A Comparison of Acute Pharmacological Effects of Methylone and MDMA Administration in Humans and Oral Fluid Concentrations as Biomarkers of Exposure
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Design and Treatments
2.3. Procedures
2.4. Physiological Effects
2.5. Subjective Effects
2.6. Oral Concentrations
2.7. Statistical Analysis
3. Results
3.1. Participants
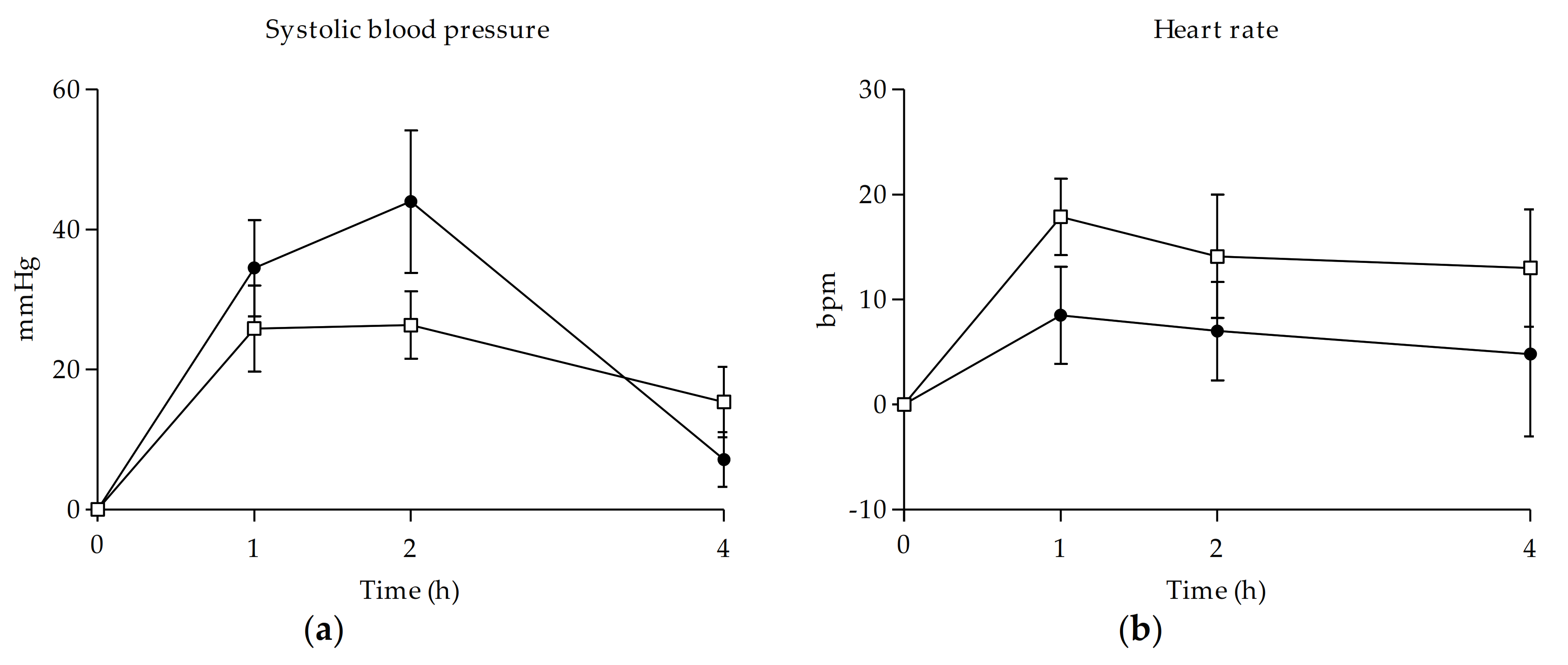
3.2. Physiological Effects
3.3. Subjective Effects
3.4. Oral Fluid Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banks, M.L.; Worst, T.J.; Sprague, J. Synthetic Cathinones and amphetamine analogues: What’s the rave about? J. Emerg. Med. 2014, 46, 632–642. [Google Scholar] [CrossRef]
- Evans-Brown, M.; Sedefov, R. Responding to new psychoactive substances in the european union: Early warning, risk assessment, and control measures. In Handbook of Experimental Pharmacology; Springer: New York, NY, USA, 2018; Volume 252, pp. 3–49. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European Drug Report–Trends and Developments; Publications Office of the European Union: Luxembourg, 2020; ISBN 978-92-9497-544-7.
