Light Quality-Dependent Regulation of Non-Photochemical Quenching in Tomato Plants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Pre-Illumination of Ascorbic Acid- and Dithiothreitol-Infiltrated Leaf Samples
2.3. Measurement of Chlorophyll Fluorescence (ChF) Induction Kinetics
2.4. Antioxidant Enzyme Activity Assay and MDA Measurements
2.5. Pigment Determination
2.6. Western Blot Analysis
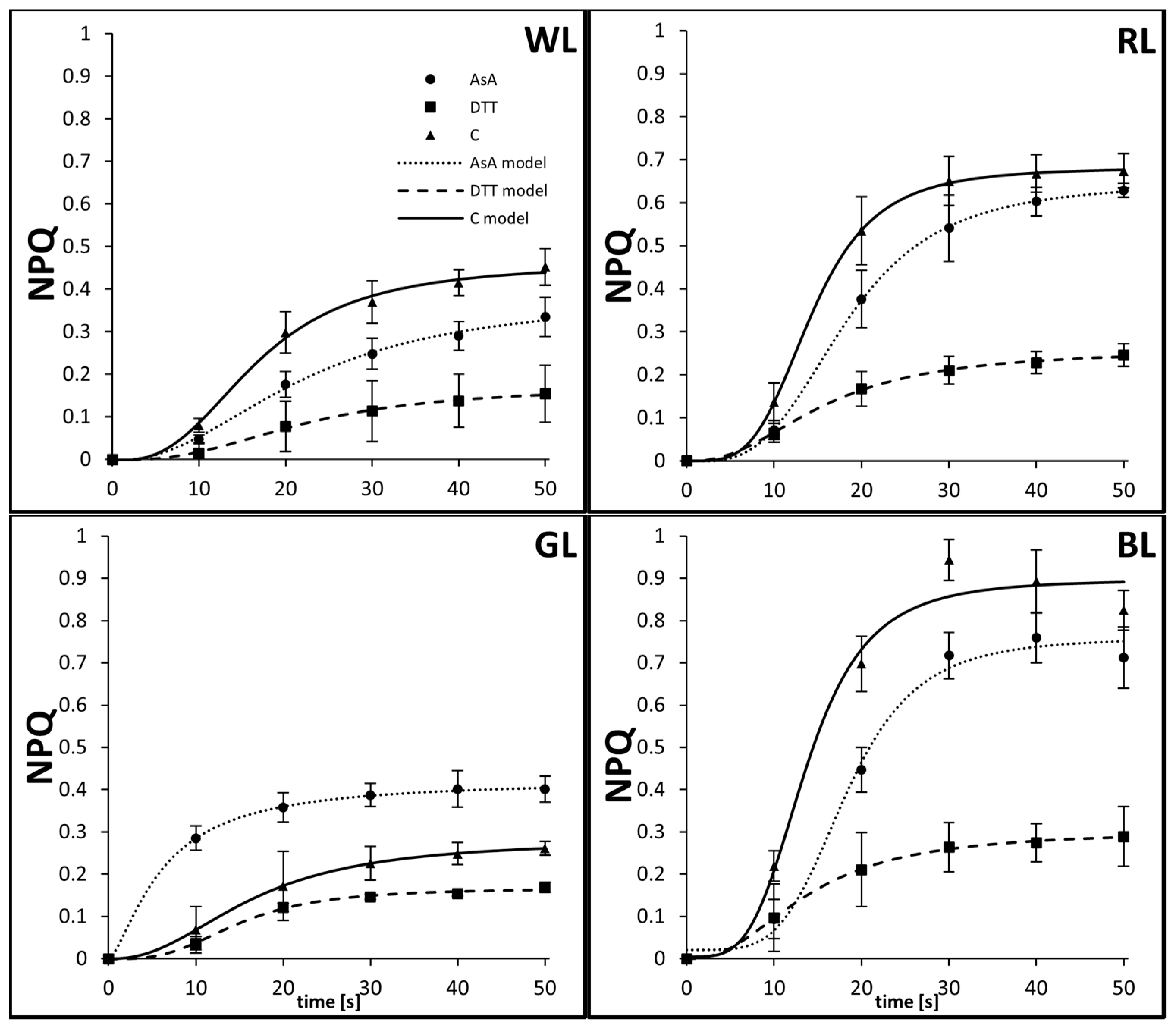
2.7. Model for Fitting of Experimental Data of Fast qE NPQ
- Logistic regression equation for fitting data (x) of fast qE NPQ induction:
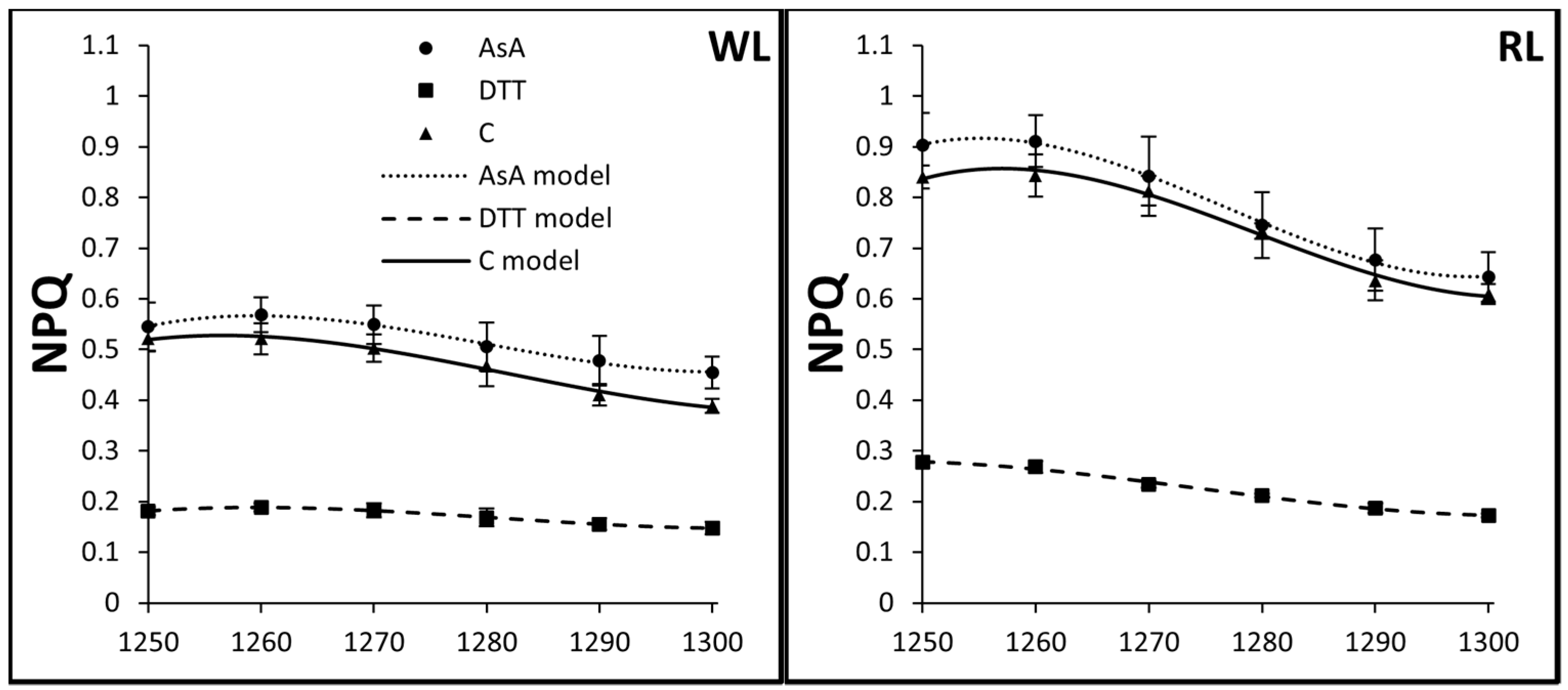
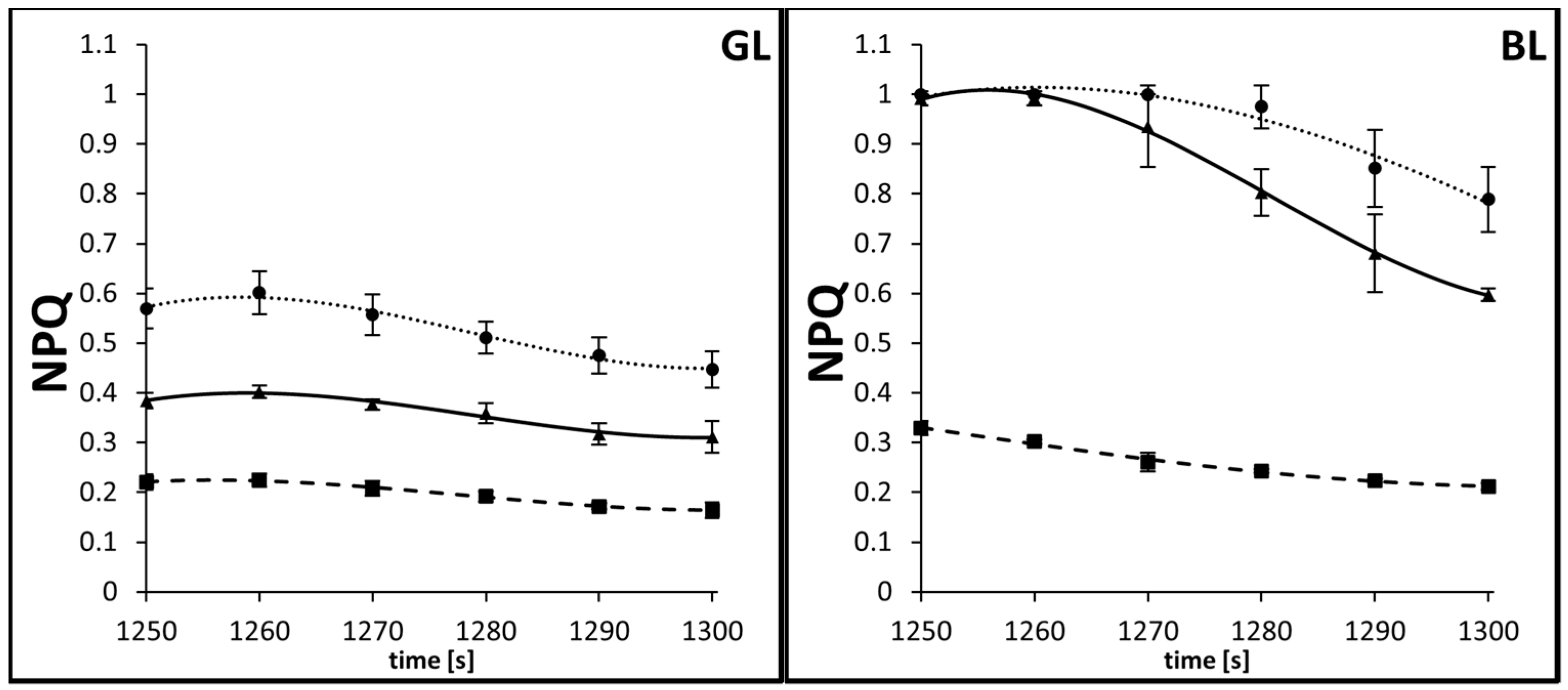
- Polynomial cubic regression equation for fitting data (x) of fast qE NPQ relaxation:
2.8. Statistical Analysis
3. Results
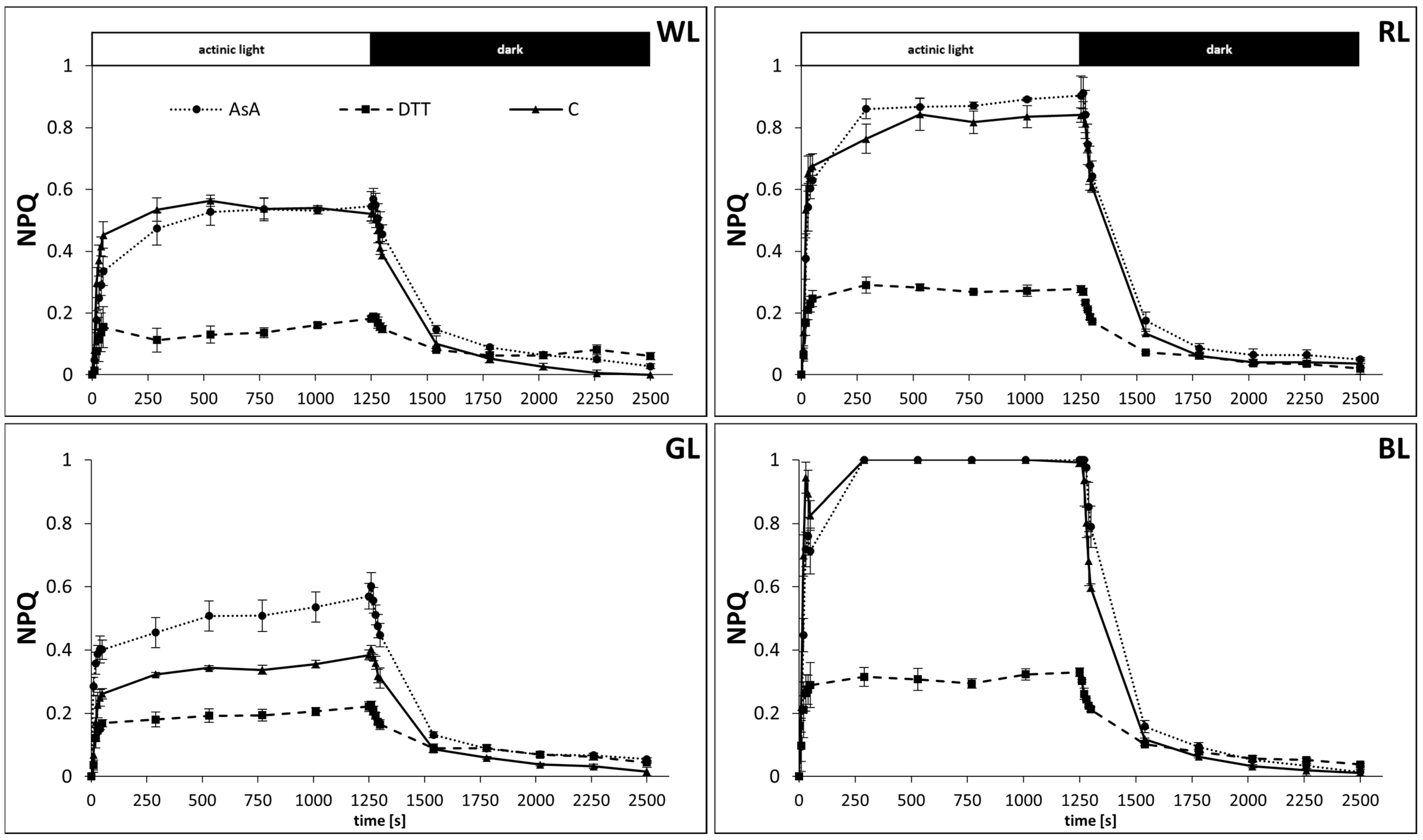
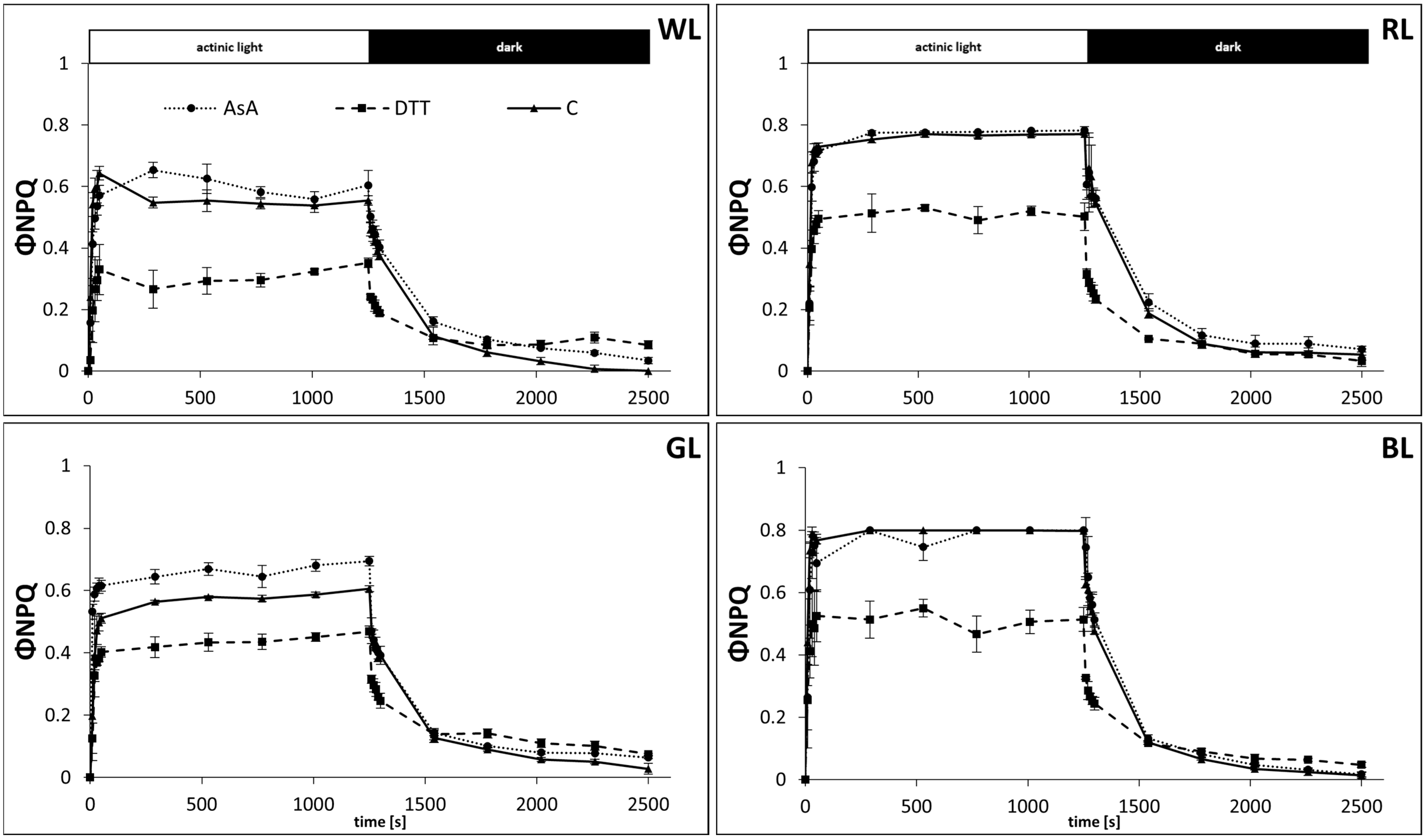
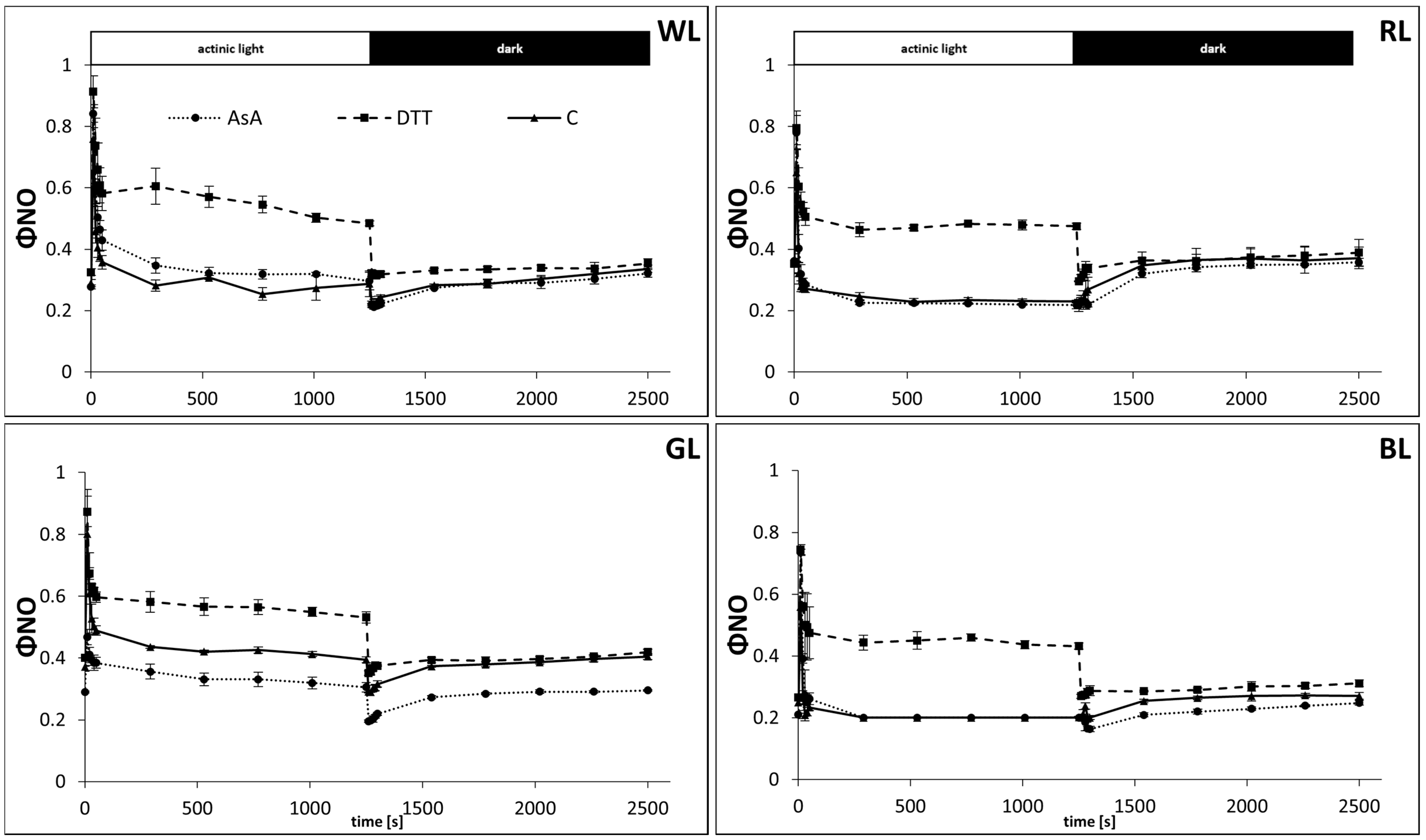
3.1. NPQ Formation in the Presence of AsA and DTT
3.2. Kinetics of Rapid Phase of NPQ Induction
3.3. Kinetics of Rapid and Middle Phases of NPQ Relaxation
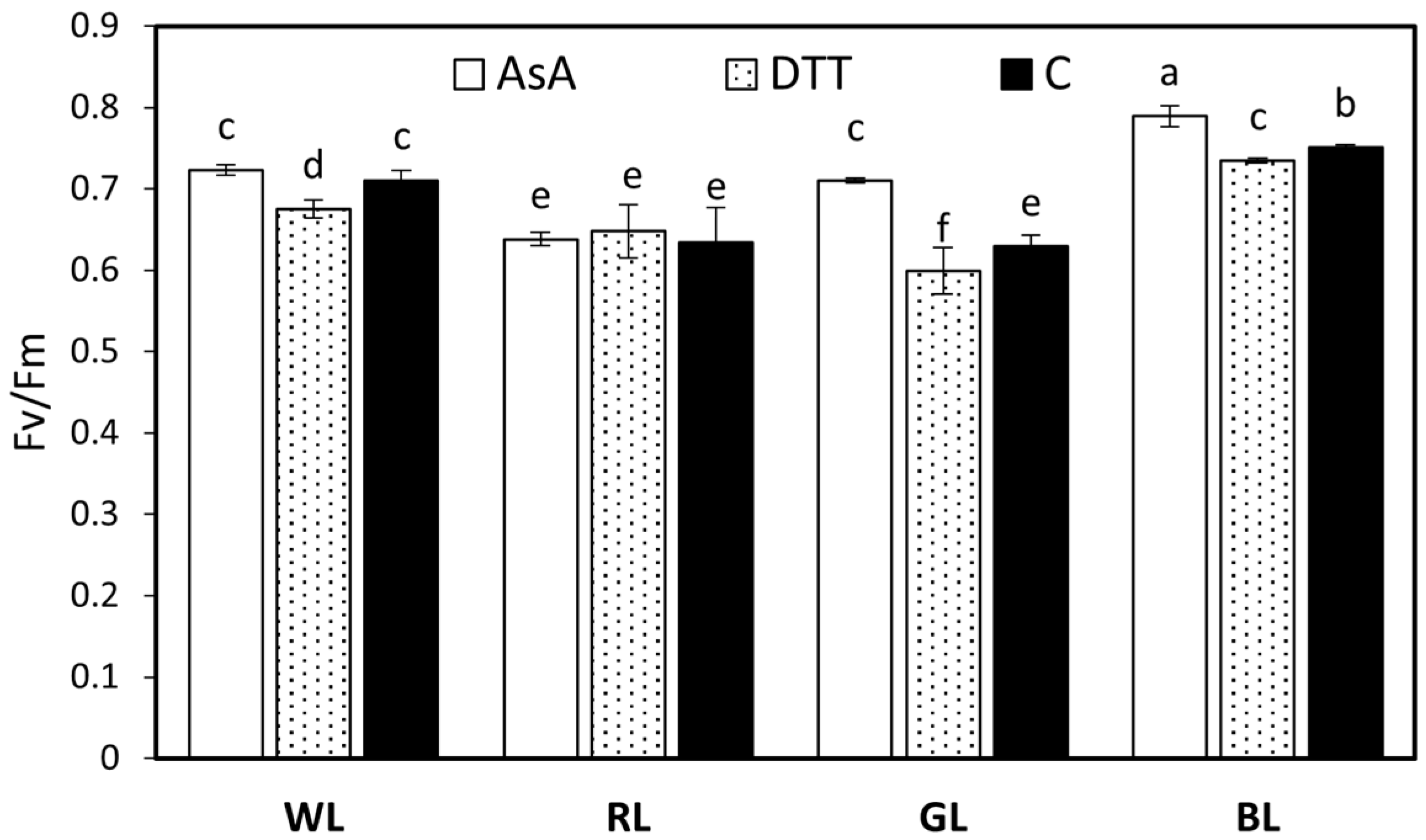
3.4. Maximum Quantum Yield and Photosynthetic Energy Partitioning in PSII
3.5. Photosynthetic Pigment Accumulation
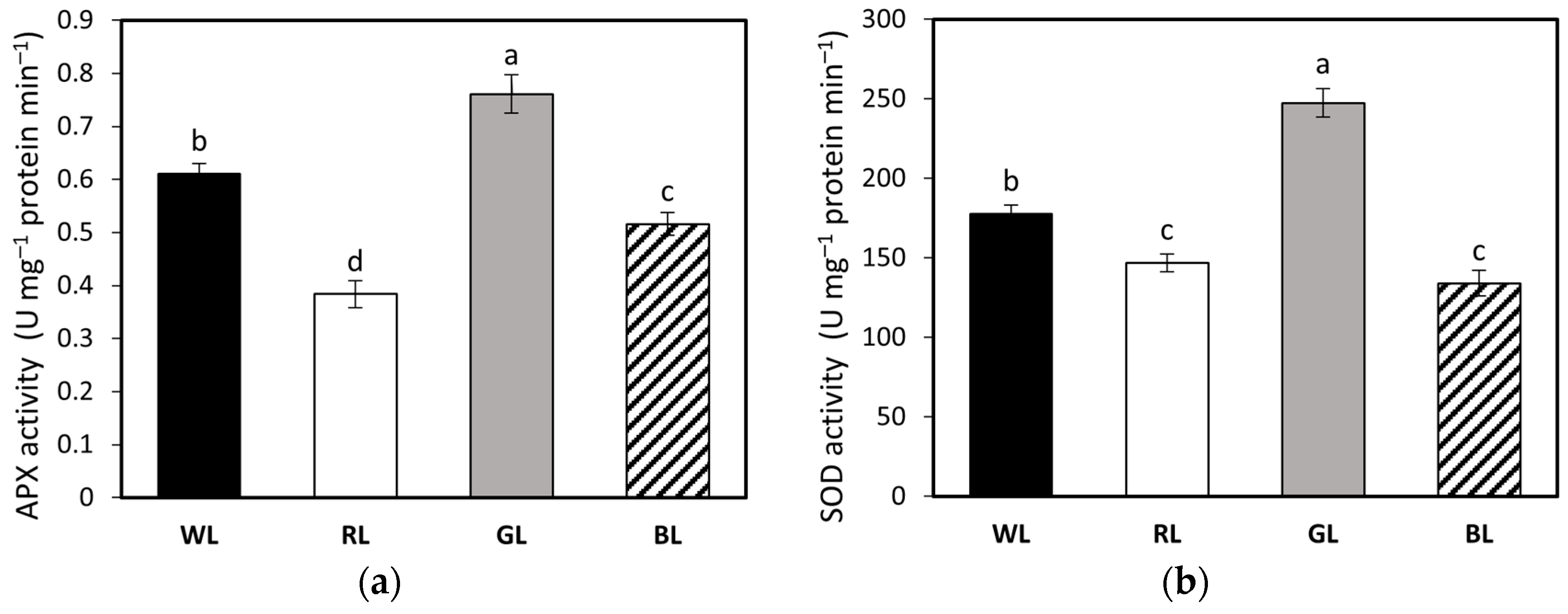
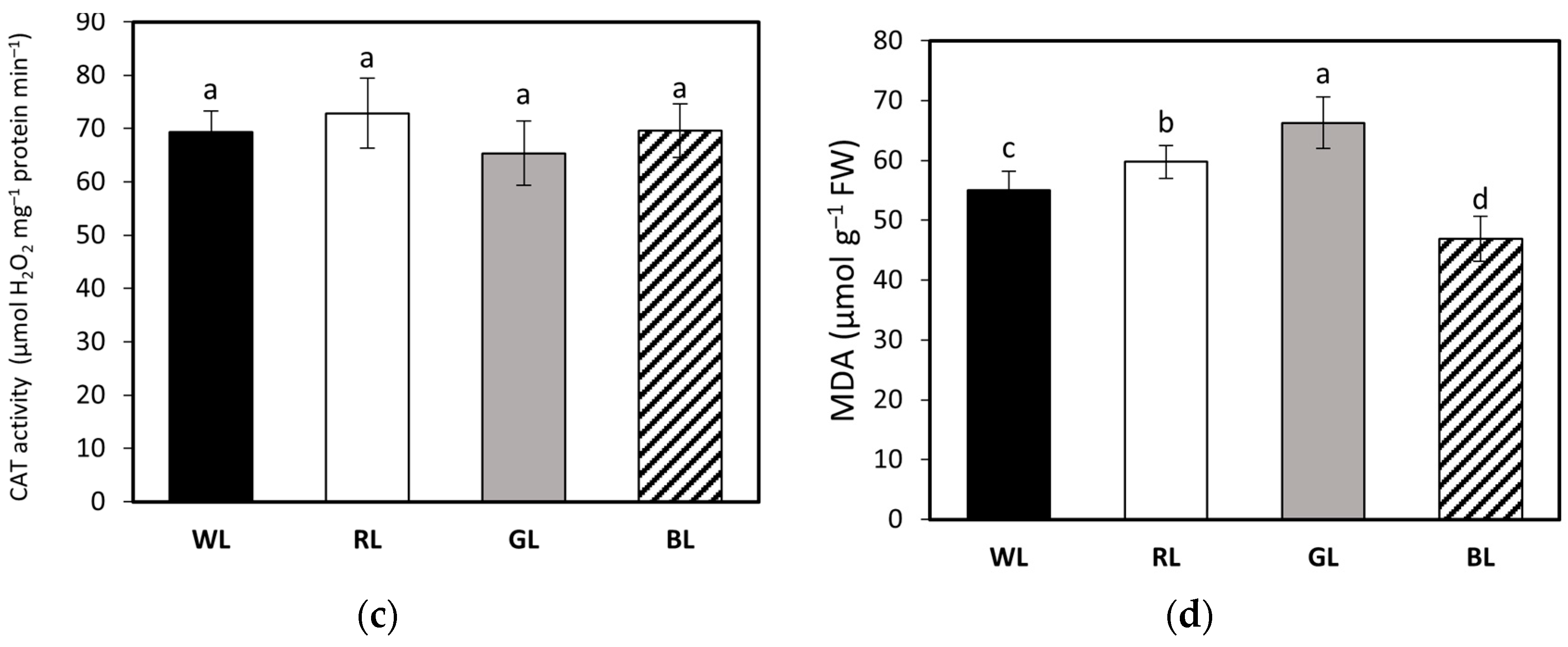
3.6. Activity of Antioxidant Enzymes APX, SOD, and CAT; MDA Accumulation
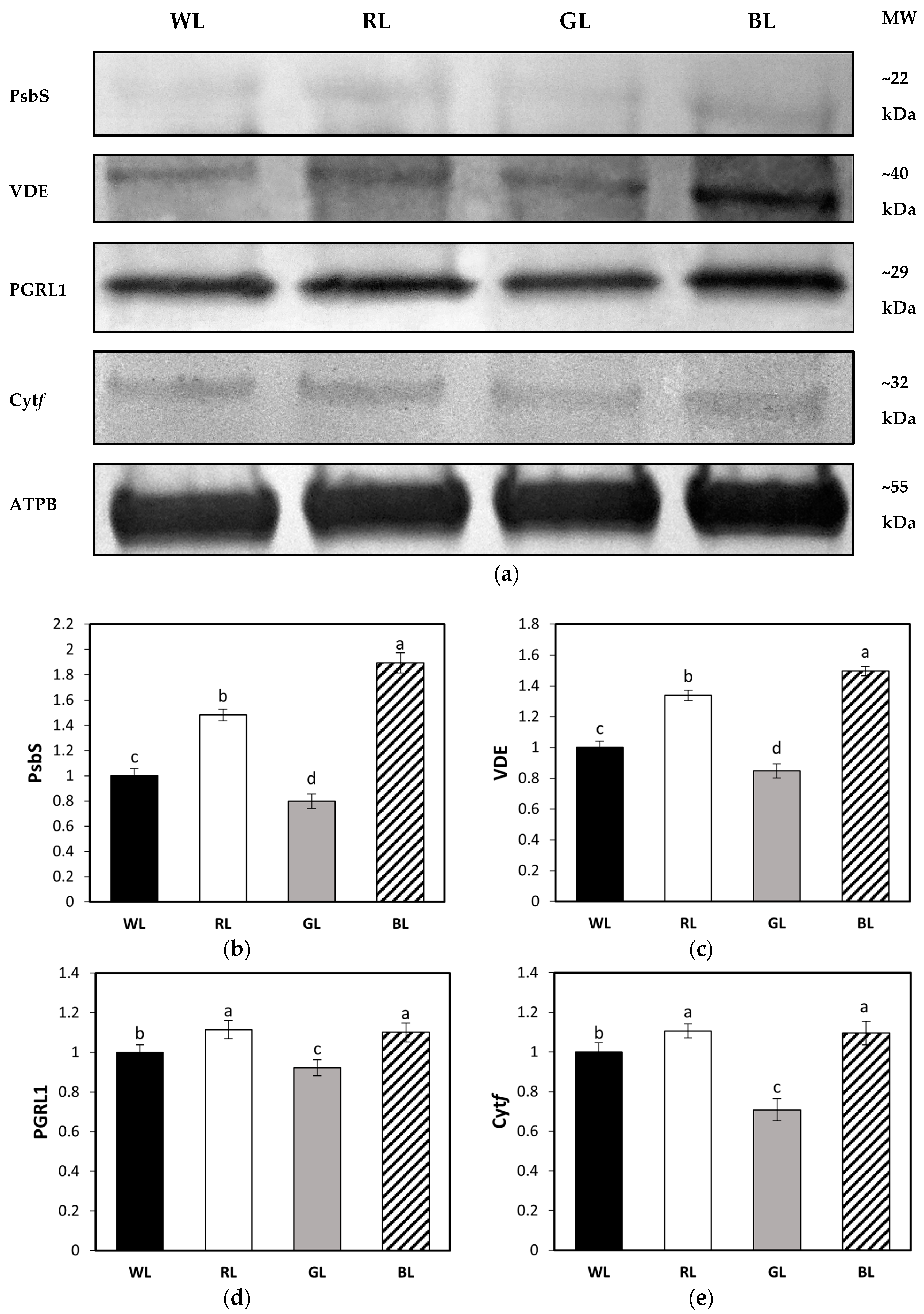
3.7. Accumulation of Leaf Proteins Related to NPQ
4. Discussion
4.1. Photosynthetic Capacity under Different Type of Light Quality
4.2. Effect of Light Quality on qE NPQ Component
4.3. Effect of Light Quality on qZ NPQ Component
4.4. Correlation between Antioxidant Activity, Lipid Peroxidation and Excitation Energy Quenching under Different Light Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Müller-Moulé, P.; Conklin, P.L.; Niyogi, K.K. Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol. 2002, 128, 970–977. [Google Scholar] [CrossRef] [Green Version]
