Targeted Lipidomic Analysis of Aqueous Humor Reveals Signaling Lipid-Mediated Pathways in Primary Open-Angle Glaucoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Subjects
2.3. AH and TF Collection
2.4. Lipid Extraction
2.5. UPLC-MS/MS Analysis
2.6. Statistics
2.7. Transcriptome Analysis
3. Results
3.1. Characteristics of the Experimental Groups
3.2. Profile of Signaling Lipids Secreted in AH
3.3. POAG-Related Alterations in Signaling Lipids of AH
3.4. POAG Stage and IOP-Dependent Alterations in Signaling Lipids of AH
3.5. Alterations in Oxylipin Content of TF, Associated with POAG
3.6. Bioinformatics Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leske, M.C. Open-Angle Glaucoma—An Epidemiologic Overview. Ophthalmic Epidemiol. 2007, 14, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Iomdina, E.N.; Tikhomirova, N.K.; Bessmertny, A.M.; Serebryakova, M.V.; Baksheeva, V.E.; Zalevsky, A.O.; Kotelin, V.I.; Kiseleva, O.A.; Kosakyan, S.M.; Zamyatnin, A.A.; et al. Alterations in proteome of human sclera associated with primary open-angle glaucoma involve proteins participating in regulation of the extracellular matrix. Mol. Vis. 2020, 26, 623–640. [Google Scholar]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Casson, R.J. Possible role of excitotoxicity in the pathogenesis of glaucoma. Clin. Exp. Ophthalmol. 2006, 34, 54–63. [Google Scholar] [CrossRef]
- Li, Y.; Andereggen, L.; Yuki, K.; Omura, K.; Yin, Y.; Gilbert, H.-Y.; Erdogan, B.; Asdourian, M.S.; Shrock, C.; de Lima, S.; et al. Mobile zinc increases rapidly in the retina after optic nerve injury and regulates ganglion cell survival and optic nerve regeneration. Proc. Natl. Acad. Sci. USA 2017, 114, E209–E218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieshaber, M.C.; Mozaffarieh, M.; Flammer, J. What Is the Link Between Vascular Dysregulation and Glaucoma? Surv. Ophthalmol. 2007, 52, S144–S154. [Google Scholar] [CrossRef]
- Vohra, R.; Tsai, J.C.; Kolko, M. The Role of Inflammation in the Pathogenesis of Glaucoma. Surv. Ophthalmol. 2013, 58, 311–320. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [Green Version]
- Lima, I.V.D.A.; Bastos, L.; Limborço-Filho, M.; Fiebich, B.L.; De Oliveira, A.C.P. Role of Prostaglandins in Neuroinflammatory and Neurodegenerative Diseases. Mediat. Inflamm. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Famitafreshi, H.; Karimian, M. Prostaglandins as the Agents That Modulate the Course of Brain Disorders. Degener. Neurol. Neuromuscul. Dis. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Kawasaki, A.; Han, M.-H.; Wei, J.-Y.; Hirata, K.; Otori, Y.; Barnstable, C.J. Protective effect of arachidonic acid on glutamate neurotoxicity in rat retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1835–1842. [Google Scholar]