- Valente, M.J.; Guedes De Pinho, P.; De Lourdes Bastos, M.; Carvalho, F.; Carvalho, M. Khat and synthetic cathinones: A review. Arch. Toxicol. 2014, 88, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Karila, L.; Megarbane, B.; Cottencin, O.; Lejoyeux, M. Synthetic Cathinones: A New Public Health Problem. Curr. Neuropharmacol. 2014, 13, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.M.; Nelson, L.S. The Toxicology of Bath Salts: A Review of Synthetic Cathinones. J. Med. Toxicol. 2012, 8, 33–42. [Google Scholar] [CrossRef]
- Bossong, M.G.; Van Dijk, J.P.; Niesink, R.J.M. Methylone and mCPP, two new drugs of abuse? Addict. Biol. 2005, 10, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Ayestas, M.A.; Partilla, J.S.; Sink, J.R.; Shulgin, A.T.; Daley, P.F.; Brandt, S.D.; Rothman, R.B.; Ruoho, A.E.; Cozzi, N.V. The Designer Methcathinone Analogs, Mephedrone and Methylone, are Substrates for Monoamine Transporters in Brain Tissue. Neuropsychopharmacology 2012, 37, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Štefková, K.; Židková, M.; Horsley, R.R.; Pinterová, N.; Šíchová, K.; Uttl, L.; Balíková, M.; Danda, H.; Kuchar, M.; Pálenícek, T. Pharmacokinetic, ambulatory, and hyperthermic effects of 3,4-methylenedioxy-n-methylcathinone (Methylone) in rats. Front. Psychiatry 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Hondebrink, L.; Zwartsen, A.; Westerink, R.H.S. Effect fingerprinting of new psychoactive substances (NPS): What can we learn from in vitro data? Pharmacol. Ther. 2018, 182, 193–224. [Google Scholar] [CrossRef]
- Cozzi, N.V.; Sievert, M.K.; Shulgin, A.T.; Jacob, P.; Ruoho, A.E. Inhibition of plasma membrane monoamine transporters by β- ketoamphetamines. Eur. J. Pharmacol. 1999, 381, 63–69. [Google Scholar] [CrossRef]
- Sogawa, C.; Sogawa, N.; Ohyama, K.; Kikura-Hanajiri, R.; Goda, Y.; Sora, I.; Kitayama, S. Methylone and Monoamine Transporters: Correlation with Toxicity. Curr. Neuropharmacol. 2011, 9, 58–62. [Google Scholar] [CrossRef][Green Version]
- López-Arnau, R.; Martínez-Clemente, J.; Pubill, D.; Escubedo, E.; Camarasa, J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: Butylone, mephedrone and methylone. Br. J. Pharmacol. 2012, 167, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Simmler, L.D.; Buser, T.A.; Donzelli, M.; Schramm, Y.; Dieu, L.H.; Huwyler, J.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 2013, 168, 458–470. [Google Scholar] [CrossRef]
- Pedersen, A.J.; Petersen, T.H.; Linnet, K. In vitro metabolism and pharmacokinetic studies on methylone. Drug Metab. Dispos. 2013, 41, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Schmid, Y.; Vizeli, P.; Hysek, C.M.; Prestin, K.; Meyer Zu Schwabedissen, H.E.; Liechti, M.E. CYP2D6 function moderates the pharmacokinetics and pharmacodynamics of 3,4-methylene-dioxymethamphetamine in a controlled study in healthy individuals. Pharm. Genom. 2016, 26, 397–401. [Google Scholar] [CrossRef]
