Suspension of Amorphous Calcium Phosphate Nanoparticles Impact Commitment of Human Adipose-Derived Stem Cells In Vitro
Abstract
:Simple Summary
Abstract
1. Introduction
- (a)
- Gene expression of osteo-associated commitment would be enhanced with more aCaP nanoparticles in DMEM in a dose-dependent manner, while gene expression of endothelial cell commitment, chondrogenic and adipogenic commitment would be reduced in the presence of such nanoparticles.
- (b)
- Gene expression changes would be more prominent at two weeks compared to 1 week.
- (c)
- Human ASCs of three donors would behave individually different from each other with regard to gene expression changes—however, similar trends would occur.
2. Materials and Methods
2.1. Synthesis of aCaP Nanoparticles
2.2. Cell Isolation
2.3. Multilineage Cell Differentiation
2.4. ASC Cultivation with aCaP Nanoparticles
2.5. Quantitative Real Time PCR
2.6. Measurement of Free Calcium and Phosphate Ion Concentrations
2.7. Statistics
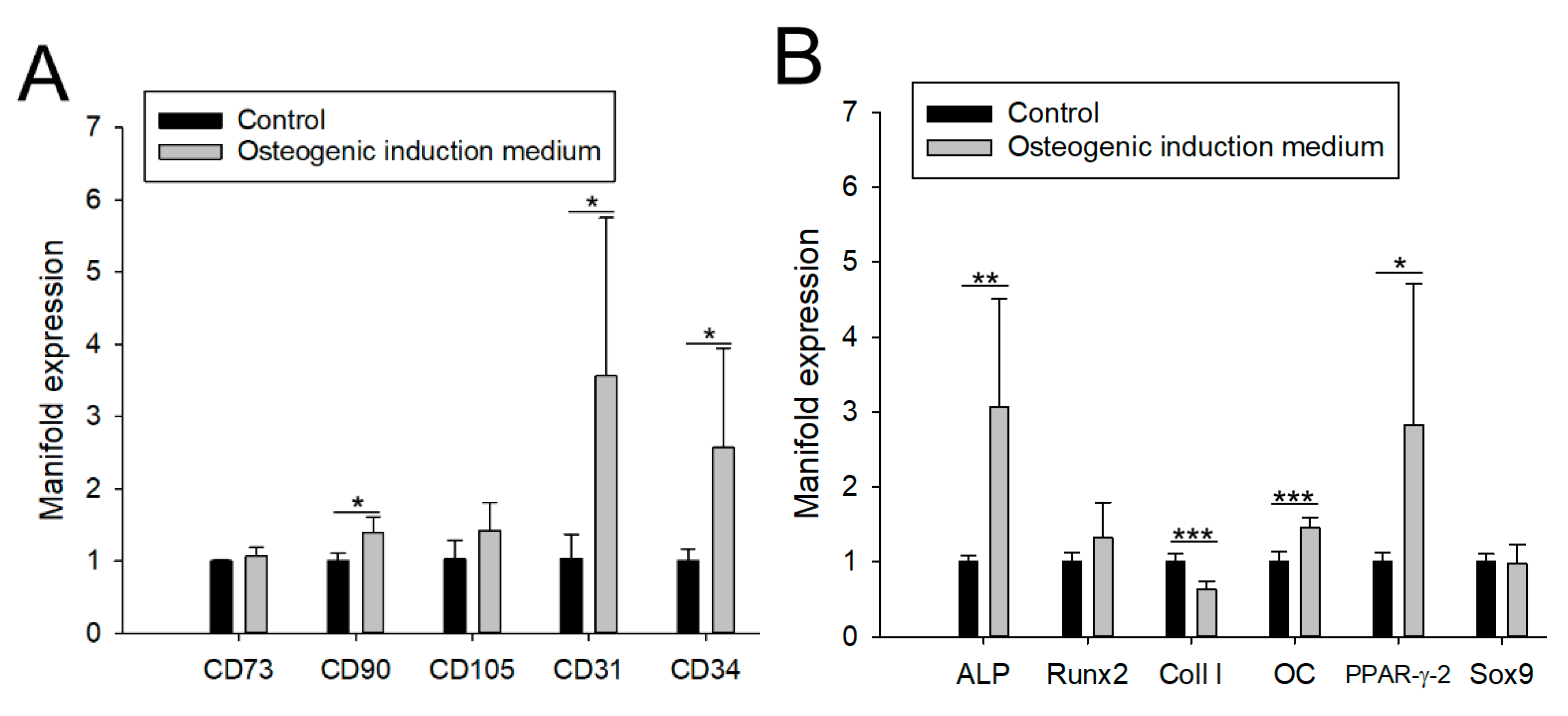
3. Results
3.1. Impact of aCaP Nanoparticles on Gene Expression of Stem Cells
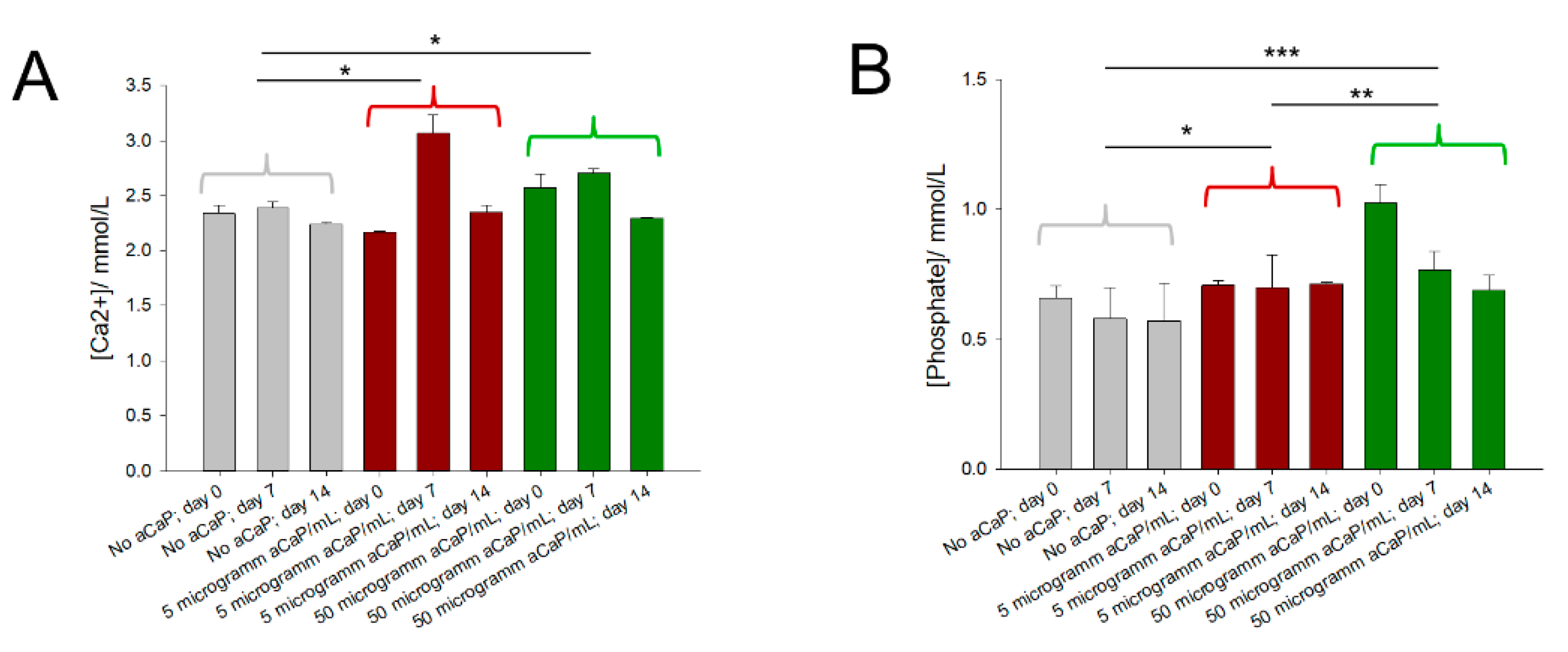
3.2. Free Calcium and Phosphate Concentrations
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Li, H.; Li, J.; Ye, J. Construction and properties of poly(lactic-co-glycolic acid)/calcium phosphate cement composite pellets with microspheres-in-pellet structure for bone repair. Ceram. Int. 2016, 42, 5587–5592. [Google Scholar] [CrossRef]
- Schamel, M.; Bernhardt, A.; Quade, M.; Wuerkner, C.; Gbureck, U.; Moseke, C.; Gelinsky, M.; Lode, A. Cu2+, Co2+ and Cr3+ doping of a calcium phosphate cement influences materials properties and response of human mesenchymal stromal cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Owen, G.R.; Dard, M.; Larjava, H. Hydoxyapatite/beta-tricalcium phosphate biphasic ceramics as regenerative material for the repair of complex bone defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2493–2512. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.T.; Jang, Y.S.; Kim, Y.K.; Kim, S.Y.; Lee, M.H.; Bae, T.S. Osteogenesis-Related Gene Expression and Guided Bone Regeneration of a Strontium-Doped Calcium-Phosphate-Coated Titanium Mesh. ACS Biomater. Sci. Eng. 2019, 5, 6715–6724. [Google Scholar] [CrossRef] [PubMed]
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef]
- Alves Cardoso, D.; Jansen, J.A.; Leeuwenburgh, S.C. Synthesis and application of nanostructured calcium phosphate ceramics for bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2316–2326. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.H.; Liu, X.Y.; Tang, R.K.; Xu, H.Y. Mystery of the transformation from amorphous calcium phosphate to hydroxyapatite. Chem. Commun. 2010, 46, 7415–7420. [Google Scholar] [CrossRef]
