Thermal and Oxygen Flight Sensitivity in Ageing Drosophila melanogaster Flies: Links to Rapamycin-Induced Cell Size Changes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Flies
2.2. Isolines
2.3. Induction of Two Phenotypes
2.4. Flight Performance
2.5. Morphological Measurements
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olson, S.L.; Schwieterman, E.W.; Reinhard, C.T.; Lyons, T.W. Earth: Atmospheric evolution of a habitable planet. In Handbook of Exoplanets; Deeg, H., Belmonte, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 2817–2853. ISBN 978-3-319-55332-0. [Google Scholar]
- Harrison, J.F.; Kaiser, A.; VandenBrooks, J.M. Atmospheric oxygen level and the evolution of insect body size. Proc. R Soc. B Biol. Sci. 2010, 277, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.E. On the Wing; Oxford University Press: New York, NY, USA, 2015; ISBN 9780199996773. [Google Scholar]
- Verberk, W.C.E.P.; Bilton, D.T. Can Oxygen set thermal limits in an insect and drive gigantism? PLoS ONE 2011, 6, e22610. [Google Scholar] [CrossRef] [PubMed]
- Woods, H.A.; Hill, R.I. Temperature-dependent oxygen limitation in insect eggs. J. Exp. Biol. 2004, 207, 2267–2276. [Google Scholar] [CrossRef]
- Stearns, S. The Evolution of Life Histories; Oxford University Press: New York, NY, USA, 1992; ISBN 0198577419. [Google Scholar]
- Kozłowski, J. Optimal allocation of resources to growth and reproduction: Implications for age and size at maturity. Trends Ecol. Evol. 1992, 7, 15–19. [Google Scholar] [CrossRef]
- Edgar, B.A. How flies get their size: Genetics meets physiology. Nat. Rev. Genet. 2006, 7, 907–916. [Google Scholar] [CrossRef]
- VandenBrooks, J.M.; Munoz, E.E.; Weed, M.D.; Ford, C.F.; Harrison, M.A.; Harrison, J.F. Impacts of Paleo-Oxygen Levels on the Size, Development, Reproduction, and Tracheal Systems of Blatella germanica. Evol. Biol. 2012, 39, 83–93. [Google Scholar] [CrossRef]
- Dudley, R. The evolutionary physiology of animal flight: Paleobiological and present perspectives. Annu. Rev. Physiol. 2000, 62, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Hoback, W.W.; Stanley, D.W. Insects in hypoxia. J. Insect Physiol. 2001, 47, 533–542. [Google Scholar] [CrossRef]
- Dillon, M.E.; Frazier, M.R. Drosophila melanogaster locomotion in cold thin air. J. Exp. Biol. 2006, 209, 364–371. [Google Scholar] [CrossRef]
- Anderson, J.F.; Ultsch, G.R. Respiratory gas concentrations in the microhabitats of some florida arthropods. Comp. Biochem. Physiol. Part A Physiol. 1987, 88, 585–588. [Google Scholar] [CrossRef]
- Frazier, M.R.; Woods, H.A.; Harrison, J.F. Interactive effects of rearing temperature and oxygen on the development of Drosophila melanogaster. Physiol. Biochem. Zool. 2001, 74, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Rubalcaba, J.G.; Verberk, W.C.E.P.; Jan Hendriks, A.; Saris, B.; Arthur Woods, H. Oxygen limitation may affect the temperature and size dependence of metabolism in aquatic ectotherms. Proc. Natl. Acad. Sci. USA 2020, 117, 31963–31968. [Google Scholar] [CrossRef]
- Klok, C.J.; Sinclair, B.J.; Chown, S.L. Upper thermal tolerance and oxygen limitation in terrestrial arthropods. J. Exp. Biol. 2004, 207, 2361–2370. [Google Scholar] [CrossRef]
- Verberk, W.C.E.P.; Atkinson, D.; Hoefnagel, K.N.; Hirst, A.G.; Horne, C.R.; Siepel, H. Shrinking body sizes in response to warming: Explanations for the temperature—size rule with special emphasis on the role of oxygen. Biol. Rev. 2020, 96, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Verberk, W.C.E.P.; Bilton, D.; Calosi, P.; Spicer, J. Oxygen supply in aquatic ectotherms: Partial pressure and solubility together explain biodiversity and size patterns. Ecology 2011, 92, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Verberk, W.C.E.P.; Overgaard, J.; Ern, R.; Bayley, M.; Wang, T.; Boardman, L.; Terblanche, J.S. Does oxygen limit thermal tolerance in arthropods? A critical review of current evidence. Comp. Biochem. Physiol. Part A 2016, 192, 64–78. [Google Scholar] [CrossRef]
- Harrison, J.F.; Greenlee, K.J.; Verberk, W.C.E.P. Functional Hypoxia in Insects: Definition, Assessment, and Consequences for Physiology, Ecology, and Evolution. Annu. Rev. Entomol. 2018, 63, 303–325. [Google Scholar] [CrossRef]
- Harrison, J.F.; Frazier, M.R.; Henry, J.R.; Kaiser, A.; Klok, C.J.; Rascón, B. Responses of terrestrial insects to hypoxia or hyperoxia. Respir. Physiol. Neurobiol. 2006, 154, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Privalova, V.; Szlachcic, E.; Sobczyk, Ł.; Szabla, N.; Czarnoleski, M. Oxygen Dependence of Flight Performance in Ageing Drosophila melanogaster. Biology 2021, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.B.; Jew, C.J.; Wegner, N.C. Modeling Variable Phanerozoic Oxygen Effects on Physiology and Evolution. Adv. Experiemntal Med. Biol. 2016, 903, 409–426. [Google Scholar] [CrossRef]