- Elmore, J.S.; Dillon-Carter, O.; Partilla, J.S.; Ellefsen, K.N.; Concheiro, M.; Suzuki, M.; Rice, K.C.; Huestis, M.A.; Baumann, M.H. Pharmacokinetic Profiles and Pharmacodynamic Effects for Methylone and Its Metabolites in Rats. Neuropsychopharmacology 2017, 42, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Centazzo, N.; Chojnacki, M.R.; Elmore, J.S.; Rodriguez, R.; Acosta, T.; Suzuki, M.; Rice, K.C.; Baumann, M.H.; Concheiro, M. Brain Concentrations of Methylone and Its Metabolites after Systemic Methylone Administration: Relationship to Pharmacodynamic Effects. J. Pharmacol. Exp. Ther. 2021, 377, 398–406. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime (UNODC). World Drug Report 2020. Drug Use and Heath Consequences; Sales No. E.20.XI.6; United Nations Publications: Vienna, Austria, 2020; ISBN 9789211483451. [Google Scholar]
- Madras, B.K. The Growing Problem of New Psychoactive Substances (NPS). Curr. Top Behav. Neurosci. 2017, 32, 1–18. [Google Scholar] [CrossRef]
- Palamar, J.J.; Acosta, P.; Calderón, F.F.; Sherman, S.; Cleland, C.M. Assessing self-reported use of new psychoactive substances: The impact of gate questions. Am. J. Drug Alcohol Abuse 2017, 43, 609–617. [Google Scholar] [CrossRef]
- National Forensic Laboratory Information System (NFLIS). Special Report: Synthetic Cannabinoids and Synthetic Cathinones Reported in NFLIS, 2013–2015; U.S. Drug Enforcement Administration: Springfield, IL, USA, 2016; p. 12.
- World Health Organization (WHO). Methylone (bk–MDMA). Critical Review Report; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- De Felice, L.J.; Glennon, R.A.; Negus, S.S. Synthetic Cathinones: Chemical Phylogeny, Physiology, and Neuropharmacology. Life Sci. 2014, 97, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Watterson, L.R.; Burrows, B.T.; Hernandez, R.D.; Moore, K.N.; Grabenauer, M.; Marusich, J.A.; Olive, M.F. Effects of α-pyrrolidinopentiophenone and 4-methyl-N-ethylcathinone, two synthetic cathinones commonly found in second-generation “bath salts,” on intracranial self-stimulation thresholds in rats. Int. J. Neuropsychopharmacol. 2015, 18, 1–7. [Google Scholar] [CrossRef]
- Karila, L.; Billieux, J.; Benyamina, A.; Lançon, C.; Cottencin, O. The effects and risks associated to mephedrone and methylone in humans: A review of the preliminary evidences. Brain Res. Bull. 2016, 126, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Cawrse, B.M.; Levine, B.; Jufer, R.A.; Fowler, D.R.; Vorce, S.P.; Dickson, A.J.; Holler, J.M. Distribution of methylone in four postmortem cases. J. Anal. Toxicol. 2012, 36, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Boulanger-Gobeil, C.; St-Onge, M.; Laliberté, M.; Auger, P.L. Seizures and Hyponatremia Related to Ethcathinone and Methylone Poisoning. J. Med. Toxicol. 2012, 8, 59–61. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, I.M.; Hamm, C.E.; Aldridge, L.; Nelson, C.L. Acute methylone intoxication in an accidental drowning—A case report. Forensic Sci. Int. 2013, 231, 8–10. [Google Scholar] [CrossRef]