- Steen, C.J.; Morris, J.M.; Short, A.H.; Niyogi, K.K.; Fleming, G.R. Complex roles of PsbS and xanthophylls in the regulation of nonphotochemical quenching in Arabidopsis thaliana under fluctuating light. J. Phys. Chem. B 2020, 124, 10311–10325. [Google Scholar] [CrossRef]
- Malnoë, A. Photoinhibition or photoprotection of photosynthesis? Update on the (newly termed) sustained quenching component qH. Environ. Exp. Bot. 2018, 154, 123–133. [Google Scholar] [CrossRef]
- Sukhova, E.; Khlopkov, A.; Vodeneev, V.; Sukhov, V. Simulation of a nonphotochemical quenching in plant leaf under different light intensities. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148138. [Google Scholar] [CrossRef]
- Hamdani, S.; Khan, N.; Perveen, S.; Qu, M.; Jiang, J.; Zhu, X.G. Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light. Photosyn. Res. 2019, 139, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Nicol, L.; Croce, R. The PsbS protein and low pH are necessary and sufficient to induce quenching in the light-harvesting complex of plants LHCII. Sci. Rep. 2021, 11, 7415. [Google Scholar] [CrossRef] [PubMed]
- Kress, E.; Jahns, P. The dynamics of energy dissipation and xanthophyll conversion in Arabidopsis indicate an indirect photoprotective role of zeaxanthin in slowly inducible and relaxing components of non-photochemical quenching of excitation energy. Front. Plant Sci. 2017, 8, 2094. [Google Scholar] [CrossRef]
- Ferroni, L.; Colpo, A.; Baldisserotto, C.; Pancaldi, S. In an ancient vascular plant the intermediate relaxing component of NPQ depends on a reduced stroma: Evidence from dithiothreitol treatment. J. Photochem. Photobiol. B Biol. 2021, 215, 112114. [Google Scholar] [CrossRef]
- Kohzuma, K.; Hikosaka, K. Physiological validation of photochemical reflectance index (PRI) as a photosynthetic parameter using Arabidopsis thaliana mutants. Biochem. Biophys. Res. Commun. 2018, 498, 52–57. [Google Scholar] [CrossRef]