- Plazio, E.; Margol, T.; Nowicki, P. Intersexual differences in density-dependent dispersal and their evolutionary drivers. J. Evol. Biol. 2020, 33, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Dong, H.; Zhao, K. A balance between aerodynamic and olfactory performance during flight in Drosophila. Nat. Commun. 2018, 9, 3215. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.F.; Roberts, S.P. Flight respiration and energetics. Annu. Rev. Physiol. 2000, 62, 179–205. [Google Scholar] [CrossRef]
- Harrison, J.F.; Woods, H.A.; Roberts, S.P. Ecological and Environmental Physiology of Insects; Oxford University Press: New York, NY, USA, 2012; ISBN 9780199225958. [Google Scholar]
- Ginzberg, M.B.; Kafri, R.; Kirschner, M. On being the right (cell) size. Science 2015, 348, 1245075. [Google Scholar] [CrossRef] [PubMed]
- Antoł, A.; Labecka, A.M.; Horváthová, T.; Sikorska, A.; Szabla, N.; Bauchinger, U.; Kozłowski, J.; Czarnoleski, M. Effects of thermal and oxygen conditions during development on cell size in the common rough woodlice Porcellio Scaber. Ecol. Evol. 2020, 10, 9552–9566. [Google Scholar] [CrossRef]
- Atkinson, D.; Morley, S.A.; Hughes, R.N. From cells to colonies: At what levels of body organization does the “temperature-size rule” apply? Evol. Dev. 2006, 8, 202–214. [Google Scholar] [CrossRef]
- Czarnoleski, M.; Labecka, A.M.; Dragosz-Kluska, D.; Pis, T.; Pawlik, K.; Kapustka, F.; Kilarski, W.M.; Kozłowski, J. Concerted evolution of body mass and cell size: Similar patterns among species of birds (Galliformes) and mammals (Rodentia). Biol. Open 2018, 7, bio029603. [Google Scholar] [CrossRef]
- Maciak, S.; Bonda-Ostaszewska, E.; Czarnoleski, M.; Konarzewski, M.; Kozlowski, J. Mice divergently selected for high and low basal metabolic rates evolved different cell size and organ mass. J. Evol. Biol. 2014, 27, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Davison, J. An analysis of cell growth and metabolism in the crayfish (Procambarus alleni). Biol. Bull. 1956, 110, 264–273. [Google Scholar] [CrossRef]
- Woods, H.A. Egg-Mass Size and Cell Size: Effects of Temperature on Oxygen Distribution. Am. Zool. 1999, 39, 244–252. [Google Scholar] [CrossRef]
- Czarnoleski, M.; Labecka, A.M.; Starostová, Z.; Sikorska, A.; Bonda-Ostaszewska, E.; Woch, K.; Kubička, L.; Kratochvíl, L.; Kozlowski, J. Not all cells are equal: Effects of temperature and sex on the size of different cell types in the Madagascar ground gecko Paroedura Picta. Biol. Open 2017, 6, 1149–1154. [Google Scholar] [CrossRef]
- Czarnoleski, M.; Ejsmont-Karabin, J.; Angilletta, M.J., Jr.; Kozlowski, J. Colder rotifers grow larger but only in oxygenated waters. Ecosphere 2015, 6, 1–5. [Google Scholar] [CrossRef]
- Czarnoleski, M.; Cooper, B.S.; Kierat, J.; Angilletta, M.J., Jr. Flies developed small bodies and small cells in warm and in thermally fluctuating environments. J. Exp. Biol. 2013, 216, 2896–2901. [Google Scholar] [CrossRef]
- Hermaniuk, A.; Rybacki, M.; Taylor, J.R.E. Low Temperature and Polyploidy Result in Larger Cell and Body Size in an Ectothermic Vertebrate. Physiol. Biochem. Zool. 2016, 89, 118–129. [Google Scholar] [CrossRef]
- Hermaniuk, A.; Van De Pol, I.L.E.; Verberk, W.C.E.P. Are acute and acclimated thermal effects on metabolic rate modulated by cell size? A comparison between diploid and triploid zebrafish larvae. J. Exp. Biol. 2021, 224, jeb227124. [Google Scholar] [CrossRef] [PubMed]
- Szarski, H. Cell size and the concept of wasteful and frugal evolutionary strategies. J. Theor. Biol. 1983, 105, 201–209. [Google Scholar] [CrossRef]
- Walczyńska, A.; Labecka, A.M.; Sobczyk, M.; Czarnoleski, M.; Kozłowski, J. The Temperature-Size Rule in Lecane inermis (Rotifera) is adaptive and driven by nuclei size adjustment to temperature and oxygen combinations. J. Therm. Biol. 2015, 54, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, J.; Konarzewski, M.; Gawelczyk, A.T. Cell size as a link between noncoding DNA and metabolic rate scaling. Proc. Natl. Acad. Sci. USA 2003, 100, 14080–14085. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, J.; Konarzewski, M.; Czarnoleski, M. Coevolution of body size and metabolic rate in vertebrates: A life-history perspective. Biol. Rev. 2020, 95, 1393–1417. [Google Scholar] [CrossRef]
- Engl, E.; Attwell, D. Non-signalling energy use in the brain. J. Physiol. 2015, 593, 3417–3429. [Google Scholar] [CrossRef]
- Rolfe, D.F.; Brown, G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [CrossRef]
- Wu, B.J.; Else, P.L.; Storlien, L.H.; Hulbert, A.J. Molecular activity of Na+/K+-ATPase from different sources is related to the packing of membrane lipids. J. Exp. Biol. 2001, 204, 4271–4280. [Google Scholar] [CrossRef]
- Czarnoleski, M.; Dragosz-Kluska, D.; Angilletta, M.J., Jr. Flies developed smaller cells when temperature fluctuated more frequently. J. Therm. Biol. 2015, 54, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Sam, R.; Ryan, E.; Alvarado, K.; Villa-Cuesta, E. Rapamycin as a potential treatment for succinate dehydrogenase deficiency. Heliyon 2019, 5, e01217. [Google Scholar] [CrossRef] [PubMed]