- Shimomura, E.T.; Briones, A.J.; Warren, W.S.; Addison, J.W.; Knittel, J.L.; Shoemaker, S.A.; King, T.D.; Bosy, T.Z. Case report of methylone, oxymorphone and ethanol in a fatality case with tissue distribution. J. Anal. Toxicol. 2016, 40, 543–545. [Google Scholar] [CrossRef]
- Barrios, L.; Grison-Hernando, H.; Boels, D.; Bouquie, R.; Monteil-Ganiere, C.; Clement, R. Death following ingestion of methylone. Int. J. Leg. Med. 2016, 130, 381–385. [Google Scholar] [CrossRef] [PubMed]
- deRoux, S.J.; Dunn, W.A. “Bath Salts” the New York City Medical Examiner Experience: A 3-Year Retrospective Review. J. Forensic Sci. 2017, 62, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Liakoni, E.; Müller, S.; Stoller, A.; Ricklin, M.; Liechti, M.E.; Exadaktylos, A.K. Presentations to an urban emergency department in Bern, Switzerland associated with acute recreational drug toxicity. Scand. J. Trauma. Resusc. Emerg. Med. 2017, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, H.; Langel, K.; Favretto, D.; Verstraete, A.G. Detection of illicit drugs in oral fluid from drivers as biomarker for drugs in blood. Forensic Sci. Int. 2015, 256, 42–45. [Google Scholar] [CrossRef]
- Busardo, F.P.; Pichini, S.; Pellegrini, M.; Montana, A.; Lo Faro, A.F.; Zaami, S.; Graziano, S. Correlation between Blood and Oral Fluid Psychoactive Drug Concentrations and Cognitive Impairment in Driving under the Influence of Drugs. Curr. Neuropharmacol. 2017, 16. [Google Scholar] [CrossRef]
- Navarro, M.; Pichini, S.; Farré, M.; Ortuño, J.; Roset, P.N.; Segura, J.; De La Torre, R. Usefulness of saliva for measurement of 3,4-methylenedioxymethamphetamine and its metabolites: Correlation with plasma drug concentrations and effect of salivary pH. Clin. Chem. 2001, 47, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Pichini, S.; Navarro, M.; Farré, M.; Ortuño, J.; Nolasc Roset, P.; Pacifici, R.; Zuccaro, P.; Segura, J.; de la Torre, R. On-Site Testing of 3,4-Methylenedioxymethamphet-amine (Ecstasy) in Saliva with Drugwipe and Drugread: A Controlled Study in Recreational Users. Clin. Chem. 2002, 48, 147–176. [Google Scholar] [CrossRef]
- González, D.; Riba, J.; Bouso, J.C.; Gómez-Jarabo, G.; Barbanoj, M.J. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend. 2006, 85, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Papaseit, E.; Olesti, E.; Pérez-Mañá, C.; Torrens, M.; Grifell, M.; Ventura, M.; Pozo, O.J.; de Sousa Fernandes Perna, E.B.; Ramaekers, J.G.; de la Torre, R.; et al. Acute Effects of 2C-E in Humans: An Observational Study. Front. Pharmacol. 