- Wang, F.; Yan, J.; Ahammed, G.J.; Wang, X.; Bu, X.; Xiang, H.; Li, Y.; Lu, J.; Liu, Y.; Qi, H.; et al. PGR5/PGRL1 and NDH mediate far-red light-induced photoprotection in response to chilling stress in tomato. Front. Plant Sci. 2020, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.N.; Wang, F.; Wang, J.W.; Sun, L.J.; Gao, W.R.; Song, X.S. Overexpression of the ChVDE gene, encoding a violaxanthin de-epoxidase, improves tolerance to drought and salt stress in transgenic Arabidopsis. 3 Biotech 2019, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Arnoux, P.; Morosinotto, T.; Saga, G.; Bassi, R.; Pignol, D. A structural basis for the pH-dependent xanthophyll cycle in Arabidopsis thaliana. Plant Cell 2009, 21, 2036–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acebron, K.; Matsubara, S.; Jedmowski, C.; Emin, D.; Muller, O.; Rascher, U. Diurnal dynamics of nonphotochemical quenching in Arabidopsis npq mutants assessed by solar-induced fluorescence and reflectance measurements in the field. New Phytol. 2021, 229, 2104–2119. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.A.; Belgio, E.; Ruban, A.V. Comparison of the protective effectiveness of NPQ in Arabidopsis plants deficient in PsbS protein and zeaxanthin. J. Exp. Bot. 2015, 66, 1259–1270. [Google Scholar] [CrossRef] [Green Version]
- Bian, Z.; Yang, Q.; Li, T.; Cheng, R.; Barnett, Y.; Lu, C. Study of the beneficial effects of green light on lettuce grown under short-term continuous red and blue light-emitting diodes. Physiol. Plant. 2018, 164, 226–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, L.H.; Lee, D.G.; Jung, S. Light quality-dependent regulation of photoprotection and antioxidant properties in rice seedlings grown under different light-emitting diodes. Photosynthetica 2021, 59, 12–22. [Google Scholar] [CrossRef]
- Hallin, E.I.; Hasan, M.; Guo, K.; Åkerlund, H.E. Molecular studies on structural changes and oligomerisation of violaxanthin de-epoxidase associated with the pH-dependent activation. Photosyn. Res. 2016, 129, 29–41. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [Green Version]
- Jahns, P.; Latowski, D.; Strzalka, K. Mechanism and regulation of the violaxanthin cycle: The role of antenna proteins and membrane lipids. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Murchie, E.H.; Ruban, A.V. Dynamic non-photochemical quenching in plants: From molecular mechanism to productivity. Plant J. 2020, 101, 885–896. [Google Scholar] [CrossRef] [Green Version]