- Katewa, S.D.; Kapahi, P. Role of TOR signaling in aging and related biological processes in Drosophila melanogaster. Exp. Gerontol. 2011, 46, 382–390. [Google Scholar] [CrossRef]
- Emran, S.; Yang, M.; He, X.; Zandveld, J.; Piper, M.D.W. Target of rapamycin signalling mediates the lifespan-extending effects of dietary restriction by essential amino acid alteration. Aging 2014, 6, 390–398. [Google Scholar] [CrossRef]
- Bové, J.; Martínez-Vicente, M.; Vila, M. Fighting neurodegeneration with rapamycin: Mechanistic insights. Nat. Rev. Neurosci. 2011, 12, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Cully, M.; Downward, J. Assessing cell size and cell cycle regulation in cells with altered TOR activity. Methods Mol. Biol. 2012, 821, 227–237. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Rapamycin and quasi-programmed aging: Four years later. Cell Cycle 2010, 9, 1859–1862. [Google Scholar] [CrossRef]
- Beauchamp, E.M.; Platanias, L.C. The evolution of the TOR pathway and its role in cancer. Oncogene 2013, 32, 3923–3932. [Google Scholar] [CrossRef]
- Lloyd, A.C. The regulation of cell size. Cell 2013, 154, 1194–1205. [Google Scholar] [CrossRef]
- Schramm, B.W.; Labecka, A.M.; Gudowska, A.; Antoł, A.; Sikorska, A.; Szabla, N.; Bauchinger, U.; Kozłowski, J.; Czarnoleski, M. Concerted evolution of body mass, cell size and metabolic rate among carabid beetles. J. Insect Physiol. 2021, 132, 104272. [Google Scholar] [CrossRef]
- Montagne, J.; Stewart, M.J.; Stocker, H.; Hafen, E.; Kozma, S.C.; Thomas, G. Drosophila S6 kinase: A regulator of cell size. Science 1999, 285, 2126–2129. [Google Scholar] [CrossRef]
- Oldham, S.; Montagne, J.; Radimerski, T.; Thomas, G.; Hafen, E. Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes Dev. 2000, 14, 2689–2694. [Google Scholar] [CrossRef]
- Zhang, H.; Stallock, J.P.; Ng, J.C.; Reinhard, C.; Neufeld, T.P. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev. 2000, 14, 2712–2724. [Google Scholar] [CrossRef] [PubMed]
- Mołoń, M.; Dampc, J.; Kula-Maximenko, M.; Zebrowski, J.; Mołoń, A.; Dobler, R.; Durak, R.; Skoczowski, A. Effects of Temperature on Lifespan of Drosophila melanogaster from Different Genetic Backgrounds: Links between Metabolic Rate and Longevity. Insects 2020, 11, 470. [Google Scholar] [CrossRef]
- Vance, J.T.; Williams, J.B.; Elekonich, M.M.; Roberts, S.R. The effects of age and behavioral development on honey bee (Apis mellifera) flight performance. J. Exp. Biol. 2009, 212, 2604–2611. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.R.; Papadopoulos, N.; Kouloussis, N.; Katsoyannos, B.; Müller, H.-G.; Wang, J.-L.; Tseng, Y.-K. Age-specific and lifetime behavior patterns in Drosophila melanogaster and the Mediterranean fruit fly, Ceratitis Capitata. Exp. Gerontol. 2006, 41, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-L.; Li, X.-K.; Shen, X.-J.; Wang, M.-L.; Wu, K.-M. Flight Performance of Mamestra brassicae (Lepidoptera: Noctuidae) under Different Biotic and Abiotic Conditions. J. Insect Sci. 2020, 20, 2. [Google Scholar] [CrossRef]
- Gaviraghi, A.; Oliveira, M.F. A simple and reliable method for longitudinal assessment of untethered mosquito induced flight activity. J. Insect Physiol. 2020, 126, 104098. [Google Scholar] [CrossRef]
- Petrosyan, A.; Hsieh, I.-H.; Saberi, K. Age-dependent stability of sensorimotor functions in the life-extended Drosophila mutant Methuselah. Behav. Genet. 2007, 37, 585–594. [Google Scholar] [CrossRef]
- Miller, M.S.; Lekkas, P.; Braddock, J.M.; Farman, G.P.; Ballif, B.A.; Irving, T.C.; Maughan, D.W.; Vigoreaux, J.O. Aging enhances indirect flight muscle fiber performance yet decreases flight ability in Drosophila. Biophys. J. 2008, 95, 2391–2401. [Google Scholar] [CrossRef] [PubMed]
- Aita, R.C.; Kees, A.M.; Aukema, B.H.; Hutchison, W.D.; Koch, R.L. Effects of Starvation, Age, and Mating Status on Flight Capacity of Laboratory-Reared Brown Marmorated Stink Bug (Hemiptera: Pentatomidae). Environ. Entomol. 2021, 50, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Mouser, J.; Pitt, J.; Promislow, D.; Kaeberlein, M. Rapamycin enhances survival in a Drosophila model of mitochondrial disease. Oncotarget 2016, 7, 80131–80139. [Google Scholar] [CrossRef] [PubMed]
- Unwin, D.M.; Ellington, C.P. An optical tachometer for measurement of the wing-beat frequency of free-flying insects. J. Exp. Biol. 1979, 82, 377–378. [Google Scholar] [CrossRef]
- Dobzhansky, T. The influence of the quantity and quality of chromosomal material on the size of the cells in Drosophila melanogaster. Wilhelm Roux. Arch. Entwickl. Mech. Org. 1929, 115, 363–379. [Google Scholar] [CrossRef]
- Azevedo, R.B.R.; James, A.C.; McCabe, J.; Partridge, L. Latitudinal Variation of Wing: Thorax Size Ratio and Wing-Aspect Ratio in Drosophila melanogaster. Evolution 1998, 52, 1353–1362. [Google Scholar] [CrossRef]
- Starmer, W.T.; Wolf, L.L. Causes of variation in wing loading among Drosophila species. Biol. J. Linn. Soc. 1989, 37, 247–261. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.r-project.org/833 (accessed on 5 February 2021).