2020, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Papaseit, E.; Olesti, E.; Pérez-Mañá, C.; Torrens, M.; Fonseca, F.; Grifell, M.; Ventura, M.; de la Torre, R.; Farré, M. Acute pharmacological effects of oral and intranasal mephedrone: An observational study in humans. Pharmaceuticals 2021, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Warrick, B.J.; Wilson, J.; Hedge, M.; Freeman, S.; Leonard, K.; Aaron, C. Lethal Serotonin Syndrome After Methylone and Butylone Ingestion. J. Med. Toxicol. 2012, 8, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Studerus, E.; Vizeli, P.; Harder, S.; Ley, L.; Liechti, M.E. Prediction of MDMA response in healthy humans: A pooled analysis of placebo-controlled studies. J. Psychopharmacol. 2021, 35, 556–565. [Google Scholar] [CrossRef]
- Kirkpatrick, M.G.; Baggott, M.J.; Mendelson, J.E.; Galloway, G.P.; Liechti, M.E.; Hysek, C.M.; De Wit, H. MDMA effects consistent across laboratories. Psychopharmacology 2014, 231, 3899–3905. [Google Scholar] [CrossRef]
- Papaseit, E.; Pérez-Mañá, C.; Mateus, J.A.; Pujadas, M.; Fonseca, F.; Torrens, M.; Olesti, E.; De La Torre, R.; Farre, M. Human pharmacology of mephedrone in comparison with MDMA. Neuropsychopharmacology 2016, 41, 2704–2713. [Google Scholar] [CrossRef]
- Holze, F.; Vizeli, P.; Müller, F.; Ley, L.; Duerig, R.; Varghese, N.; Eckert, A.; Borgwardt, S.; Liechti, M.E. Distinct acute effects of LSD, MDMA, and d-amphetamine in healthy subjects. Neuropsychopharmacology 2020, 45, 462–471. [Google Scholar] [CrossRef]
- Clark, C.M.; Frye, C.G.; Wardle, M.C.; Norman, G.J.; de Wit, H. Acute effects of MDMA on autonomic cardiac activity and their relation to subjective prosocial and stimulant effects. Psychophysiology 2015, 52, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, K.P.C.; Dolder, P.C.; Ramaekers, J.G.; Liechti, M.E. Multifaceted empathy of healthy volunteers after single doses of MDMA: A pooled sample of placebo-controlled studies. J. Psychopharmacol. 2017, 31, 589–598. [Google Scholar] [CrossRef]
- van Wel, J.H.P.; Kuypers, K.P.C.; Theunissen, E.L.; Bosker, W.M.; Bakker, K.; Ramaekers, J.G. Effects of acute MDMA intoxication on mood and impulsivity: Role of the 5-HT2 and 5-HT1 receptors. PLoS ONE 2012, 7, e40187. [Google Scholar] [CrossRef] [PubMed]
- Bershad, A.K.; Mayo, L.M.; Van Hedger, K.; McGlone, F.; Walker, S.C.; de Wit, H. Effects of MDMA on attention to positive social cues and pleasantness of affective touch. Neuropsychopharmacology 2019, 44, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Poyatos, L.; Papaseit, E.; Pérez-Mañá, C.; Martín, S.; Hladun, O.; Vila, A.; Siles, A.; Fuster, D.; Barriocanal, A.M.; Pichini, S.; et al. Human pharmacology of methylone: A pilot phase I dose-finding study. European Association of Clinical Pharmacology and Therapeutics. Eur. J. Clin. Pharmacol. 2021, 77, 8. [Google Scholar] [CrossRef]