- Lotsari, A.; Rajasekharan, A.K.; Halvarsson, M.; Andersson, M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat. Commun. 2018, 9, 4170. [Google Scholar] [CrossRef] [Green Version]
- Buschmann, J.; Härter, L.; Gao, S.; Hemmi, S.; Welti, M.; Hild, N.; Schneider, O.D.; Stark, W.J.; Lindenblatt, N.; Werner, C.M.L.; et al. Tissue engineered bone grafts based on biomimetic nanocomposite PLGA/amorphous calcium phosphate scaffold and human adipose-derived stem cells. Injury 2012, 43, 1689–1697. [Google Scholar] [CrossRef] [Green Version]
- Schneider, O.D.; Weber, F.; Brunner, T.J.; Loher, S.; Ehrbar, M.; Schmidlin, P.R.; Stark, W.J. In vivo and in vitro evaluation of flexible, cottonwool-like nanocomposites as bone substitute material for complex defects. Acta Biomater. 2009, 5, 1775–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groninger, O.; Hess, S.; Mohn, D.; Schneider, E.; Stark, W.; Marsmann, S.; Wolint, P.; Calcagni, M.; Cinelli, P.; Buschmann, J. Directing Stem Cell Commitment by Amorphous Calcium Phosphate Nanoparticles Incorporated in PLGA: Relevance of the Free Calcium Ion Concentration. Int. J. Mol. Sci. 2020, 21, 2627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.W.; Zhao, J.K.; Zhang, X.; Zhang, H.; Peng, L.; Xu, B.C. In Vitro Calcium Pretreatment Enhances Bone Formation of Human Adipose Derived Stromal Cells In Vivo. J. Biomater. Tissue Eng. 2016, 6, 20–26. [Google Scholar] [CrossRef]
- Gurumurthy, B.; Bierdeman, P.C.; Janorkar, A.V. Spheroid model for functional osteogenic evaluation of human adipose derived stem cells. J. Biomed. Mater. Res. Part A 2017, 105, 1230–1236. [Google Scholar] [CrossRef]
- Bodle, J.C.; Hanson, A.D.; Loboa, E.G. Adipose-Derived Stem Cells in Functional Bone Tissue Engineering: Lessons from Bone Mechanobiology. Tissue Eng. Part B Rev. 2011, 17, 195–211. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Schack, L.M.; Noack, S.; Winkler, R.; Wissmann, G.; Behrens, P.; Wellmann, M.; Jagodzinski, M.; Krettek, C.; Hoffmann, A. The Phosphate Source Influences Gene Expression and Quality of Mineralization during In Vitro Osteogenic Differentiation of Human Mesenchymal Stem Cells. PLoS ONE 2013, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Boskey, A.L.; Roy, R. Cell Culture Systems for Studies of Bone and Tooth Mineralization. Chem. Rev. 2008, 108, 4716–4733. [Google Scholar] [CrossRef] [Green Version]
- Hatt, L.P.; Thompson, K.; Müller, W.E.G.; Stoddart, M.J.; Armiento, A.R. Calcium polyphosphate nanoparticles act as an effective inorganic phosphate source during osteogenic differentiation of human mesenchymal stem cells. Int. J. Mol. Sci. 2019, 20, 22. [Google Scholar]