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2019; ISBN 9781544336473. [Google Scholar]
- Wickham, H. Ggplot2 Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 9780387981406. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.5.3. 2020. Available online: https://cran.r-project.org/package=emmeans (accessed on 10 December 2020).
- Guertin, D.A.; Guntur, K.V.P.; Bell, G.W.; Thoreen, C.C.; Sabatini, D.M. Functional Genomics Identifies TOR-Regulated Genes that Control Growth and Division. Curr. Biol. 2006, 16, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Ginzberg, M.B.; Chang, N.; D’souza, H.; Patel, N.; Kafri, R.; Kirschner, M.W. Cell size sensing in animal cells coordinates anabolic growth rates and cell cycle progression to maintain cell size uniformity. eLife 2018, 7, e26957. [Google Scholar] [CrossRef]
- Fingar, D.C.; Salama, S.; Tsou, C.; Harlow, E.; Blenis, J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002, 16, 1472–1487. [Google Scholar] [CrossRef]
- Ohanna, M.; Sobering, A.K.; Lapointe, T.; Lorenzo, L.; Praud, C.; Petroulakis, E.; Sonenberg, N.; Kelly, P.A.; Sotiropoulos, A.; Pende, M. Atrophy of S6K1-/- skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat. Cell Biol. 2005, 7, 286–294. [Google Scholar] [CrossRef]
- Wu, M.Y.W.; Cully, M.; Andersen, D.; Leevers, S.J. Insulin delays the progression of Drosophila cells through G2/M by activating the dTOR/dRaptor complex. EMBO J. 2007, 26, 371–379. [Google Scholar] [CrossRef]
- French, V.; Feast, M.; Partridge, L. Body size and cell size in Drosophila: The developmental response to temperature. J. Insect Physiol. 1998, 44, 1081–1089. [Google Scholar] [CrossRef]
- Potter, S.; Sifers, J.; Yocom, E.; Blümich, S.L.E.; Potter, R.; Nadolski, J.; Harrison, D.A.; Cooper, R.L. Effects of inhibiting mTOR with rapamycin on behavior, development, neuromuscular physiology and cardiac function in larval Drosophila. Biol. Open 2019, 8, bio046508. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.C.; Schuldiner, O.; Neufeld, T.P. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell 2004, 7, 167–178. [Google Scholar] [CrossRef]
- Angilletta, M.J., Jr. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: New York, NY, USA, 2009; ISBN 9780198570875. [Google Scholar]
- Roff, D. Life History Evolution; Sinauer Associates: Sunderland, MA, USA, 2002; ISBN 0878937560. [Google Scholar]
- Atkinson, D. Temperature and Organism Size—A Biological Law for Ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar] [CrossRef]
- Angilletta, M.J., Jr.; Niewiarowski, P.H.; Dunham, A.E.; Leaché, A.D.; Porter, W.P. Bergmann’s Clines in Ectotherms: Illustrating a Life-History Perspective with Sceloporine Lizards. Am. Nat. 2004, 164, E168–E183. [Google Scholar] [CrossRef] [PubMed]
- Heinze, J.; Foitzik, S.; Fischer, B.; Wanke, T.; Kipyatkov, V.E. The significance of latitudinal variation in body size in a holarctic ant, Leptothorax acervorum. Ecography 2003, 26, 349–355. [Google Scholar] [CrossRef]