- Nishio, J.N. Why are higher plants green? Evolution of the higher plant photosynthetic pigment complement. Plant Cell Environ. 2000, 23, 539–548. [Google Scholar] [CrossRef]
- Busch, B.L.; Schmitz, G.; Rossmann, S.; Piron, F.; Ding, J.; Bendahmane, A.; Theres, K. Shoot branching and leaf dissection in tomato are regulated by homologous gene modules. Plant Cell 2011, 23, 3595–3609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinnola, A.; Dall’Osto, L.; Gerotto, C.; Morosinotto, T.; Bassi, R.; Alboresi, A. Zeaxanthin binds to light-harvesting complex stress-related protein to enhance nonphotochemical quenching in Physcomitrella patens. Plant Cell 2013, 25, 3519–3534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anielska-Mazur, A.; Bernaś, T.; Gabryś, H. In vivo reorganization of the actin cytoskeleton in leaves of Nicotiana tabacum L. transformed with plastin-GFP. Correlation with light-activated chloroplast responses. BMC Plant Biol. 2009, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Kalituho, L.; Beran, K.C.; Jahns, P. The transiently generated nonphotochemical quenching of excitation energy in Arabidopsis leaves is modulated by zeaxanthin. Plant Physiol. 2007, 143, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Nilkens, M.; Kress, E.; Lambrev, P.; Miloslavina, Y.; Müller, M.; Holzwarth, A.R.; Jahns, P. Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 466–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosyn. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Duan, B.; Yang, Y.; Lu, Y.; Korpelainen, H.; Berninger, F.; Li, C. Interactions between water deficit, ABA, and provenances in Picea asperata. J. Exp. Bot. 2007, 58, 3025–3036. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.L.; Guo, Y.K.; Bai, J.G.; Shang, L.; Wang, X.J. Effects of long-term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivars under low light. Physiol. Plant 2008, 132, 467–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [Green Version]