- Kunisch, E.; Gunnella, F.; Wagner, S.; Dees, F.; Maenz, S.; Bossert, J.; Jandt, K.D.; Kinne, R.W. The poly (l-lactid-co-glycolide; PLGA) fiber component of brushite-forming calcium phosphate cement induces the osteogenic differentiation of human adipose tissue-derived stem cells. Biomed. Mater. 2019, 14, 055012. [Google Scholar] [CrossRef]
- Baumgartner, W.; Otto, L.; Hess, S.C.; Stark, W.J.; Märsmann, S.; Bürgisser, G.M.; Calcagni, M.; Cinelli, P.; Buschmann, J. Cartilage/bone interface fabricated under perfusion: Spatially organized commitment of adipose-derived stem cells without medium supplementation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107B, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Rohanova, D.; Boccaccini, A.R.; Horkavcova, D.; Bozdechova, P.; Bezdicka, P.; Castoralova, M. Is non-buffered DMEM solution a suitable medium for in vitro bioactivity tests? J. Mater. Chem. B 2014, 2, 5068–5076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loher, S.; Stark, W.J.; Maciejewski, M.; Baiker, A.; Pratsinis, S.E.; Reichardt, D.; Maspero, F.; Krumeich, F.; Gunther, D. Fluoro-apatite and calcium phosphate nanoparticles by flame synthesis. Chem. Mater. 2005, 17, 36–42. [Google Scholar] [CrossRef]
- Buschmann, J.; Gao, S.; Härter, L.; Hemmi, S.; Welti, M.; Werner, C.M.L.; Calcagni, M.; Cinelli, P.; Wanner, G.A. Yield and proliferation rate of adipose-derived stem cells as a function of age, BMI and harvest site: Increasing the yield by using adherent and supernatant fractions? Cytotherapy 2013, 15, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Franklin, D.M.; Leddy, H.A.; Robey, P.G.; Storms, R.W.; Gimble, J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 2001, 189, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.C.; Stark, W.J.; Mohn, D.; Cohrs, N.H.; Märsmann, S.; Calcagni, M.; Cinelli, P.; Buschmann, J. Gene expression in human adipose-derived stem cells: Comparison of 2D films, 3D electrospun meshes or co-cultured scaffolds with two-way paracrine effects. Eur. Cells Mat 2017, 34, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Canepa, D.D.; Casanova, E.; Arvaniti, E.; Tosevski, V.; Märsmann, S.; Eggerschwiler, B.; Halvachizadeh, S.; Buschmann, J.; Barth, A.A.; Plock, J.; et al. Identification of ALP+/CD73+ defining markers for enhanced osteogenic potential in human adipose-derived mesenchymal stromal cells by mass cytometry. Stem Cell. Res. Ther. 2021, 12, 7. [Google Scholar] [CrossRef]
- Middleton, J.; Americh, L.; Gayon, R.; Julien, D.; Mansat, M.; Mansat, P.; Anract, P.; Cantagrel, A.; Cattan, P.; Reimund, J.M.; et al. A comparative study of endothelial cell markers expressed in chronically inflamed human tissues: MECA-79, Duffy antigen receptor for chemokines, von Willebrand factor, CD31, CD34, CD105 and CD146. J. Pathol. 2005, 206, 260–268. [Google Scholar] [CrossRef]