- Zwaan, B.J.; Azevedo, R.B.R.; James, A.C.; Van ’T Land, J.; Partridge, L. Cellular of wing size variation in Drosophila melanogaster: A comparison of latitudinal clines on two continents. Heredity 2000, 84, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C. Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Größe; Vandhoeck und Ruprecht: Götingen, Germany, 1848. [Google Scholar]
- Heinrich, E.C.; Farzin, M.; Klok, C.J.; Harrison, J.F. The effect of developmental stage on the sensitivity of cell and body size to hypoxia in Drosophila melanogaster. J. Exp. Biol. 2011, 214, 1419–1427. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, D.; Xue, J.; Chen, J.; Morcillo, P.; Lambert, J.D.; White, K.P.; Haddad, G.G. Experimental Selection for Drosophila Survival in Extremely Low O2 Environment. PLoS ONE 2007, 2, e490. [Google Scholar] [CrossRef] [PubMed]
- Vijendravarma, R.K.; Narasimha, S.; Kawecki, T.J. Plastic and evolutionary responses of cell size and number to larval malnutrition in Drosophila melanogaster. J. Evol. Biol. 2011, 24, 897–903. [Google Scholar] [CrossRef]
- Partridge, L.; Barrie, B.; Fowler, K.; French, V. Evolution and Development of Body Size and Cell Size in Drosophila melanogaster in Response to Temperature. Evolution 1994, 48, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.B.R.; French, V.; Partridge, L. Temperature modulates epidermal cell size in Drosophila melanogaster. J. Insect Physiol. 2002, 48, 231–237. [Google Scholar] [CrossRef]
- Czarnoleski, M.; Labecka, A.M.; Kozłowski, J. Thermal plasticity of body size and cell size in snails from two subspecies of Cornu aspersum. J. Molluscan Stud. 2016, 82, 235–243. [Google Scholar] [CrossRef]
- Stevenson, R.D.; Hill, M.F.; Bryant, P.J. Organ and cell allometry in Hawaiian Drosophila: How to make a big fly. Proc. R. Soc. B Biol. Sci. 1995, 259, 105–110. [Google Scholar] [CrossRef]
- Kozlowski, J.; Czarnoleski, M.; François-Krassowska, A.; Maciak, S.; Pis, T. Cell size is positively correlated between different tissues in passerine birds and amphibians, but not necessarily in mammals. Biol. Lett. 2010, 6, 792–796. [Google Scholar] [CrossRef]
- Frazier, M.R.; Harrison, J.F.; Kirkton, S.D.; Roberts, S.P. Cold rearing improves cold-flight performance in Drosophila via changes in wing morphology. J. Exp. Biol. 2008, 211, 2116–2122. [Google Scholar] [CrossRef]
- Stalker, H.D.; Carson, H.L. Seasonal variation in the morphology of Drosophila robusta Sturtevant. Evolution 1949, 3, 330–343. [Google Scholar] [CrossRef]
- Dudley, R. The Biomechanics of Insect Flight: Form, Function, Evolution; Princeton University Press: Princeton, NJ, USA, 2000; ISBN 9780691186344. [Google Scholar]
- Pétavy, G.; Morin, J.-P.; Moreteau, B.; David, J.R. Growth temperature and phenotypic plasticity in two Drosophila sibling species: Probable adaptive changes in flight capacities. J. Evol. Biol. 1997, 10, 875–887. [Google Scholar] [CrossRef]
- Dillon, M.E.; Frazier, M.R.; Dudley, R. Into thin air: Physiology and evolution of alpine insects. Integr. Comp. Biol. 2006, 46, 49–61. [Google Scholar] [CrossRef]
- Noach, E.J.K.; Van Der Klis, H.; De Jong, G.; Scharloo, W. Wing beat frequencies in Drosophila melanogaster selected for different wing lengths. Neth. J. Zool. 1997, 47, 241–253. [Google Scholar] [CrossRef]