- Skowron, E.; Trojak, M. Effect of exogenously-applied abscisic acid, putrescine and hydrogen peroxide on drought tolerance of barley. Biologia 2021, 76, 453–468. [Google Scholar] [CrossRef]
- Pilarska, M.; Wiciarz, M.; Jajić, I.; Kozieradzka-Kiszkurno, M.; Dobrev, P.; Vanková, R.; Niewiadomska, E. A different pattern of production and scavenging of reactive oxygen species in halophytic Eutrema salsugineum (Thellungiella salsuginea) plants in comparison to Arabidopsis thaliana and its relation to salt stress signaling. Front. Plant Sci. 2016, 7, 1179. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.Q.; Song, L.L.; Jacobs, D.F.; Mei, L.; Liu, P.; Jin, S.H.; Wu, J.S. Physiological response to drought stress in Camptotheca acuminata seedlings from two provenances. Front. Plant Sci. 2015, 6, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tercé-Laforgue, T.; Mäck, G.; Hirel, B. New insights towards the function of glutamate dehydrogenase revealed during source-sink transition of tobacco (Nicotiana tabacum) plants grown under different nitrogen regimes. Physiol. Plant 2004, 120, 220–228. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose—Responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Zeng, L.D.; Li, M.; Chow, W.S.; Peng, C.L. Susceptibility of an ascorbate-deficient mutant of Arabidopsis to high-light stress. Photosynthetica 2018, 56, 427–432. [Google Scholar] [CrossRef]
- Yin, Y.; Li, S.; Liao, W.; Lu, Q.; Wen, X.; Lu, C. Photosystem II photochemistry, photoinhibition, and the xanthophyll cycle in heat-stressed rice leaves. J. Plant Physiol. 2010, 167, 959–966. [Google Scholar] [CrossRef]
- Ermakova, M.; Lopez-Calcagno, P.E.; Raines, C.A.; Furbank, R.T.; von Caemmerer, S. Overexpression of the rieske FeS protein of the cytochrome b6f complex increases C4 photosynthesis in Setaria viridis. Commun. Biol. 2019, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- DalCorso, G.; Pesaresi, P.; Masiero, S.; Aseeva, E.; Schünemann, D.; Finazzi, G.; Joliot, P.; Barbato, R.; Leister, D. A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 2008, 132, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Yamamoto, H.; Shikanai, T. Contribution of NDH-dependent cyclic electron transport around photosystem I to the generation of proton motive force in the weak mutant allele of pgr5. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Suorsa, M.; Rossi, F.; Tadini, L.; Labs, M.; Colombo, M.; Kater, M.M.; Leister, D.; Finazzi, G.; Aro, E.M.; Barbato, R.; et al. PGR5-PGRL1-dependent cyclic electron transport modulates linear electron transport rate in Arabidopsis thaliana. Mol. Plant 2016, 9, 271–288. [Google Scholar] [CrossRef] [Green Version]
- Trinh, M.D.L.; Sato, R.; Masuda, S. Genetic characterization of a flap1 null mutation in Arabidopsis npq4 and pgr5 plants suggests that the regulatory role of FLAP1 involves the control of proton homeostasis in chloroplasts. Photosynth. Res. 2019, 139, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Munekage, Y.; Hojo, M.; Meurer, J.; Endo, T.; Tasaka, M.; Shikanai, T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 2002, 110, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Zaks, J.; Amarnath, K.; Kramer, D.M.; Niyogi, K.K.; Fleming, G.R. A kinetic model of rapidly reversible nonphotochemical quenching. Proc. Natl. Acad. Sci. USA 2012, 109, 15757–15762. [Google Scholar] [CrossRef] [Green Version]
- Essemine, J.; Govindachary, S.; Joly, D.; Ammar, S.; Bouzid, S.; Carpentier, R. Effect of moderate and high light on photosystem II function in Arabidopsis thaliana depleted in digalactosyl-diacylglycerol. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 1367–1373. [Google Scholar] [CrossRef] [Green Version]
- Finazzi, G.; Johnson, G.N.; Dallosto, L.; Joliot, P.; Wollman, F.A.; Bassi, R. A zeaxanthin-independent nonphotochemical quenching mechanism localized in the photosystem II core complex. Proc. Natl. Acad. Sci. USA 2004, 101, 12375–12380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhold, C.; Niczyporuk, S.; Beran, K.C.; Jahns, P. Short-term down-regulation of zeaxanthin epoxidation in Arabidopsis thaliana in response to photo-oxidative stress conditions. Biochim. Biophys. Acta Bioenerg. 