- López-Arnau, R.; Martínez-Clemente, J.; Carbó, M.; Pubill, D.; Escubedo, E.; Camarasa, J. An integrated pharmacokinetic and pharmacodynamic study of a new drug of abuse, methylone, a synthetic cathinone sold as “bath salts”. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 45, 64–72. [Google Scholar] [CrossRef]
- Desrosiers, N.A.; Barnes, A.J.; Hartman, R.L.; Scheidweiler, K.B.; Kolbrich-Spargo, E.A.; Gorelick, D.A.; Goodwin, R.S.; Huestis, M.A. Intramural Research Program National Institute on Drug Abuse, National Institutes of Health 251 Bayview Boulevard, Suite 200 Room 05A-721. Anal. Bioanal. Chem. 2013, 405, 4067–4076. [Google Scholar] [CrossRef]
- Olesti, E.; Farré, M.; Carbó, M.; Papaseit, E.; Perez-Mañá, C.; Torrens, M.; Yubero-Lahoz, S.; Pujadas, M.; Pozo, Ó.J.; de la Torre, R. Dose-Response Pharmacological Study of Mephedrone and Its Metabolites: Pharmacokinetics, Serotoninergic Effects, and Impact of CYP2D6 Genetic Variation. Clin. Pharmacol. Ther. 2019, 106, 596–604. [Google Scholar] [CrossRef]
- López-Arnau, R.; Martínez-Clemente, J.; Pubill, D.; Escubedo, E.; Camarasa, J. Serotonergic impairment and memory deficits in adolescent rats after binge exposure of methylone. J. Psychopharmacol. 2014, 28, 1053–1063. [Google Scholar] [CrossRef]
- Eshleman, A.J.; Wolfrum, K.M.; Hatfield, M.G.; Johnson, R.A.; Murphy, K.V.; Janowsky, A. Substituted methcathinone differ in transporter and receptor interactions. Biochem. Pharmacol. 2013, 85, 1803–1815. [Google Scholar] [CrossRef]
- La Maida, N.; Papaseit, E.; Martínez, L.; Pérez-Mañá, C.; Poyatos, L.; Pellegrini, M.; Pichini, S.; Pacifici, R.; Ventura, M.; Galindo, L.; et al. Acute pharmacological effects and oral fluid biomarkers of the synthetic cannabinoid ur-144 and thc in recreational users. Biology 2021, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Busardò, F.P.; Pérez-Acevedo, A.P.; Pacifici, R.; Mannocchi, G.; Gottardi, M.; Papaseit, E.; Pérez-Mañá, C.; Martin, S.; Poyatos, L.; Pichini, S.; et al. Disposition of phytocannabinoids, their acidic precursors and their metabolites in biological matrices of healthy individuals treated with vaporized medical cannabis. Pharmaceuticals 2021, 14, 59. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Mean ± SD | T-Student | Dunnet’s Test | |||
|---|---|---|---|---|---|---|
| Methylone | MDMA | p Value | Methylone | MDMA | ||
| Physiological effects | ||||||
| SBP (mmHg) | Emax | 31.25 ± 14.77 | 46.83 ± 20.83 | 0.126 | a, b, c | a, b |
| Tmax | 1.5 (1.0–4.0) | 2.0 (1.0–2.0) | 0.659 | |||
| AUC0–4h | 80.81 ± 42.89 | 107.67 ± 44.34 | 0.275 | |||
| DBP (mmHg) | Emax | 19.63 ± 13.96 | 32.17 ± 11.29 | 0.097 | a, b, c | a, b |
| Tmax | 2.0 (1.0–4.0) | 2.0 (1.0–2.0) | 0.150 | |||
| AUC0–4h | 55.19 ± 31.02 | 78.92 ± 30.33 | 0.178 | |||
| HR (bpm) | Emax | 20.50 ± 19.78 | 10.67 ± 18.22 | 0.360 | a | NS |