- Yue, L.; Daxiang, J.; Weixing, X.; Longfei, W.; Weijian, C.; Jixi, X.; Jinyong, D.; Dongcheng, R. PPAR-gamma; and Wnt Regulate the Differentiation of MSCs into Adipocytes and Osteoblasts Respectively. Curr. Stem Cell Res. Ther. 2018, 13, 185–192. [Google Scholar]
- Lefebvre, V.; Dvir-Ginzberg, M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect. Tissue Res. 2017, 58, 2–14. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Karalashvili, L.; Kakabadze, A.; Uhryn, M.; Vyshnevska, H.; Ediberidze, K.; Kakabadze, Z. Bone Grafts for Reconstruction of bone defects (Reveiw). Georgian Med. News 2018, 282, 44–49. [Google Scholar]
- Roberts, S.J.; Geris, L.; Kerckhofs, G.; Desmet, E.; Schrooten, J.; Luyten, F.P. The combined bone forming capacity of human periosteal derived cells and calcium phosphates. Biomaterials 2011, 32, 4393–4405. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.; Zhao, Y.; Zhang, F.; Li, X.; Wang, L.; Weir, M.D.; Ma, J.; Reynolds, M.A.; Gu, N.; et al. Novel magnetic calcium phosphate-stem cell construct with magnetic field enhances osteogenic differentiation and bone tissue engineering. Mater. Sci. Eng. C 2019, 98, 30–41. [Google Scholar] [CrossRef]
- Zhang, Y. An In Vitro Comparative Study of Multisource Derived Human Mesenchymal Stem Cells for Bone Tissue Engineering. Stem Cells Dev. 2018, 27, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.; Larochette, N.; Bourguignon, M.; El-Hafci, H.; Potier, E.; Petite, H.; Logeart-Avramoglou, D. Osteogenic potential of adipogenic predifferentiated human bone-marrow-derived multipotent stromal cells for bone tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, e1511–e1524. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Gou, W.; Lu, Q.; Peng, J.; Lu, S. Role of mesenchymal stem cells in bone regeneration and fracture repair: A review. Int. Orthop. 2013, 37, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgio, F.; Rimmer, N.; Pieles, U.; Buschmann, J.; Beaufils-Hugot, M. Characterization and in ovo vascularization of a 3D-printed hydroxyapatite scaffold with different extracellular matrix coatings under perfusion culture. Biol. Open 2018, 7, bio034488. [Google Scholar] [CrossRef] [Green Version]
- Yuasa, M.; Yamada, T.; Taniyama, T.; Masaoka, T.; Xuetao, W.; Yoshii, T.; Horie, M.; Yasuda, H.; Uemura, T.; Okawa, A.; et al. Dexamethasone enhances osteogenic differentiation of bone marrow- and muscle-derived stromal cells and augments ectopic bone formation induced by bone morphogenetic protein-2. PLoS ONE 2015, 10, e0116462. [Google Scholar] [CrossRef] [Green Version]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and beta-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell. Res. Ther. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoemann, C.D.; El-Gabalawy, H.; McKee, M.D. In vitro osteogenesis assays: Influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol. Biol. 2009, 57, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.S.; Glenske, K.; Wolf, V.; Fietz, D.; Mazurek, S.; Hanke, T.; Moritz, A.; Arnhold, S.; Wenisch, S. Osteogenic differentiation capacity of human mesenchymal stromal cells in response to extracellular calcium with special regard to connexin 43. Annals Anat. Anat. Anz. 2017, 209, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Elashry, M.I.; Baulig, N.; Heimann, M.; Bernhardt, C.; Wenisch, S.; Arnhold, S. Osteogenic differentiation of equine adipose tissue derived mesenchymal stem cells using CaCl2. Res. Vet. Sci. 2018, 117, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Levingstone, T.J.; Herbaj, S.; Redmond, J.; McCarthy, H.O.; Dunne, N.J. Calcium Phosphate Nanoparticles-Based Systems for RNAi Delivery: Applications in Bone Tissue Regeneration. Nanomaterials 2020, 10, 146. [Google Scholar] [CrossRef] [Green Version]
- Suga, H.; Matsumoto, D.; Eto, H.; Inoue, K.; Aoi, N.; Kato, H.; Araki, J.; Yoshimura, K. Functional Implications of CD34 Expression in Human Adipose-Derived Stem/Progenitor Cells. Stem Cells Dev. 2009, 18, 1201–1209. [Google Scholar] [CrossRef] [Green Version]
- Clayton, Z.E.; Tan, R.P.; Miravet, M.M.; Lennartsson, K.; Cooke, J.P.; Bursill, C.A.; Wise, S.G.; Patel, S. Induced pluripotent stem cell-derived endothelial cells promote angiogenesis and accelerate wound closure in a murine excisional wound healing model. Biosci. Rep. 2018, 38, 4. [Google Scholar] [CrossRef] [Green Version]
- Orbay, H.; Devi, K.; Williams, P.A.; Dehghani, T.; Silva, E.A.; Sahar, D.E. Comparison of Endothelial Differentiation Capacities of Human and Rat Adipose-Derived Stem Cells. Plast. Reconstr. Surg. 2016, 138, 1231–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Cao, Y.; Zou, J.; Zhu, F.; Gao, Y.; Zheng, X.; Wang, H.; Zhang, T.; Wu, T. Improved osteogenesis and angiogenesis of a novel copper ions doped calcium phosphate cement. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111032. [Google Scholar] [CrossRef]
- Tian, T.; Xie, W.; Gao, W.; Wang, G.; Zeng, L.; Miao, G.; Lei, B.; Lin, Z.; Chen, X. Micro-Nano Bioactive Glass Particles Incorporated Porous Scaffold for Promoting Osteogenesis and Angiogenesis in vitro. Front. Chem. 2019, 7, 186. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.W.; Wang, Q.; Kang, N.; Wu, J.G.; Gu, C.M.; Bi, J.H.; Lv, T.; Xie, F.N.; Hu, J.W.; Liu, X.; et al. The effects of different vascular carrier patterns on the angiogenesis and osteogenesis of BMSC-TCP-based tissue-engineered bone in beagle dogs. J. Tissue Eng. Regen. Med. 2017, 11, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.