2008, 1777, 462–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.; Fang, S.; Zhou, M.; Qin, J. Responses of morphology, gas exchange, photochemical activity of photosystem II, and antioxidant balance in Cyclocarya paliurus to light spectra. Front. Plant Sci. 2018, 9, 1704. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, Z.; Jahan, M.S.; Wen, Y.; Yao, X.; Ding, H.; Guo, S.; Xu, Z. RNA-Seq analysis reveals the growth and photosynthetic responses of rapeseed (Brassica napus L.) under red and blue LEDs with supplemental yellow, green, or white light. Hortic. Res. 2020, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Kreslavski, V.D.; Strokina, V.V.; Pashkovskiy, P.P.; Balakhnina, T.I.; Voloshin, R.A.; Alwasel, S.; Kosobryukhov, A.A.; Allakhverdiev, S.I. Deficiencies in phytochromes A and B and cryptochrome 1 affect the resistance of the photosynthetic apparatus to high-intensity light in Solanum lycopersicum. J. Photochem. Photobiol. B 2020, 210, 111976. [Google Scholar] [CrossRef] [PubMed]

| Light Quality | Treatment | A1 | A2 | x0 | α | Ra2 |
|---|---|---|---|---|---|---|
| WL | AsA | −0.00252 | 0.38013 | 22.09031 | 2.22938 | 0.99203 |
| DTT | −0.00167 | 0.17035 | 22.17636 | 2.58926 | 0.99382 | |
| C | −0.00274 | 0.46039 | 16.73017 | 2.78761 | 0.99076 | |
| RL | AsA | −0.00147 | 0.64659 | 18.21531 | 3.37634 | 0.99971 |
| DTT | −3.29693 × 10−4 | 0.25832 | 15.6885 | 2.34694 | 0.99808 | |
| C | 3.59786 × 10−4 | 0.68169 | 14.29079 | 3.89071 | 0.99978 | |
| GL | AsA | 1.23493 × 10−5 | 0.42252 | 6.1032 | 1.47486 | 0.99937 |
| DTT | −4.9053 × 10−4 | 0.16698 | 14.89135 | 3.05313 | 0.99232 | |
| C | 6.50152 × 10−6 | 0.2812 | 16.37096 | 2.30662 | 0.99995 | |
| BL | AsA | 0.02089 | 0.75903 | 18.24241 | 4.49567 | 0.9808 |
| DTT | 9.39587 × 10−5 | 0.30166 | 13.81813 | 2.34655 | 0.99839 | |
| C | 0.00485 | 0.8969 | 13.65348 | 3.89107 | 0.95673 |
| Light Quality | Treatment | A | B | C | D | Ra2 |
|---|---|---|---|---|---|---|
| WL | AsA | −6808.2723 | 15.96759 | −0.01248 | 3.25 × 10−6 | 0.98669 |