| Tmax | 3.0 (1.0–4.0) | 3.0 (1.0–4.0) | 0.436 | |||
| AUC0–4h | 52.06 ± 39.16 | 23.83 ± 38.73 | 0.205 | |||
| Subjective effects | ||||||
| VAS intensity (mm) | Emax | 20.0 ± 16.48 | 47 ± 11.19 | 0.005 | a, b | a, b |
| Tmax | 2.0 (1.0–2.0) | 1.5 (1.0–2.0) | 0.411 | |||
| AUC0–4h | 45.88 ± 43.66 | 111.33 ± 36.34 | 0.012 | |||
| VAS stimulated (mm) | Emax | 22.50 ± 18.81 | 50.17 ± 17.12 | 0.015 | b | a, b |
| Tmax | 2.0 (1.0–4.0) | 1.5 (1.0–4.0) | 0.160 | |||
| AUC0–4h | 50.56 ± 47.38 | 113.17 ± 40.92 | 0.024 | |||
| VAS high (mm) | Emax | 24.0 ± 21.28 | 60.17 ± 14.96 | 0.004 | a, b | a, b |
| Tmax | 2.0 (1.0–4.0) | 1.5 (1.0–2.0) | 0.032 | |||
| AUC0–4h | 55.06 ± 53.54 | 136.92 ± 41.05 | 0.009 | |||
| VAS good effects (mm) | Emax | 35.63 ± 30.63 | 67.83 ± 16.51 | 0.039 | a, b | a, b |
| Tmax | 2.0 (1.0–2.0) | 1.5 (1.0–2.0) | 0.548 | |||
| AUC0–4h | 74.06 ± 67.08 | 167.67 ± 54.01 | 0.016 | |||
| VAS content (mm) | Emax | 35.25 ± 30.38 | 74.50 ± 18.96 | 0.017 | a, b | a, b, c |
| Tmax | 1.5 (1.0–2.0) | 1.0 (1.0–2.0) | 0.133 | |||
| AUC0–4h | 80.06 ± 82.59 | 186.25 ± 52.39 | 0.018 | |||
| VAS change in lights (mm) | Emax | 4.88 ± 4.82 | 20.00 ± 25.11 | 0.118 | a, b | NS |
| Tmax | 1.0 (0.0–2.0) | 1.5 (0.0–4.0) | 0.491 | |||
| AUC0–4h | 10.88 ± 11.85 | 40.00 ± 53.35 | 0.156 | |||
| VAS different body feeling (mm) | Emax | 22.25 ± 20.60 | 50.33 ± 22.59 | 0.032 | NS | a |
| Tmax | 2.0 (0.0–4.0) | 1.0 (1.0–2.0) | 0.258 | |||
| AUC0–4h | 44.13 ± 43.86 | 95.25 ± 53.40 | 0.072 | |||
| VAS different surrounding (mm) | Emax | 4.38 ± 7.50 | 17.33 ± 17.68 | 0.085 | NS | a |
| Tmax | 0.5 (0.0–4.0) | 1.0 (0.0–1.0) | 0.581 | |||
| AUC0–4h | 5.69 ± 10.39 | 27.33 ± 39.71 | 0.161 | |||
| VAS dizziness (mm) | Emax | 2.13 ± 2.53 | 13.00 ± 9.84 | 0.010 | a | NS |
| Tmax | 0.5 (0.0–4.0) | 1.0 (0.0–4.0) | 0.034 | |||
| AUC0–4h | 2.63 ± 3.02 | 20.17 ± 20.68 | 0.034 | |||
| VAS headache (mm) | Emax | 20.25 ± 28.93 | 5.83 ± 7.68 | 0.261 | c | NS |
| Tmax | 3.0 (0.0–4.0) | 1.0 (0.0–4.0) | 0.629 | |||
| AUC0–4h | 23.63 ± 28.94 | 10.58 ± 18.86 | 0.357 | |||
| VAS face flushing (mm) | Emax | 31.25 ± 21.91 | 45.00 ± 22.74 | 0.275 | b | a |
| Tmax | 2.0 (1.0–4.0) | 1.5 (1.0–4.0) | 0.406 | |||
| AUC0–4h | 64.50 ± 56.75 | 89.33 ± 58.31 | 0.439 | |||
| ARCI PCAG (score) | Emax | −1.25 ± 1.58 | −0.83 ± 3.54 | 0.771 | a | NS |
| Tmax | 1.0 (1.0–4.0) | 1.0 (1.0–2.0) | 0.105 | |||
| AUC0–4h | −2.75 ± 3.73 | −1.58 ± 8.39 | 0.730 | |||
| ARCI MBG (score) | Emax | 7.5 ± 5.13 | 8.0 ± 3.35 | 0.839 | a, b | a, b |
| Tmax | 2.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.011 | |||
| AUC0–4h | 16.63 ± 13.21 | 20.83 ±10.05 | 0.528 | |||
| ARCI BG (score) | Emax | 4.63 ± 3.34 | 4.67 ± 2.25 | 0.979 | a, b | a, b |
| Tmax | 2.0 (1.0–4.0) | 1.0 (1.0–2.0) | 0.030 | |||
| AUC0–4h | 10.06 ± 7.83 | 9.83 ± 6.38 | 0.954 | |||