-J.; Su, Y. Nuclear matrix-targeting of the osteogenic factor Runx2 is essential for its recognition and activation of the alkaline phosphatase gene. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 2839–2852. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, S.; Shao, X.; Shi, S.; Zhang, Q.; Xue, C.; Lin, Y.; Zhu, B.; Cai, X. Regulating Osteogenesis and Adipogenesis in Adipose-Derived Stem Cells by Controlling Underlying Substrate Stiffness. J. Cell. Physiol. 2017, 233, 3418–3428. [Google Scholar] [CrossRef]
- Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef]
- Gao, S.P.; Calcagni, M.; Welti, M.; Hemmi, S.; Hild, N.; Stark, W.J.; Meier Buergisser, G.; Wanner, G.A.; Cinelli, P.; Buschmann, J. Proliferation of ASC-derived endothelial cells in a 3D electrospun mesh: Impact of bone-biomimetic nanocomposite and co-culture with ASC-derived osteoblasts. Injury 2014, 45, 974–980. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. A review on the dissolution models of calcium apatites. Prog. Cryst. Growth Charact. Mater. 2002, 44, 45–61. [Google Scholar] [CrossRef]

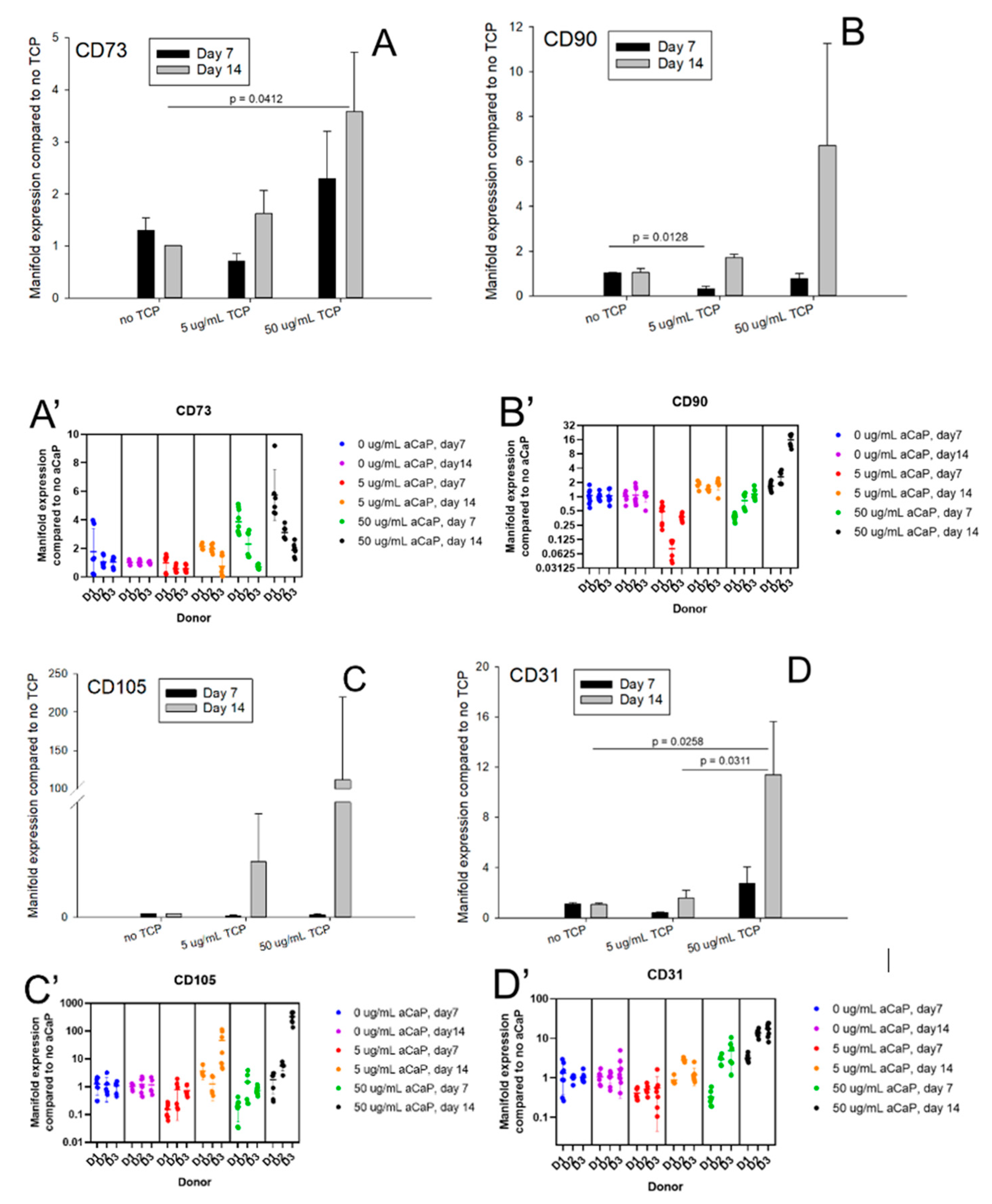
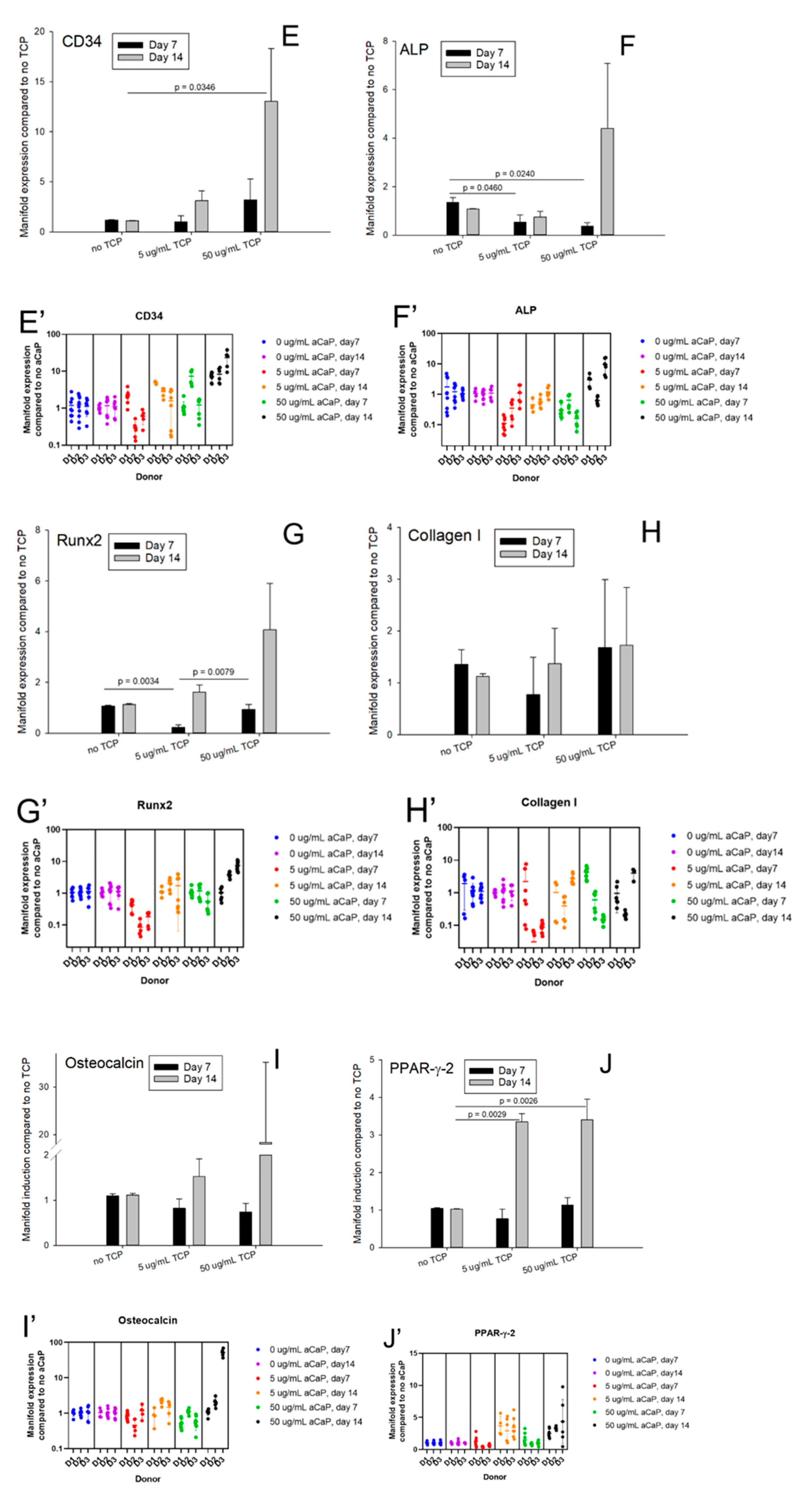
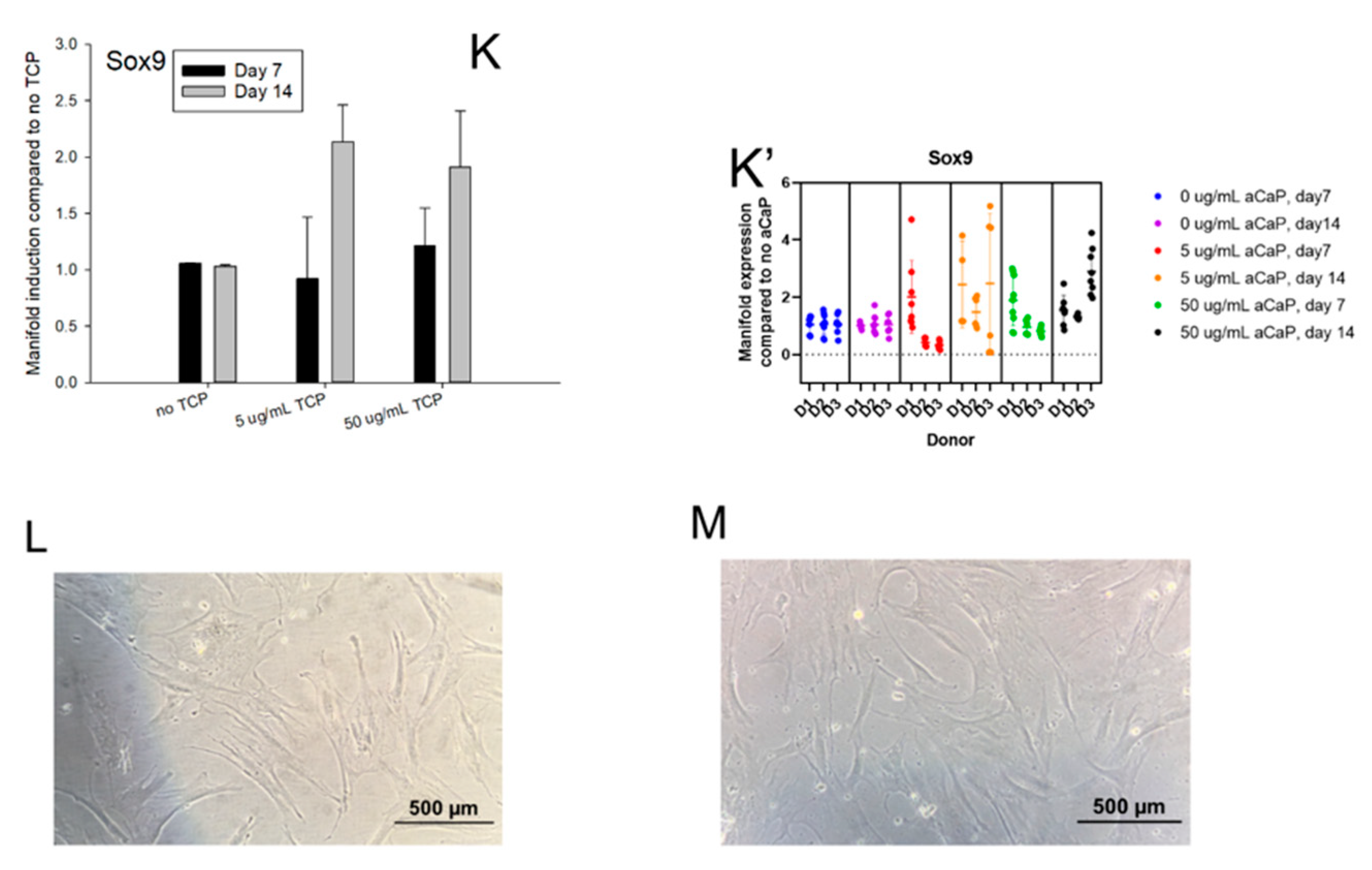
| D1 | D2 | D3 | D1 | D2 | D3 | |
|---|---|---|---|---|---|---|
| from 0 to 5 µg/mL | from 0 to 5 µg/mL | |||||
| Day 7 | Day 14 | |||||
| CD73 | - | - | ↓ ** | - | - | - |
| CD90 | - | - | ↓ *** | ↑ ** | - | - |
| CD105 | - | - | - | - | - | - |
| CD31 | - | - | - | - | - | - |
| CD34 | ↑ * | - | - | - | - | - |
| ALP | - | - | - | - | ↓ * | - |
| Runx2 | ↓ ** | - | ↓ ** | - | - | - |
| Collagen I | - | ↓ ** | ↓ *** | - | ↓ * | - |
| Osteocalcin | - | ↓ *** | - | - | ↑ *** | - |
| PPAR-γ-2 | - | ↓ *** | ↓ * | - | - | - |
| Sox9 | - | ↓ ** | ↓ *** | - | - | - |
| D1 | D2 | D3 | D1 | D2 | D3 | |
|---|---|---|---|---|---|---|
| from 5 to 50 µg/mL | from 5 to 50 µg/mL | |||||
| Day 7 | Day 14 | |||||
| CD73 | - | - | - | ↑ ** | - | ↑ ** |
| CD90 | - | - | ↑ *** | - | - | ↑ *** |
| CD105 | - | - | - | - | ↑ *** | ↑ *** |
| CD31 | - | - | ↑ ** | ↑ *** | - | ↑ *** |
| CD34 | ↓ ** | - | - | - | - | ↑ *** |
| ALP | - | - | ↓ ** | ↑ ** | - | ↑ *** |
| Runx2 | ↑ *** | - | - | - | ↑ ** | ↑ *** |
| Collagen I | - | - | - | - | - | - |
| Osteocalcin | ↓ ** | ↑ *** | ↓ * | - | - | ↑ *** |
| PPAR-γ-2 | - | ↑ ** | - | - | - | - |
| Sox9 | - | ↑ * | ↑ ** | - | - | - |
| D1 | D2 | D3 | D1 | D2 | D3 | |
|---|---|---|---|---|---|---|
| from 1 to 2 Weeks | from 1 to 2 Weeks | |||||
| at 5 µg/mL aCaP | at 50 µg/mL a CaP | |||||
| CD73 | ↑ *** | ↑ *** | - | - | - | ↑ ** |