- Heinrich, B. Thermoregulation in endothermic insects. Science 1974, 185, 747–756. [Google Scholar] [CrossRef]
- Esch, H.; Goller, F.; Heinrich, B. How do bees shiver? Sci. Nat. 1991, 78, 325–328. [Google Scholar] [CrossRef]
- Heinrich, B.; Pantle, C. Thermoregulation in small flies (Syrphus sp.): Basking and shivering. J. Exp. Biol. 1975, 62, 599–610. [Google Scholar] [CrossRef]
- Kammer, A.E.; Heinrich, B. Insect Flight Metabolism. Adv. Insect Phys. 1978, 13, 133–228. [Google Scholar] [CrossRef]
- Shiehzadegan, S.; Le Vinh Thuy, J.; Szabla, N.; Angilletta, M.J., Jr.; VandenBrooks, J.M. More oxygen during development enhanced flight performance but not thermal tolerance of Drosophila melanogaster. PLoS ONE 2017, 12, e0177827. [Google Scholar] [CrossRef]
- Joos, B.; Lighton, J.R.B.; Harrison, J.F.; Suarez, R.K.; Roberts, S.P. Effects of ambient oxygen tension on flight performance, metabolism, and water loss of the honeybee. Physiol. Zool. 1997, 70, 167–174. [Google Scholar] [CrossRef]
- Henry, J.R.; Harrison, J.F. Effects of body size on the oxygen sensitivity of dragonfly flight. J. Exp. Biol. 2014, 217, 3447–3456. [Google Scholar] [CrossRef]
- Harrison, J.F.; Lighton, J.R.B. Oxygen-sensitive flight metabolism in the dragonfly Erythemis simplicicollis. J. Exp. Biol. 1998, 201, 1739–1744. [Google Scholar] [CrossRef]
- Rascón, B.; Harrison, J.F. Oxygen partial pressure effects on metabolic rate and behavior of tethered flying locusts. J. Insect Physiol. 2005, 51, 1193–1199. [Google Scholar] [CrossRef]
- Cooper, B.S.; Czarnoleski, M.; Angilletta, M.J., Jr. Acclimation of thermal physiology in natural populations of Drosophila melanogaster: A test of an optimality model. J. Evol. Biol. 2010, 23, 2346–2355. [Google Scholar] [CrossRef] [PubMed]
- Klepsatel, P.; Gáliková, M.; De Maio, N.; Huber, C.D.; Schlötterer, C.; Flatt, T. Variation in thermal performance and reaction norms among populations of Drosophila melanogaster. Evolution 2013, 67, 3573–3587. [Google Scholar] [CrossRef]
- Condon, C.; Cooper, B.S.; Yeaman, S.; Angilletta, M.J., Jr. Temporal variation favors the evolution of generalists in experimental populations of Drosophila melanogaster. Evolution 2014, 68, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Estay, S.A.; Lima, M.; Bozinovic, F. The role of temperature variability on insect performance and population dynamics in a warming world. Oikos 2014, 123, 131–140. [Google Scholar] [CrossRef]
- Petavy, G.; David, J.R.; Gibert, P.; Moreteau, B. Viability and rate of development at different temperatures in Drosophila: A comparison of constant and alternating thermal regimes. J. Therm. Biol. 2001, 26, 29–39. [Google Scholar] [CrossRef]
- De Araujo, L.I.; Karsten, M.; Terblanche, J.S. Exploring thermal fl ight responses as predictors of flight ability and geographic range size in Drosophila. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 236, 110532. [Google Scholar] [CrossRef]