- Brust, A.-K.; Ulbrich, H.K.; Seigel, G.M.; Pfeiffer, N.; Grus, F.H. Effects of Cyclooxygenase Inhibitors on Apoptotic Neuroretinal Cells. Biomark. Insights 2008, 3. [Google Scholar] [CrossRef]
- Sakai, Y.; Tanaka, T.; Seki, M.; Okuyama, S.; Fukuchi, T.; Yamagata, K.; Takei, N.; Nawa, H.; Abe, H. Cyclooxygenase-2 plays a critical role in retinal ganglion cell death after transient ischemia: Real-time monitoring of RGC survival using Thy-1-EGFP transgenic mice. Neurosci. Res. 2009, 65, 319–325. [Google Scholar] [CrossRef]
- Doucette, L.; Walter, M.A. Prostaglandins in the eye: Function, expression, and roles in glaucoma. Ophthalmic Genet. 2016, 38, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Lütjen-Drecoll, E.; Tamm, E. Morphological study of the anterior segment of cynomolgus monkey eyes following treatment with prostaglandin F2α. Exp. Eye Res. 1988, 47, 761–769. [Google Scholar] [CrossRef]
- Schachtschabel, U.; Lindsey, J.D.; Weinreb, R.N. The mechanism of action of prostaglandins on uveoscleral outflow. Curr. Opin. Ophthalmol. 2000, 11, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Schmidl, D.; Schmetterer, L.; Garhöfer, G.; Popa-Cherecheanu, A. Pharmacotherapy of Glaucoma. J. Ocul. Pharmacol. Ther. 2015, 31, 63–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scozzafava, A.; Supuran, C.T. Glaucoma and the Applications of Carbonic Anhydrase Inhibitors. Alzheimer’s Disease 2014, 75, 349–359. [Google Scholar] [CrossRef]
- Umeno, A.; Tanito, M.; Kaidzu, S.; Takai, Y.; Horie, M.; Yoshida, Y. Comprehensive measurements of hydroxylinoleate and hydroxyarachidonate isomers in blood samples from primary open-angle glaucoma patients and controls. Sci. Rep. 2019, 9, 2171. [Google Scholar] [CrossRef]
- Goel, M. Aqueous Humor Dynamics: A Review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Buisset, A.; Gohier, P.; Leruez, S.; Muller, J.; Amati-Bonneau, P.; Lenaers, G.; Bonneau, D.; Simard, G.; Procaccio, V.; Annweiler, C.; et al. Metabolomic Profiling of Aqueous Humor in Glaucoma Points to Taurine and Spermine Deficiency: Findings from the Eye-D Study. J. Proteome Res. 2019, 18, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Myer, C.; Perez, J.; Abdelrahman, L.; Mendez, R.; Khattri, R.B.; Junk, A.K.; Bhattacharya, S.K. Differentiation of soluble aqueous humor metabolites in primary open angle glaucoma and controls. Exp. Eye Res. 2020, 194, 108024. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Azbukina, N.V.; Astakhova, A.A.; Goriainov, S.; Chistyakov, V.V.; Tiulina, V.V.; Baksheeva, V.; Kotelin, V.; Fedoseeva, E.V.; Zamyatnin, A.; et al. Comparative lipidomic analysis of inflammatory mediators in the aqueous humor and tear fluid of humans and rabbits. Metabolomics 2020, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, D.V.; Gancharova, O.S.; Baksheeva, V.E.; Tiulina, V.V.; Goriainov, S.V.; Azbukina, N.V.; Tsarkova, M.S.; Zamyatnin, J.A.A.; Philippov, P.P.; Sergeeva, M.G.; et al. Inflammation in Dry Eye Syndrome: Identification and Targeting of Oxylipin-Mediated Mechanisms. Biomedicines 2020, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, D.V.; Baksheeva, V.E.; Tiulina, V.V.; Goriainov, S.V.; Azbukina, N.V.; Gancharova, O.S.; Arifulin, E.A.; Komarov, S.V.; Chistyakov, V.V.; Tikhomirova, N.K.; et al. Mechanisms and Treatment of Light-Induced Retinal Degeneration-Associated Inflammation: Insights from Biochemical Profiling of the Aqueous Humor. Int. J. Mol. Sci. 2020, 21, 704. [Google Scholar] [CrossRef] [Green Version]
- Pieragostino, D.; Bucci, S.; Agnifili, L.; Fasanella, V.; D’Aguanno, S.; Mastropasqua, A.; Ciancaglini, M.; Mastropasqua, L.; Di Ilio, C.; Sacchetta, P.; et al. Differential protein expression in tears of patients with primary open angle and pseudoexfoliative glaucoma. Mol. BioSyst. 2012, 8, 1017–1028. [Google Scholar] [CrossRef]
- Pieragostino, D.; Agnifili, L.; Fasanella, V.; D’Aguanno, S.; Mastropasqua, R.; Di Ilio, P.S.C.; Sacchetta, P.; Urbani, A.; Del Boccio, P. Shotgun proteomics reveals specific modulated protein patterns in tears of patients with primary open angle glaucoma naïve to therapy. Mol. BioSyst. 2013, 9, 1108–1116. [Google Scholar] [CrossRef]
- Mastropasqua, R.; Agnifili, L.; Mastropasqua, L. Structural and Molecular Tear Film Changes in Glaucoma. Curr. Med. Chem. 2019, 26, 4225–4240. [Google Scholar] [CrossRef]
- Egorov, E.; Astakhov, Y.; Shuko, A. National Glaucoma Guidelines; GEOTAR Media: Moscow, Russia, 2011; p. 297. [Google Scholar]
- Fiehn, O.; Robertson, D.; Griffin, J.; Van Der Werf, M.; Nikolau, B.; Morrison, N.; Sumner, L.W.; Goodacre, R.; Hardy, N.W.; Taylor, C.; et al. The metabolomics standards initiative (MSI). Metabolomics 2007, 3, 175–178. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [Green Version]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Research 2016, 4, 1521. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Stocchero, M.; Locci, E.; D’Aloja, E.; Nioi, M.; Baraldi, E.; Giordano, G. PLS2 in Metabolomics. Metabolites 2019, 9, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S. LXII Edward Jackson Lecture: Open Angle Glaucoma after Vitrectomy. Am. J. Ophthalmol. 2006, 141, 1033–1043.e1. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Chen, D.; Guo, J.; Liao, S.; Luo, X.; Zhang, Y.; Guo, R.; Xu, C.; Huang, D.; Zhang, Y.; et al. Transcriptome-wide study of the response of human trabecular meshwork cells to the substrate stiffness increase. J. Cell. Biochem. 2020, 121, 3112–3123. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Gupta, V.B.; Chick, J.M.; Greco, T.M.; Wu, Y.; Chitranshi, N.; Wall, R.V.; Hone, E.; Deng, L.; Dheer, Y.; et al. Age-related neurodegenerative disease associated pathways identified in retinal and vitreous proteome from human glaucoma eyes. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funke, S.; Perumal, N.; Beck, S.; Gabel-Scheurich, S.; Schmelter, C.; Teister, J.; Gerbig, C.; Gramlich, O.W.; Pfeiffer, N.; Grus, F.H. Glaucoma related Proteomic Alterations in Human Retina Samples. Sci. Rep. 2016, 6, 29759. [Google Scholar] [CrossRef] [Green Version]
- Vranka, J.A.; Staverosky, J.A.; Reddy, A.P.; Wilmarth, P.A.; David, L.L.; Acott, T.S.; Russell, P.; Raghunathan, V.K. Biomechanical Rigidity and Quantitative Proteomics Analysis of Segmental Regions of the Trabecular Meshwork at Physiologic and Elevated Pressures. Investig. Opthalmol. Vis. Sci. 2018, 59, 246–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, G.; Arcuri, J.; Wang, H.; Ziebarth, N.; Zode, G.; Lee, R.K.; Bhattacharya, S.K. Endogenous ocular lipids as potential modulators of intraocular pressure. J. Cell. Mol. Med. 2020, 24, 3856–3900. [Google Scholar] [CrossRef] [Green Version]
- Cabrerizo, J.; Urcola, J.A.; Vecino, E. Changes in the Lipidomic Profile of Aqueous Humor in Open-Angle Glaucoma. J. Glaucoma 2017, 26, 349–355. [Google Scholar] [CrossRef]
- Chistyakov, D.; Azbukina, N.; Goriainov, S.; Gancharova, O.; Tiulina, V.; Baksheeva, V.; Iomdina, E.; Philippov, P.; Sergeeva, M.; Senin, I.; et al. Inflammatory metabolites of arahidonic acid in tear fluid in UV-induced corneal damage. Biomeditsinskaya Khimiya 2019, 65, 33–40. [Google Scholar] [CrossRef]
- Lynes, M.D.; Leiria, L.O.; Lundh, M.; Bartelt, A.; Shamsi, F.; Huang, T.L.; Takahashi, H.; Hirshman, M.F.; Schlein, C.; Lee, A.; et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 2017, 23, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.; Middelbeek, R.J.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1111–1120.e3. [Google Scholar] [CrossRef] [Green Version]
