Effects of Extender Type, Storage Time, and Temperature on Bull Semen Parameters
Abstract
Simple Summary
Abstract
1. Introduction
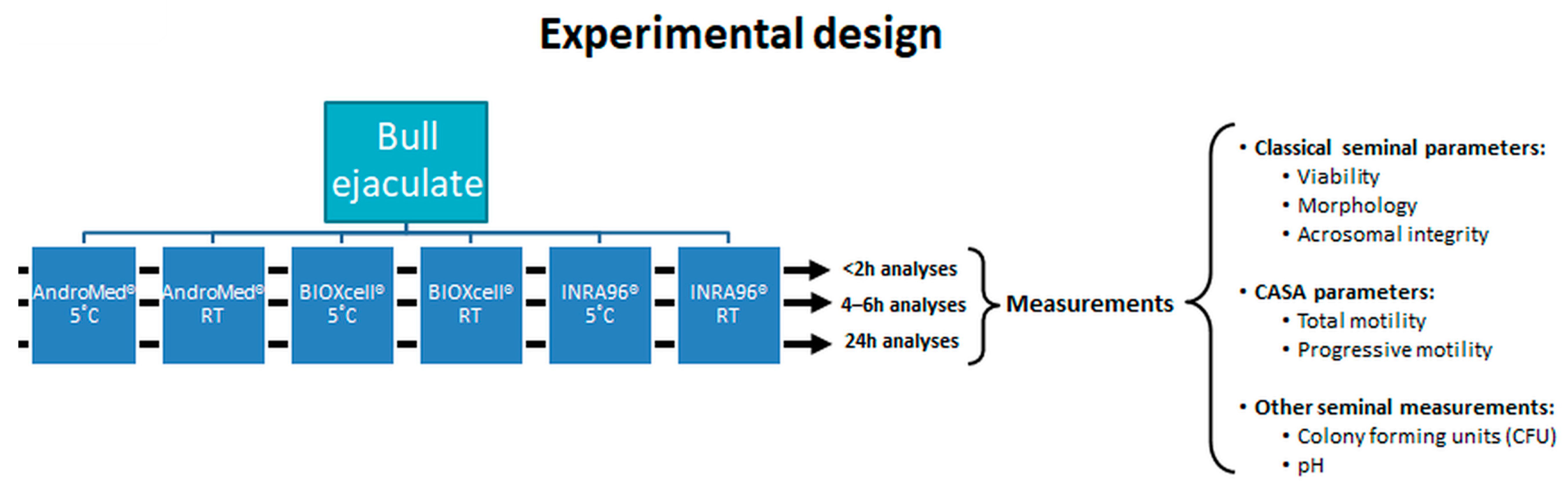
2. Material and Methods
2.1. Animals and Herds
2.2. Ejaculate Collection
2.3. Semen Storage Conditions
2.4. Semen Quality Assessment
2.5. Statistical Analysis
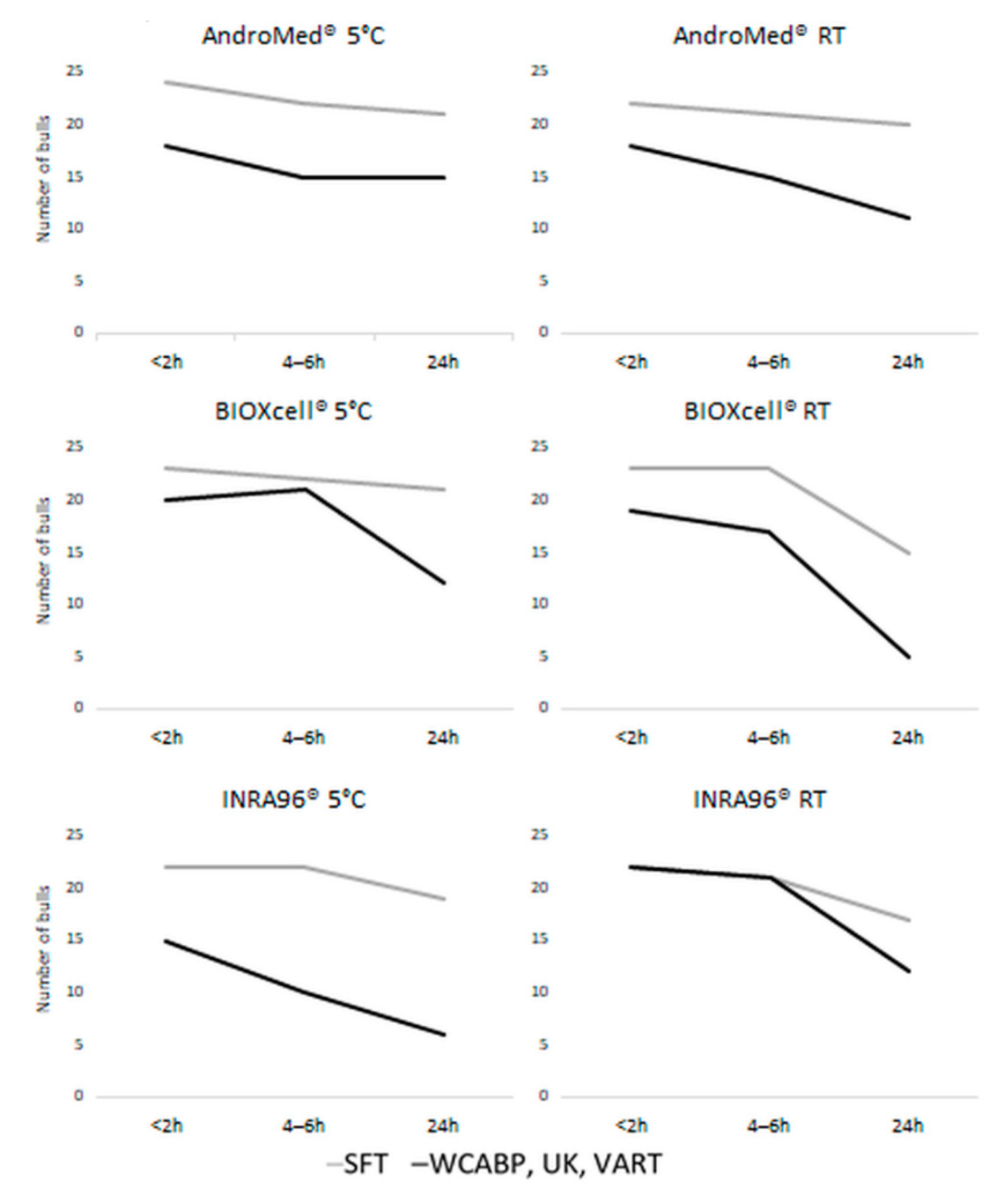
3. Results
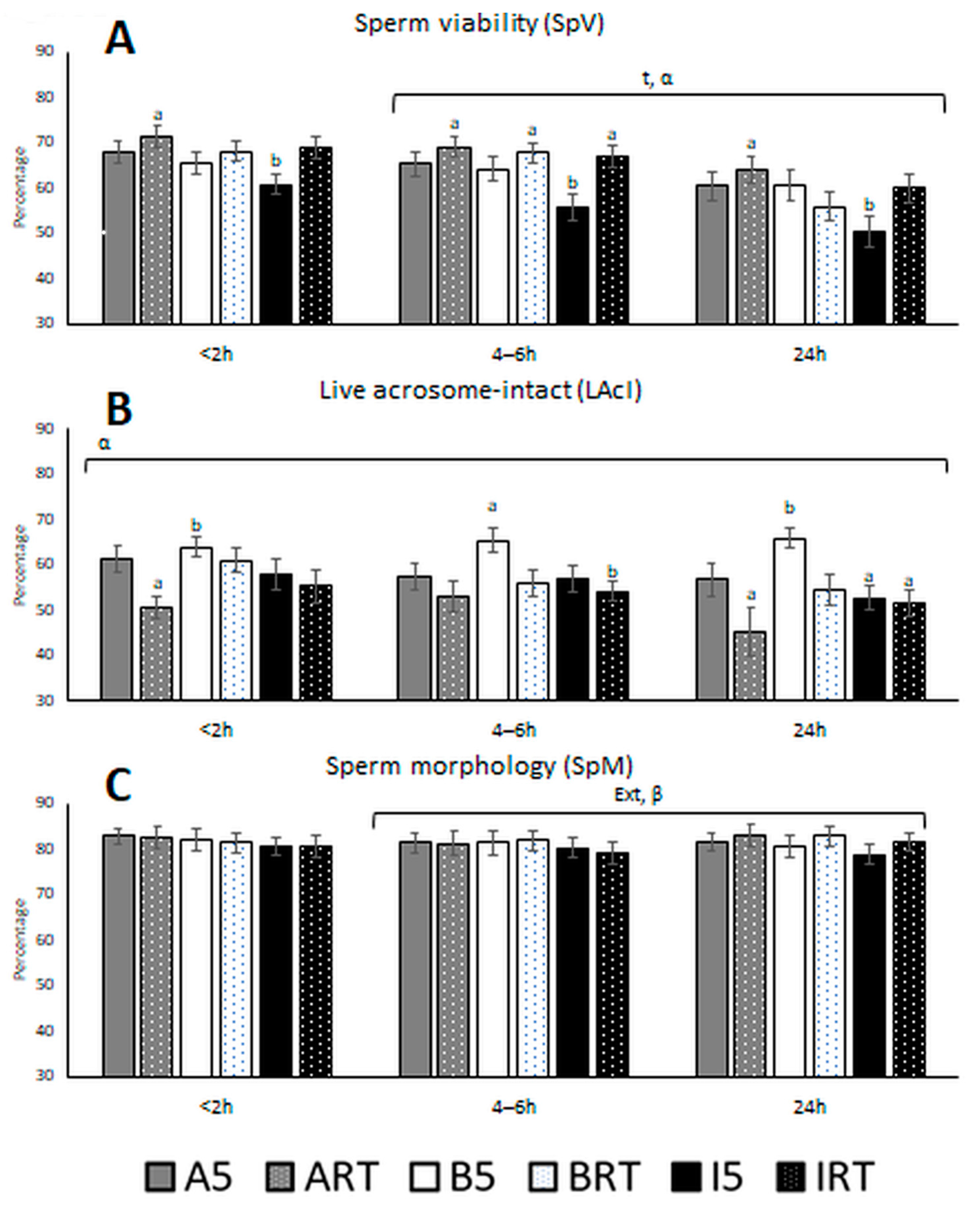
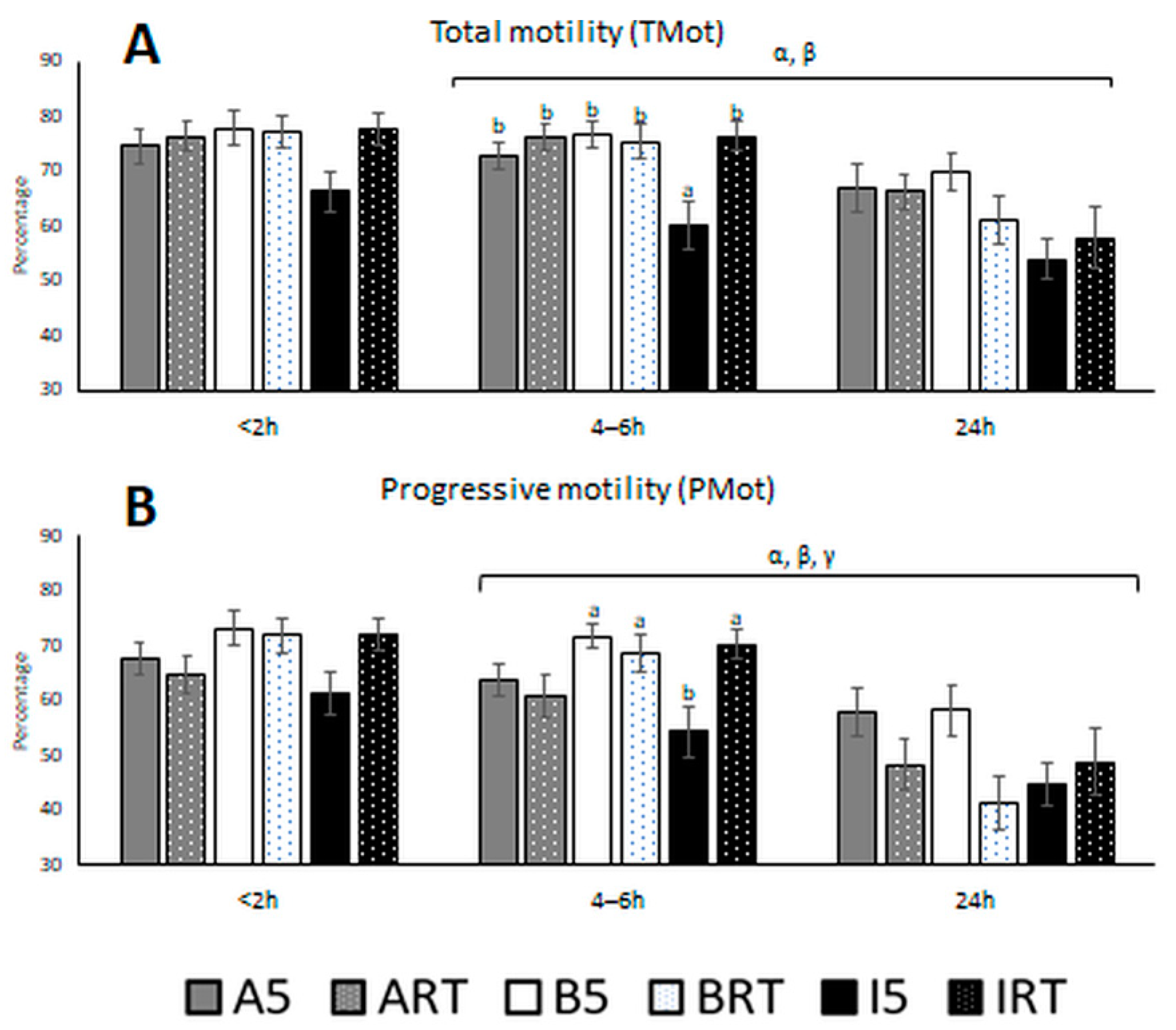
3.1. Semen Quality Based on Classical and Motility Parameters
3.2. Correlations of Semen Parameters with One Another and with Themselves over Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foote, R.H. Fertility estimation: A review of past experience and future prospects. Anim. Reprod. Sci. 2003, 75, 119–139. [Google Scholar] [CrossRef]