| ARCI A (score) | Emax | 5.38 ± 3.29 | 5.83 ± 1.17 | 0.752 | a, b | a, b, c |
| Tmax | 1.5 (1.0–4.0) | 1.0 (1.0–2.0) | 0.106 | |||
| AUC0–4h | 12.56 ± 7.77 | 15.75 ± 2.79 | 0.360 | |||
| VESSPA S (score) | Emax | 3.38 ± 2.50 | 2.83 ± 2.23 | 0.683 | b, c | a, c |
| Tmax | 4.0 (1.0–4.0) | 1.0 (1.0–1.0) | 0.061 | |||
| AUC0–4h | 7.06 ± 6.45 | 5.92 ± 6.09 | 0.742 | |||
| VESSPA ANX (score) | Emax | 7.50 ± 4.69 | 6.67 ± 4.37 | 0.741 | b, c | a, b |
| Tmax | 3.0 (1.0–4.0) | 1.0 (1.0–2.0) | 0.019 | |||
| AUC0–4h | 17.94 ± 11.17 | 16.50 ± 8.82 | 0.800 | |||
| VESSPA SOC (score) | Emax | 12.25 ± 8.31 | 14.50 ± 5.96 | 0.585 | a, b | a, b |
| Tmax | 2.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.298 | |||
| AUC0–4h | 31.31 ± 25.40 | 38.67 ± 17.84 | 0.557 | |||
| VESSPA ACT (score) | Emax | 9.75 ± 6.25 | 13.33 ± 4.84 | 0.268 | a, b | a, b |
| Tmax | 1.0 (1.0–2.0) | 1.5 (1.0–2.0) | 0.877 | |||
| AUC0–4h | 24.44 ± 17.87 | 32.58 ± 15.71 | 0.393 | |||
| VESSPA PS (score) | Emax | 2.63 ± 2.20 | 1.33 ± 1.21 | 0.221 | b | NS |
| Tmax | 1.0 (0.0–2.0) | 2.0 (0.0–2.0) | 0.362 | |||
| AUC0–4h | 5.63 ± 5.58 | 2.25 ± 2.36 | 0.192 | |||
| Oral Fluid Concentrations | Methylone | MDMA |
|---|---|---|
| Cmax | 15,514.00 ± 9748.86 | 2936.37 ± 2761.57 |
| Tmax | 2.0 (2.0–2.0) | 2.0 (2.0–4.0) |
| AUC0–4 h | 40,623.79 ± 20,001.70 | 6586.44 ± 5229.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poyatos, L.; Papaseit, E.; Olesti, E.; Pérez-Mañá, C.; Ventura, M.; Carbón, X.; Grifell, M.; Fonseca, F.; Torrens, M.; de la Torre, R.; et al. A Comparison of Acute Pharmacological Effects of Methylone and MDMA Administration in Humans and Oral Fluid Concentrations as Biomarkers of Exposure. Biology 2021, 10, 788. https://doi.org/10.3390/biology10080788
Poyatos L, Papaseit E, Olesti E, Pérez-Mañá C, Ventura M, Carbón X, Grifell M, Fonseca F, Torrens M, de la Torre R, et al. A Comparison of Acute Pharmacological Effects of Methylone and MDMA Administration in Humans and Oral Fluid Concentrations as Biomarkers of Exposure. Biology. 2021; 10(8):788. https://doi.org/10.3390/biology10080788
Chicago/Turabian StylePoyatos, Lourdes, Esther Papaseit, Eulalia Olesti, Clara Pérez-Mañá, Mireia Ventura, Xoán Carbón, Marc Grifell, Francina Fonseca, Marta Torrens, Rafael de la Torre, and et al. 2021. "A Comparison of Acute Pharmacological Effects of Methylone and MDMA Administration in Humans and Oral Fluid Concentrations as Biomarkers of Exposure" Biology 10, no. 8: 788. https://doi.org/10.3390/biology10080788
APA StylePoyatos, L., Papaseit, E., Olesti, E., Pérez-Mañá, C., Ventura, M., Carbón, X., Grifell, M., Fonseca, F., Torrens, M., de la Torre, R., & Farré, M. (2021). A Comparison of Acute Pharmacological Effects of Methylone and MDMA Administration in Humans and Oral Fluid Concentrations as Biomarkers of Exposure. Biology, 10(8), 788. https://doi.org/10.3390/biology10080788