- Marowsky, A.; Meyer, I.; Erismann-Ebner, K.; Pellegrini, G.; Mule, N.; Arand, M. Beyond detoxification: A role for mouse mEH in the hepatic metabolism of endogenous lipids. Arch. Toxicol. 2017, 91, 3571–3585. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, M.; Tanaka, Y.; Omodaka, K.; Nishiguchi, K.M.; Nakamura, O.; Tsuda, S.; Nakazawa, T. Transcriptome profiling of the rat retina after optic nerve transection. Sci. Rep. 2016, 6, 28736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasiliou, V.; Gonzalez, F.J. Role of CYP1B1 in Glaucoma. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 333–358. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, K.; Kodani, S.D.; Hammock, B.D.; Zhao, L. Cytochrome P450-derived linoleic acid metabolites EpOMEs and DiHOMEs: A review of recent studies. J. Nutr. Biochem. 2020, 86, 108484. [Google Scholar] [CrossRef]
- Nieman, D.C.; Shanely, R.A.; Luo, B.; Meaney, M.P.; Dew, D.A.; Pappan, K.L. Metabolomics approach to assessing plasma 13- and 9-hydroxy-octadecadienoic acid and linoleic acid metabolite responses to 75-km cycling. Am. J. Physiol. Integr. Comp. Physiol. 2014, 307, R68–R74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vangaveti, V.; Baune, B.T.; Kennedy, R.L. Review: Hydroxyoctadecadienoic acids: Novel regulators of macrophage differentiation and atherogenesis. Ther. Adv. Endocrinol. Metab. 2010, 1, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Umeno, A.; Tanito, M.; Kaidzu, S.; Takai, Y.; Yoshida, Y. Involvement of free radical-mediated oxidation in the pathogenesis of pseudoexfoliation syndrome detected based on specific hydroxylinoleate isomers. Free. Radic. Biol. Med. 2020, 147, 61–68. [Google Scholar] [CrossRef]
- Falero-Perez, J.; Song, Y.-S.; Sorenson, C.M.; Sheibani, N. CYP1B1: A key regulator of redox homeostasis. Trends Cell Mol. Biol. 2018, 13, 27–45. [Google Scholar]
- Izzotti, A.; Bagnis, A.; Saccà, S.C. The role of oxidative stress in glaucoma. Mutat. Res. Mutat. Res. 2006, 612, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Hayashi, Y.; Matsumoto, N.; Nemoto-Sasaki, Y.; Koizumi, T.; Inagaki, Y.; Oka, S.; Tanikawa, T.; Sugiura, T. Coenzyme-A-Independent Transacylation System; Possible Involvement of Phospholipase A2 in Transacylation. Biology 2017, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rönkkö, S.; Rekonen, P.; Kaarniranta, K.; Puustjarvi, T.; Teräsvirta, M.; Uusitalo, H. Phospholipase A2 in chamber angle of normal eyes and patients with primary open angle glaucoma and exfoliation glaucoma. Mol. Vis. 2007, 13, 408–417. [Google Scholar] [PubMed]
- Helin, M.; Rönkkö, S.; Puustjärvi, T.; Teräsvirta, M.; Uusitalo, H. Phospholipases A2 in normal human conjunctiva and from patients with primary open-angle glaucoma and exfoliation glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 739–746. [Google Scholar] [CrossRef]
- Rosenbaum, J.T.; Boney, R.S.; Samples, J.R.; Valone, F.H. Synthesis of Platelet Activating Factor by Ocular Tissue From Inflamed Eyes. Arch. Ophthalmol. 1991, 109, 410–413. [Google Scholar] [CrossRef] [Green Version]