| CD90 | - | ↑ *** | ↑ *** | - | - | ↑ *** |
| CD105 | - | - | - | - | ↑ * | ↑ *** |
| CD31 | - | ↑ *** | ↑ *** | - | - | ↑ *** |
| CD34 | ↑ *** | - | ↑ * | - | - | ↑ *** |
| ALP | ↑ ** | - | - | - | - | ↑ ** |
| Runx2 | ↑ * | - | - | ↓ ** | - | ↑ *** |
| Collagen I | - | - | ↑ *** | - | - | ↑ *** |
| Osteocalcin | ↑ *** | ↑ *** | ↑ * | ↑ *** | - | ↑ *** |
| PPAR-γ-2 | - | - | ↑ *** | - | ↑ *** | ↑ * |
| Sox9 | - | - | ↑ ** | ↑ * | - | ↑ *** |
| A | |||||||
| DAY 0 | DAY 7 | DAY 14 | |||||
| No aCaP | [32] | 1 | 1 | 2 | 2 | 3 | 3 |
| Solubility constant at 37 °C | ionic activity product | ionic activity product | ionic activity product | ||||
| Phase | Ks0 | Qs0 | logQs0-logKs0 | Qs0 | logQs0-logKs0 | Qs0 | logQs0-logKs0 |
| Ca(H2PO4)2 H2O | 0.072443596 | 1.014 × 10−9 | −7.85 | 8.011 × 10−10 | −7.96 | 7.282 × 10−10 | −8.00 |
| CaHPO4 2H2O | 2.34423 × 10−7 | 1.540 × 10−6 | 0.82 | 1.384 × 10−6 | 0.77 | 1.278 × 10−6 | 0.74 |
| CaHPO4 | 9.54993 × 10−8 | 1.540 × 10−6 | 1.21 | 1.384 × 10−6 | 1.16 | 1.278 × 10−6 | 1.13 |
| Ca8(HPO4)2(PO4)4 5H2O | 1.25893 × 10−96 | 7.285 × 10−41 | 55.76 | 4.029 × 10−41 | 55.51 | 2.187 × 10−41 | 55.24 |
| alpha-Ca3(PO4)2 | 3.16228 × 10−26 | 5.543 × 10−15 | 11.24 | 4.585 × 10−15 | 11.16 | 3.660 × 10−15 | 11.06 |
| beta-Ca3(PO4) 2 | 3.16228 × 10−30 | 5.543 × 10−15 | 15.24 | 4.585 × 10−15 | 15.16 | 3.660 × 10−15 | 15.06 |
| Ca10-x(HPO4)x(PO4)6x(OH)2-x (0<x<1) | 7.94328 × 10−86 | 1.703 × 10−43 | 42.33 | 9.640 × 10−44 | 42.08 | 4.902 × 10−44 | 41.79 |
| Ca10(PO4)6(OH)2 | 6.3096 × 10−118 | 3.983 × 10−46 | 71.80 | 2.306 × 10−46 | 71.56 | 1.099 × 10−46 | 71.24 |
| Ca4(PO4)2O | 3.16228 × 10−40 | 1.296 × 10−17 | 22.61 | 1.097 × 10−17 | 22.54 | 8.205 × 10−18 | 22.41 |
| B | |||||||
| DAY 0 | DAY 7 | DAY 14 | |||||
| 5 uM aCaP | [32] | 4 | 4 | 5 | 5 | 6 | 6 |
| Solubility constant at 37 °C | ionic activity product | ionic activity product | ionic activity product | ||||
| Phase | Ks0 | Qs0 | logQs0-logKs0 | Qs0 | logQs0-logKs0 | Qs0 | logQs0-logKs0 |
| Ca(H2PO4)2 H2O | 0.072443596 | 1.091 × 10−9 | −7.82 | 1.495 × 10−9 | −7.69 | 1.199 × 10−9 | −7.78 |
| CaHPO4 2H2O | 2.34423 × 10−7 | 1.539 × 10−6 | 0.82 | 2.140 × 10−6 | 0.96 | 1.679 × 10−6 | 0.86 |
| CaHPO4 | 9.54993 × 10−8 | 1.539 × 10−6 | 1.21 | 2.140 × 10−6 | 1.35 | 1.679 × 10−6 | 1.25 |
| Ca8(HPO4)2(PO4)4 5H2O | 1.25893 × 10−96 | 6.255 × 10−41 | 55.70 | 9.013 × 10−40 | 56.85 | 1.240 × 10−40 | 55.99 |
| alpha-Ca3(PO4)2 | 3.16228 × 10−26 | 5.140 × 10−15 | 11.21 | 1.403 × 10−14 | 11.65 | 6.632 × 10−15 | 11.32 |
| beta-Ca3(PO4) 2 | 3.16228 × 10−30 | 5.140 × 10−15 | 15.21 | 1.403 × 10−14 | 15.65 | 6.632 × 10−15 | 15.32 |
| Ca10-x(HPO4)x(PO4)6x(OH)2-x (0<x<1) | 7.94328 × 10−86 | 1.358 × 10−43 | 42.23 | 2.761 × 10−42 | 43.54 | 2.917 × 10−43 | 42.56 |
