening | Disinfection of medical devices, genes, drug delivery, and gelling/thickening agent |
| Lemon peel [64] | Packaging material | |
| Pineapple peel [65] | Inhibit lipid oxidation | |
| Tomato peel [66] | Corrosion inhibitor | |
| Grapefruit peel [37,67,68] | Gelling agent, lipid digestibility | Wastewater treatment |
| Fig skin [69] | Anti-radical/oxidant | |
| Sugar beet pulp [70] | Drug delivery | |
| Durian rind [71] | Wastewater treatment | |
| Jackfruit peel [72] | Antioxidant | |
| Sunflower head [73] | Reduce lipid uptake | |
| Carrot waste [74,75] | Antioxidant | |
| Dragon fruit peel [76] | Antioxidant |
| Source | Application |
|---|---|
| Orange peel | Prebiotic effect [92,93] |
| Sugar beet pulp | Prebiotic effect [94,95,96] |
| Anti-inflammatory [95] | |
| Antitumoral [97] | |
| Lemon peel | Prebiotic effect [94] |
| Apple pomace | Prebiotic effect [98] |
| Citrus | Anti-diabetic [99] |
| Lipid digestibility [100] | |
| Antitumoral [101,102,103,104,105,106,107] | |
| Banana passion fruit waste | Lipid digestibility [100] |
| Pumpkin waste | Antitumoral [108] |
| Fig skin | Antitumoral [69] |
| Grapefruit peel | Antioxidant [37] |
| Bergamot peel | Prebiotic effect [109] |
| Mangosteen rind | Antioxidant [110] |
| Gabiroba pulp | Antitumoral [111] |
| Dragonfruit peel | Hypolipidemic agent [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millan-Linares, M.C.; Montserrat-de la Paz, S.; Martin, M.E. Pectins and Olive Pectins: From Biotechnology to Human Health. Biology 2021, 10, 860. https://doi.org/10.3390/biology10090860
Millan-Linares MC, Montserrat-de la Paz S, Martin ME. Pectins and Olive Pectins: From Biotechnology to Human Health. Biology. 2021; 10(9):860. https://doi.org/10.3390/biology10090860
Chicago/Turabian StyleMillan-Linares, Maria C., Sergio Montserrat-de la Paz, and Maria E. Martin. 2021. "Pectins and Olive Pectins: From Biotechnology to Human Health" Biology 10, no. 9: 860. https://doi.org/10.3390/biology10090860
APA StyleMillan-Linares, M. C., Montserrat-de la Paz, S., & Martin, M. E. (2021). Pectins and Olive Pectins: From Biotechnology to Human Health. Biology, 10(9), 860. https://doi.org/10.3390/biology10090860