- Bazan, N.G.; Allan, G. Signal transduction and gene expression in the eye: A contemporary view of the pro-inflammatory, anti-inflammatory and modulatory roles of prostaglandins and other bioactive lipids. Surv. Ophthalmol. 1997, 41, S23–S34. [Google Scholar] [CrossRef]
- Jager, G.; Van Delft, J.; Van Haeringen, N.; Verbeij, N.; Braquet, P. Antagonist of platelet-activating factor prevents prostaglandin E2 induced ocular hypertension in rabbits. Prostaglandins 1993, 45, 97–105. [Google Scholar] [CrossRef]
- Marcheselli, V.L.; Bazan, N.G. Platelet-Activating-Factor (Paf) Enhances Glutamic-Acid Release in the Retina through a Presynaptic Receptor. Investig. Ophth. Vis. Sci. 1993, 34, 1048. [Google Scholar]
- Moschos, M.M.; Nitoda, E.; Chatziralli, I.P.; Panos, G.D.; Demopoulos, C.A. Impact of prostaglandin glaucoma drops on platelet-activating factor action: An in vitro study. Drug Des. Dev. Ther. 2016, 10, 3977–3981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.; Corson, T.W. Soluble Epoxide Hydrolase Inhibition for Ocular Diseases: Vision for the Future. Front. Pharmacol. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
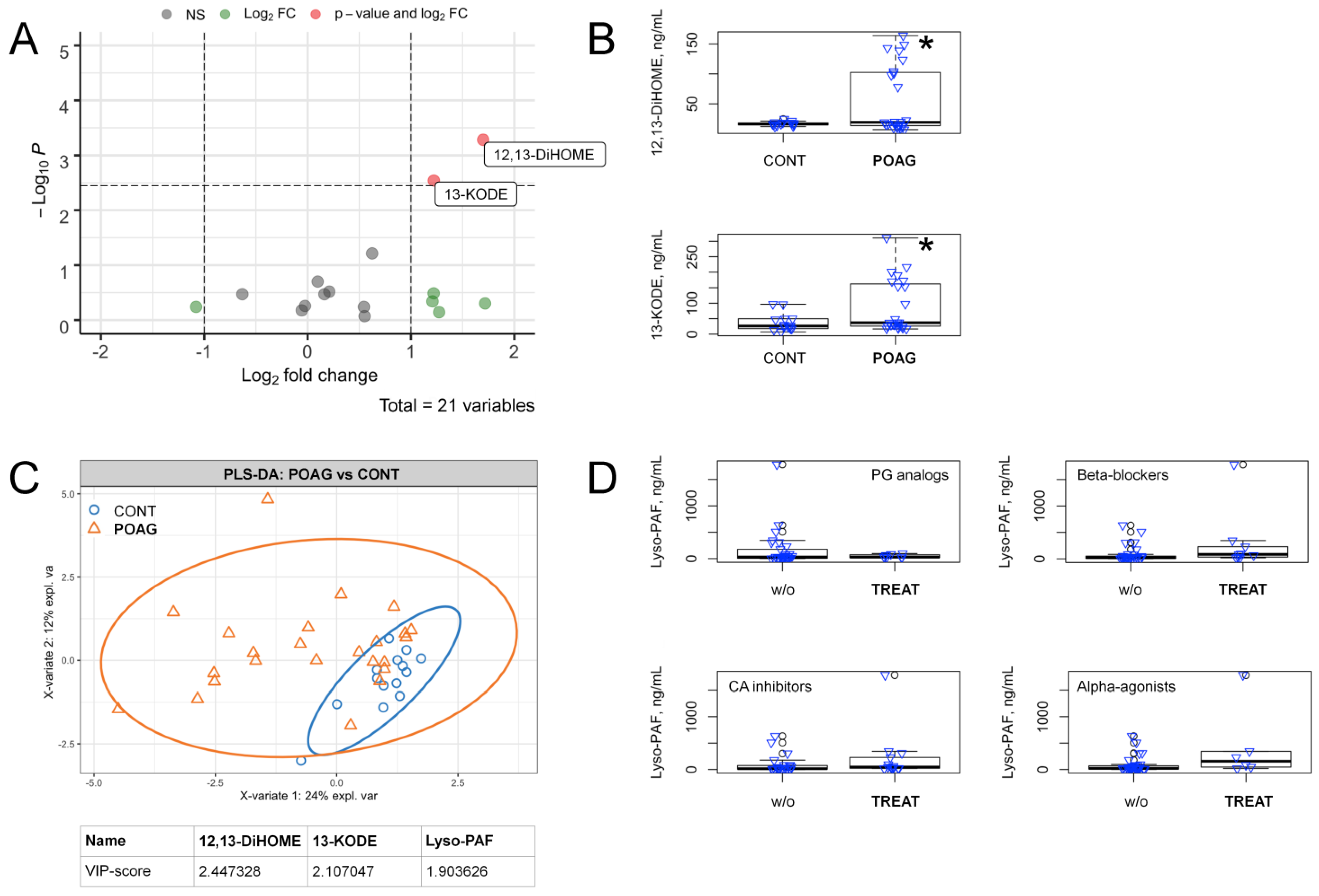



| Parameter | Control Group | Stage-Dependent POAG Subgroups (IOP) | Total POAG Group | ||||
|---|---|---|---|---|---|---|---|
| IIA (≤21 mmHg) | IIB (22–28 mHg) | IIIB (22–28 mHg) | IIIC (≥29 mmHg) | ||||
| Number of partici | 14 | 3 | 7 | 8 | 6 | 24 | |
| Mean age ± SD 1, years | 65.64 ± 13.77 | 67.0 ±2.65 | 68.86 ± 4.18 | 71.62 ± 6.8 | 63.33 ± 6.65 | 68.17 ± 9.35 | |
| Gender, % | Male | 21.43 | 0.0 | 14.29 | 50.0 | 33.33 | 29.17 |
| Female | 78.57 | 100.0 | 85.71 | 50.0 | 66.67 | 70.83 | |
| IOP ± SD, mmHg | 14.9 ± 1.8 | 17.7 ± 1.7 | 24.7 ± 2.1 | 25.5 ± 1.8 | 33.5 ± 3.4 | 26.3 ± 1.9 | |
| Cup/disc ratio | 0.54 ± 0.12 | 0.73 ± 0.05 | 0.71 ± 0.04 | 0.84 ± 0.09 | 0.85 ± 0.06 | 0.79 ± 0.06 | |
| Refr., % | Myopia | 42.86 | 33.33 | 42.86 | 12.5 | 16.67 | 25.00 |
| Comorbidities, % | HTN 2 | 42.86 | 66.67 | 42.86 | 50.0 | 50.0 | 50.00 |
| CHD 3 | 14.29 | 33.33 | 28.57 | 75.0 | 0 | 37.50 | |
| DM 4 | 7.14 | 0 | 28.57 | 0 | 16.67 | 12.50 | |
| Treatment, % | PG analogs | 0 | 0 | 0 | 62.5 | 50.0 | 33.33 |
| β-blockers | 0 | 100 | 57.14 | 37.5 | 33.33 | 37.50 | |
| CAI 5 | 0 | 100 | 71.43 | 50.0 | 66.67 | 54.16 | |
| α-agonists | 0 | 0 | 42.86 | 12.5 | 33.33 | 25.00 | |
| # | Name | Short Name | Precursor | Pathway |
|---|---|---|---|---|
| PUFAs | ||||
| 1 | Arachidonic acid | AA | Phospholipids | PLA2 |
| 2 | Docosahexaenoic acid | DHA | Diet, EPA | - |