| Ca10(PO4)6(OH)2 | 6.3096 × 10−118 | 2.948 × 10−46 | 71.67 | 8.459 × 10−45 | 73.13 | 6.862 × 10−46 | 72.04 |
| Ca4(PO4)2O | 3.16228 × 10−40 | 1.116 × 10−17 | 22.55 | 4.298 × 10−17 | 23.13 | 1.560 × 10−17 | 22.69 |
| C | |||||||
| DAY 0 | DAY 7 | DAY 14 | |||||
| 50 uM aCaP | [32] | 7 | 7 | 8 | 8 | 9 | 9 |
| Solubility constant at 37 °C | ionic activity product | ionic activity product | ionic activity product | ||||
| Phase | Ks0 | Qs0 | logQs0-logKs0 | Qs0 | logQs0-logKs0 | Qs0 | logQs0-logKs0 |
| Ca(H2PO4)2 H2O | 0.072443596 | 2.707 × 10−9 | −7.43 | 1.593 × 10−9 | −7.66 | 1.094 × 10−9 | −7.82 |
| CaHPO4 2H2O | 2.34423 × 10−7 | 2.638 × 10−6 | 1.05 | 2.075 × 10−6 | 0.95 | 1.584 × 10−6 | 0.83 |
| CaHPO4 | 9.54993 × 10−8 | 2.638 × 10−6 | 1.44 | 2.075 × 10−6 | 1.34 | 1.584 × 10−6 | 1.22 |
| Ca8(HPO4)2(PO4)4 5H2O | 1.25893 × 10−96 | 2.224 × 10−39 | 57.25 | 5.848 × 10−40 | 56.67 | 8.306 × 10−41 | 55.82 |
| alpha-Ca3(PO4)2 | 3.16228 × 10−26 | 1.788 × 10−14 | 11.75 | 1.165 × 10−14 | 11.57 | 5.754 × 10−15 | 11.26 |
| beta-Ca3(PO4) 2 | 3.16228 × 10−30 | 1.788 × 10−14 | 15.75 | 1.165 × 10−14 | 15.57 | 5.754 × 10−15 | 15.26 |
| Ca10-x(HPO4)x(PO4)6x(OH)2-x (0<x<1) | 7.94328 × 10−86 | 5.715 × 10−42 | 43.86 | 1.582 × 10−42 | 43.30 | 1.905 × 10−43 | 42.38 |
| Ca10(PO4)6(OH)2 | 6.3096 × 10−118 | 1.469 × 10−44 | 73.37 | 4.278 × 10−45 | 72.83 | 4.369 × 10−46 | 71.84 |
| Ca4(PO4)2O | 3.16228 × 10−40 | 4.595 × 10−17 | 23.16 | 3.151 × 10−17 | 23.00 | 1.320 × 10−17 | 22.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolint, P.; Näf, L.; Schibler, D.; Hild, N.; Stark, W.J.; Giovanoli, P.; Calcagni, M.; Buschmann, J. Suspension of Amorphous Calcium Phosphate Nanoparticles Impact Commitment of Human Adipose-Derived Stem Cells In Vitro. Biology 2021, 10, 675. https://doi.org/10.3390/biology10070675
Wolint P, Näf L, Schibler D, Hild N, Stark WJ, Giovanoli P, Calcagni M, Buschmann J. Suspension of Amorphous Calcium Phosphate Nanoparticles Impact Commitment of Human Adipose-Derived Stem Cells In Vitro. Biology. 2021; 10(7):675. https://doi.org/10.3390/biology10070675
Chicago/Turabian StyleWolint, Petra, Lukas Näf, Désirée Schibler, Nora Hild, Wendelin J. Stark, Pietro Giovanoli, Maurizio Calcagni, and Johanna Buschmann. 2021. "Suspension of Amorphous Calcium Phosphate Nanoparticles Impact Commitment of Human Adipose-Derived Stem Cells In Vitro" Biology 10, no. 7: 675. https://doi.org/10.3390/biology10070675
APA StyleWolint, P., Näf, L., Schibler, D., Hild, N., Stark, W. J., Giovanoli, P., Calcagni, M., & Buschmann, J. (2021). Suspension of Amorphous Calcium Phosphate Nanoparticles Impact Commitment of Human Adipose-Derived Stem Cells In Vitro. Biology, 10(7), 675. https://doi.org/10.3390/biology10070675