- Makumbe, L.D.M.; Moropa, T.P.; Manrakhan, A.; Weldon, C.W. Effect of sex, age and morphological traits on tethered flight of Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) at different temperatures. Physiol. Entomol. 2020, 45, 110–119. [Google Scholar] [CrossRef]
- Verspagen, N.; Leiva, F.P.; Janssen, I.M.; Verberk, W.C.E.P. Effects of developmental plasticity on heat tolerance may be mediated by changes in cell size in Drosophila melanogaster. Insect Sci. 2020, 27, 1244–1256. [Google Scholar] [CrossRef]
- Yang, Y.; Edery, I. Parallel clinal variation in the mid-day siesta of Drosophila melanogaster implicates continent-specific targets of natural selection. PLoS Genet. 2018, 14, e1007612. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A. Physiological climatic limits in Drosophila: Patterns and implications. J. Exp. Biol. 2010, 213, 870–880. [Google Scholar] [CrossRef]
- Hazel, J.R. Thermal adaptation in biological membranes: Is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 1995, 57, 19–42. [Google Scholar] [CrossRef]
- Jones, M.A.; Grotewiel, M. Drosophila as a model for age-related impairment in locomotor and other behaviors. Exp. Gerontol. 2011, 46, 320–325. [Google Scholar] [CrossRef]
- Grotewiel, M.S.; Martin, I.; Bhandari, P.; Cook-Wiens, E. Functional senescence in Drosophila melanogaster. Ageing Res. Rev. 2005, 4, 372–397. [Google Scholar] [CrossRef]
- Gargano, J.W.; Martin, I.; Bhandari, P.; Grotewiel, M.S. Rapid iterative negative geotaxis (RING): A new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 2005, 40, 386–395. [Google Scholar] [CrossRef]
- Economos, A.C.; Miquel, J.; Binnard, R.; Kessler, S. Quantitative analysis of mating behavior in aging male Drosophila melanogaster. Mech. Ageing Dev. 1979, 10, 233–240. [Google Scholar] [CrossRef]
- Tamura, T.; Chiang, A.S.; Ito, N.; Liu, H.-P.; Horiuchi, J.; Tully, T.; Saitoe, M. Aging Specifically Impairs amnesiac-Dependent Memory in Drosophila. Neuron 2003, 40, 1003–1011. [Google Scholar] [CrossRef]
- Lu, Y.H.; Wu, K.M.; Wyckhuys, K.A.G.; Guo, Y.Y. Comparative flight performance of three important pest Adelphocoris species of Bt cotton in China. Bull. Entomol. Res. 2009, 99, 543–550. [Google Scholar] [CrossRef]
- Lane, S.J.; Frankino, W.A.; Elekonich, M.M.; Roberts, S.P. The effects of age and lifetime flight behavior on flight capacity in Drosophila melanogaster. J. Exp. Biol. 2014, 217, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Musolin, D.L.; Saulich, A.K. Responses of insects to the current climate changes: From physiology and behavior to range shifts. Entomol. Rev. 2012, 92, 715–740. [Google Scholar] [CrossRef]
- Hansen, J.; Sato, M.; Ruedy, R. Perception of climate change. Proc. Natl. Acad. Sci. USA. 2012, 109, E2415–E2423. [Google Scholar] [CrossRef]
- Stott, P. How climate change affects extreme weather events. Science 2016, 352, 1517–1518. [Google Scholar] [CrossRef] [PubMed]
- Miler, K.; Stec, D.; Czarnoleski, M. Heat wave effects on the behavior and life-history traits of sedentary antlions. Behav. Ecol. 2020, 31, 1326–1333. [Google Scholar] [CrossRef]