| DTT | −2209.638 | 5.17863 | −0.00404 | 1.05247 × 10−6 | 0.99798 | |
| C | −4908.0904 | 11.49698 | −0.00897 | 2.33333 × 10−6 | 0.97961 | |
| RL | AsA | −13877.6622 | 32.62703 | −0.02556 | 6.67284 × 10−6 | 0.99688 |
| DTT | −2583.3524 | 6.08759 | −0.00478 | 1.25 × 10−6 | 0.98621 | |
| C | −11791.3213 | 27.66722 | −0.02163 | 5.63579 × 10−6 | 0.98203 | |
| GL | AsA | −8712.6496 | 20.45127 | −0.016 | 4.16975 × 10−6 | 0.96758 |
| DTT | −2915.1715 | 6.84801 | −0.00536 | 1.39815 × 10−6 | 0.99193 | |
| C | −5768.1806 | 13.53744 | −0.01059 | 2.75926 × 10−6 | 0.95141 | |
| BL | AsA | −1902.5802 | 4.30528 | −0.00324 | 8.0864 × 10−7 | 0.90916 |
| DTT | −620.6656 | 1.50338 | −0.00121 | 3.24074 × 10−7 | 0.98593 | |
| C | −14325.4182 | 33.57812 | −0.02622 | 6.82407 × 10−6 | 0.99645 |
| Parameter | Treatment | |||
|---|---|---|---|---|
| WL | RL | GL | BL | |
| Chl a + b (mg g−1 FW) | 2.77 ± 0.19 a | 2.62 ± 0.23 ab | 2.42 ± 0.13 b | 2.85 ± 0.18 a |
| Chl a (mg g−1 FW) | 2.18 ± 0.20 ab | 2.05 ± 0.19 ab | 1.91 ± 0.16 b | 2.27 ± 0.19 a |
| Chl b (mg g−1 FW) | 0.59 ± 0.02 a | 0.57 ± 0.03 a | 0.51 ± 0.03 b | 0.58 ± 0.03 a |
| Chl a/b | 3.69 ± 0.44 a | 3.60 ± 0.37 a | 3.75 ± 0.48 a | 3.91 ± 0.46 a |
| Carotenoids (mg g−1 FW) | 0.44 ± 0.03 a | 0.42 ± 0.03 a | 0.37 ± 0.02 b | 0.43 ± 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trojak, M.; Skowron, E. Light Quality-Dependent Regulation of Non-Photochemical Quenching in Tomato Plants. Biology 2021, 10, 721. https://doi.org/10.3390/biology10080721
Trojak M, Skowron E. Light Quality-Dependent Regulation of Non-Photochemical Quenching in Tomato Plants. Biology. 2021; 10(8):721. https://doi.org/10.3390/biology10080721
Chicago/Turabian StyleTrojak, Magdalena, and Ernest Skowron. 2021. "Light Quality-Dependent Regulation of Non-Photochemical Quenching in Tomato Plants" Biology 10, no. 8: 721. https://doi.org/10.3390/biology10080721
APA StyleTrojak, M., & Skowron, E. (2021). Light Quality-Dependent Regulation of Non-Photochemical Quenching in Tomato Plants. Biology, 10(8), 721. https://doi.org/10.3390/biology10080721