| 3 | Eicosapentaenoic acid | EPA | Diet, ALA | - |
| Oxylipins | ||||
| 4 | Leukotriene B4 | LTB4 | AA | LOX |
| 5 | 20-carboxy-leukotriene B4 | 20-carboxy-LTB4 | AA | LOX |
| 6 | 19-hydroxyeicosatetraenoic acid | 19-HETE | AA | LOX |
| 7 | 20-hydroxyeicosatetraenoic acid | 20-HETE | AA | LOX |
| 8 | Prostaglandin A2 | PGA2 | AA | COX |
| 9 | Prostaglandin J2 | PGJ2 | AA | COX |
| 10 | Thromboxane B3 | TXB3 | AA | COX |
| 11 | 9-hydroxyoctadecadienoic acid | 9-HODE | LA | LOX |
| 12 | 9-oxo-octadecadienoic acid | 9-KODE | LA | LOX |
| 13 | 13-hydroxyoctadecadienoic acid | 13-HODE | LA | LOX |
| 14 | 13-oxo-octadecadienoic acid | 13-KODE | LA | LOX |
| 15 | 9,10-dihydroxyoctadecamonoenoic acid | 9,10-DiHOME | LA | CYP |
| 16 | 9,10-epoxyoctadecamonoenoic acid | 9,10-EpOME | LA | CYP |
| 17 | 12,13-dihydroxyoctadecamonoenoic acid | 12,13-DiHOME | LA | CYP |
| 18 | 12,13-epoxyoctadecamonoenoic acid | 12,13-EpOME | LA | CYP |
| 19 | 9-hydroxyoctadecatrienoic acid | 9-HOTrE | ALA | LOX |
| 20 | 13-hydroxyoctadecatrienoic acid | 13-HOTrE | ALA | LOX |
| Phospholipid Derivatives | ||||
| 21 | Lyso-platelet-activating factor | Lyso-PAF | Phospholipids | PLA2 |
| 22 | Oleoylethanolamine | OEA | Phospholipids | NAPE-PLD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azbukina, N.V.; Chistyakov, D.V.; Goriainov, S.V.; Kotelin, V.I.; Fedoseeva, E.V.; Petrov, S.Y.; Sergeeva, M.G.; Iomdina, E.N.; Zernii, E.Y. Targeted Lipidomic Analysis of Aqueous Humor Reveals Signaling Lipid-Mediated Pathways in Primary Open-Angle Glaucoma. Biology 2021, 10, 658. https://doi.org/10.3390/biology10070658
Azbukina NV, Chistyakov DV, Goriainov SV, Kotelin VI, Fedoseeva EV, Petrov SY, Sergeeva MG, Iomdina EN, Zernii EY. Targeted Lipidomic Analysis of Aqueous Humor Reveals Signaling Lipid-Mediated Pathways in Primary Open-Angle Glaucoma. Biology. 2021; 10(7):658. https://doi.org/10.3390/biology10070658
Chicago/Turabian StyleAzbukina, Nadezhda V., Dmitry V. Chistyakov, Sergei V. Goriainov, Vladislav I. Kotelin, Elena V. Fedoseeva, Sergey Yu. Petrov, Marina G. Sergeeva, Elena N. Iomdina, and Evgeni Yu. Zernii. 2021. "Targeted Lipidomic Analysis of Aqueous Humor Reveals Signaling Lipid-Mediated Pathways in Primary Open-Angle Glaucoma" Biology 10, no. 7: 658. https://doi.org/10.3390/biology10070658
APA StyleAzbukina, N. V., Chistyakov, D. V., Goriainov, S. V., Kotelin, V. I., Fedoseeva, E. V., Petrov, S. Y., Sergeeva, M. G., Iomdina, E. N., & Zernii, E. Y. (2021). Targeted Lipidomic Analysis of Aqueous Humor Reveals Signaling Lipid-Mediated Pathways in Primary Open-Angle Glaucoma. Biology, 10(7), 658. https://doi.org/10.3390/biology10070658