- Labecka, A.M.; Czarnoleski, M. Patterns of growth, brooding and offspring size in the invasive mussel Sinanodonta woodiana (Lea, 1834) (Bivalvia: Unionidae) from an anthropogenic heat island. Hydrobiologia 2021, 848, 3093–3113. [Google Scholar] [CrossRef]
- Santamouris, M. Analyzing the heat island magnitude and characteristics in one hundred Asian and Australian cities and regions. Sci. Total Environ. 2015, 512–513, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Ohlberger, J. Climate warming and ectotherm body size—from individual physiology to community ecology. Funct. Ecol. 2013, 27, 991–1001. [Google Scholar] [CrossRef]
- Paaijmans, K.P.; Heinig, R.L.; Seliga, R.A.; Blanford, J.I.; Blanford, S.; Murdock, C.C.; Thomas, M.B. Temperature variation makes ectotherms more sensitive to climate change. Glob. Chang. Biol. 2013, 19, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Abarca, M.; Lill, J.T. Warming affects hatching time and early season survival of eastern tent caterpillars. Oecologia 2015, 179, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.B.; Nufio, C.R.; Kirk, E.M.; Kingsolver, J.G. Elevational differences in developmental plasticity determine phenological responses of grasshoppers to recent climate warming. Proc. R Soc. B Biol. Sci. 2015, 282, 20150441. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, C.; Desneux, N.; Lu, Y. Impact of Temperature on Survival Rate, Fecundity, and Feeding Behavior of Two Aphids, Aphis gossypii and Acyrthosiphon gossypii, When Reared on Cotton. Insects 2021, 12, 565. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, J.A.; Bickford, D. Shrinking body size as an ecological response to climate change. Nat. Clim. Chang. 2011, 1, 401–406. [Google Scholar] [CrossRef]
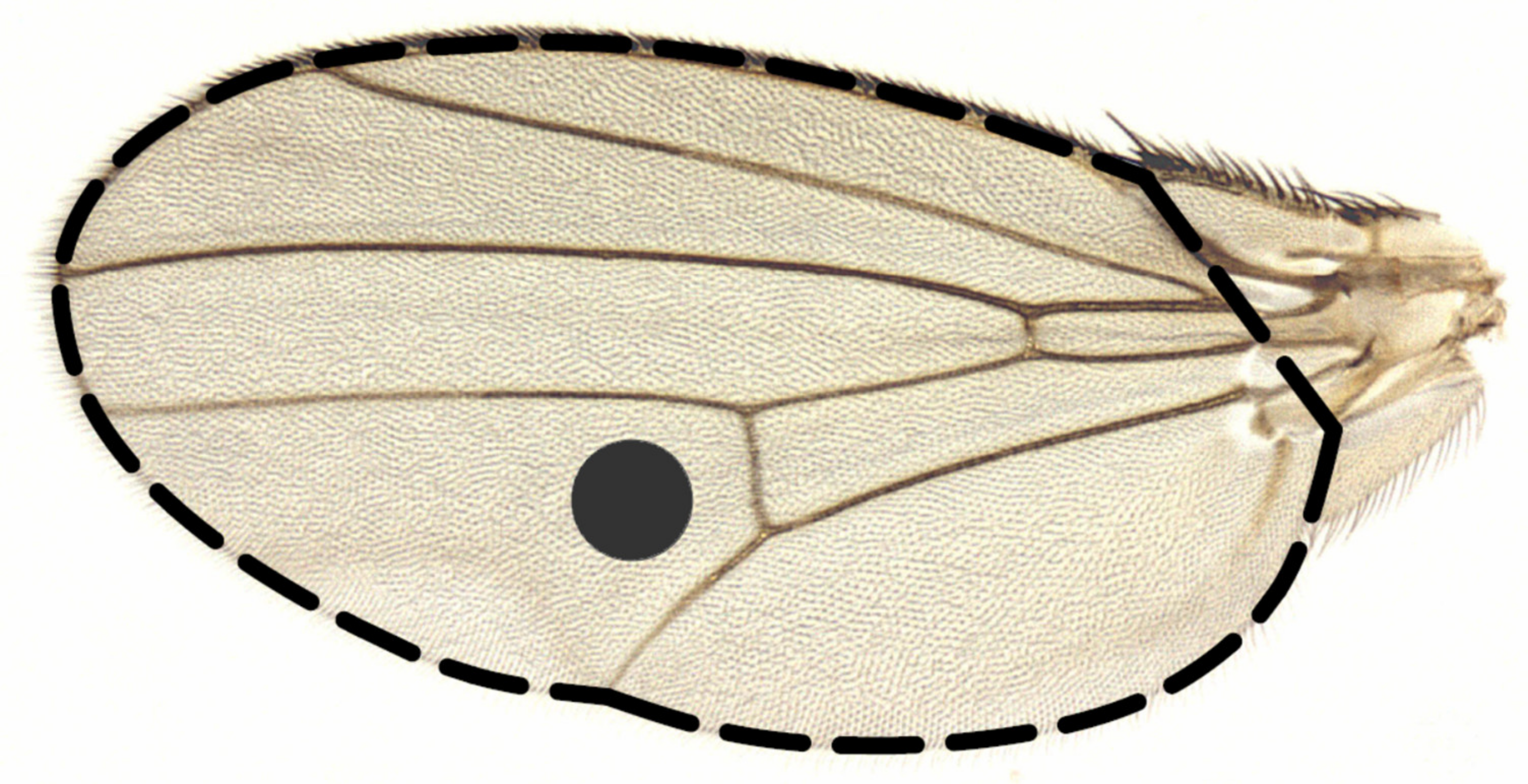
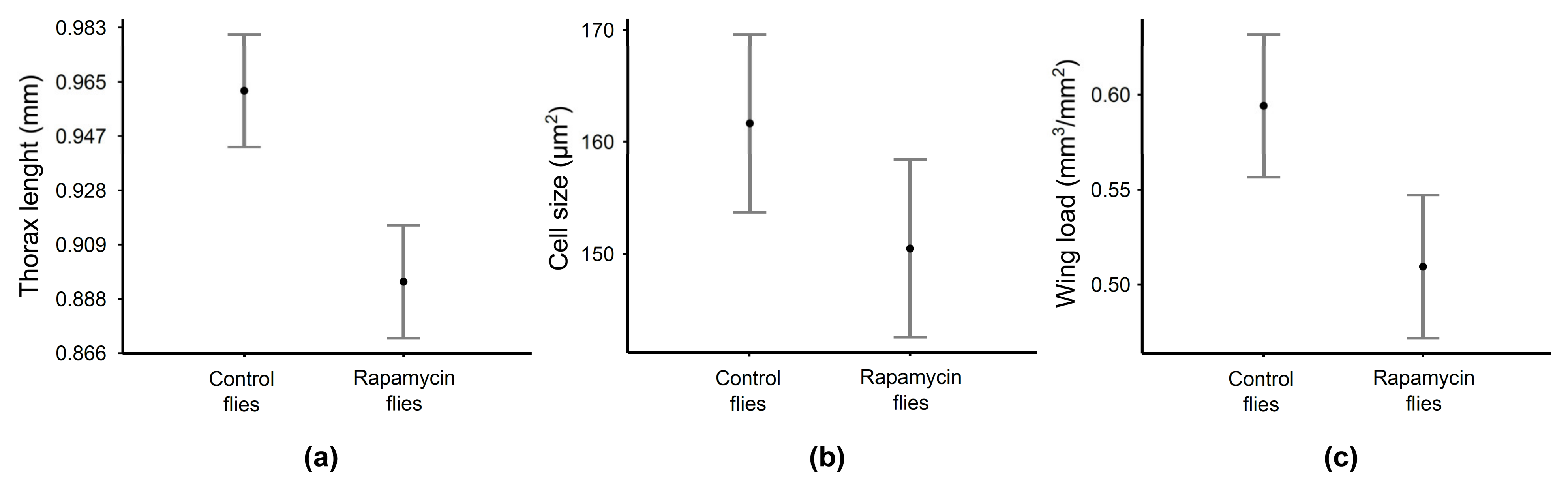
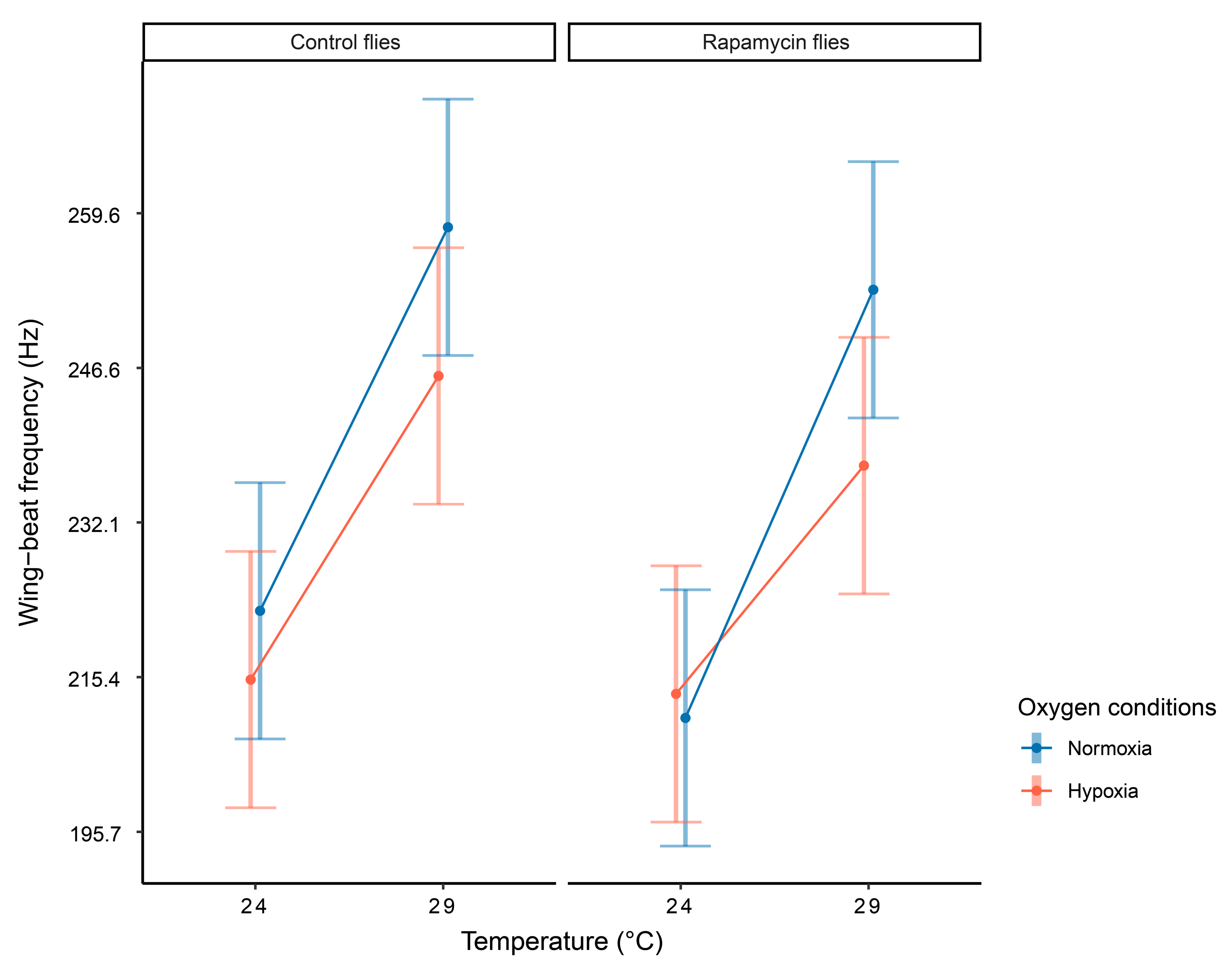
| Effect | F | Df | P |
|---|---|---|---|
| Temperature (warm vs. hot) | 77.63 | 1 | <0.0001 |
| Phenotype (small cells vs. large cells) | 1.65 | 1 | 0.199 |
| Oxygen (normoxia vs. hypoxia) | 13.10 | 1 | 0.028 |
| Age (10 vs. 25 days) | 0.97 | 1 | 0.324 |
| Temperature × oxygen × phenotype | 10.37 | 1 | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szlachcic, E.; Czarnoleski, M. Thermal and Oxygen Flight Sensitivity in Ageing Drosophila melanogaster Flies: Links to Rapamycin-Induced Cell Size Changes. Biology 2021, 10, 861. https://doi.org/10.3390/biology10090861
Szlachcic E, Czarnoleski M. Thermal and Oxygen Flight Sensitivity in Ageing Drosophila melanogaster Flies: Links to Rapamycin-Induced Cell Size Changes. Biology. 2021; 10(9):861. https://doi.org/10.3390/biology10090861
Chicago/Turabian StyleSzlachcic, Ewa, and Marcin Czarnoleski. 2021. "Thermal and Oxygen Flight Sensitivity in Ageing Drosophila melanogaster Flies: Links to Rapamycin-Induced Cell Size Changes" Biology 10, no. 9: 861. https://doi.org/10.3390/biology10090861
APA StyleSzlachcic, E., & Czarnoleski, M. (2021). Thermal and Oxygen Flight Sensitivity in Ageing Drosophila melanogaster Flies: Links to Rapamycin-Induced Cell Size Changes. Biology, 10(9), 861. https://doi.org/10.3390/biology10090861