- Kastelic, J.P. Understanding and evaluating bovine testes. Theriogenology 2014, 81, 18–23. [Google Scholar] [CrossRef]
- Kastelic, J.P.; Cook, R.B.; Coulter, G.H. Scrotal/testicular thermoregulation and the effects of increased testicular temperature in the bull. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 271–282. [Google Scholar] [CrossRef]
- Kastelic, J.P.; Thundathil, J.C. Breeding Soundness Evaluation and Semen Analysis for Predicting Bull Fertility. Reprod. Domest. Anim. 2008, 43, 368–373. [Google Scholar] [CrossRef]
- Godfrey, R.W.; Dodson, R.E. Breeding soundness evaluations of Senepol bulls in the US Virgin Islands. Theriogenology 2005, 63, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, G.; Entwistle, K.; Norman, S.; Perry, V.; Gardiner, B.; Fordyce, P. Standardising bull breeding soundness evaluations and reporting in Australia. Theriogenology 2006, 66, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Felton-Taylor, J.; Prosser, K.A.; Hernandez-Medrano, J.H.; Gentili, S.; Copping, K.J.; Macrossan, P.E.; Perry, V.E.A. Effect of breed, age, season and region on sperm morphology in 11,387 bulls submitted to breeding soundness evaluation in Australia. Theriogenology 2020, 142, 1–7. [Google Scholar] [CrossRef]
- Wiltbank, J.N.; Parish, N.R. Pregnancy rate in cows and heifers bred to bulls selected for semen quality. Theriogenology 1986, 25, 779–783. [Google Scholar] [CrossRef]
- Hopkins, F.M.; Spitzer, J.C. The new Society for Theriogenology breeding soundness evaluation system. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 283–293. [Google Scholar] [CrossRef]
- Barth, A.D. Bull Breeding Soundness Evaluation Manual, 2nd ed.; The Western Canadian Association of Bovine Practitioners: Saskatoon, SK, Canada, 2000. [Google Scholar]
- Entwistle, K.; Fordyce, G. Evaluating and Reporting Bull Fertility, 1st ed.; Australian Cattle Vets: Brisbane, Australia, 2003. [Google Scholar]
- Irons, P.C.; Nöthling, J.O.; Bertschinger, H.J. Bull breeding soundness evaluation in Southern Africa. Theriogenology 2007, 68, 842–847. [Google Scholar] [CrossRef][Green Version]
- Penny, C. Examination of Bulls for Breding Soundness. An Illustrated Guide. In Proceedings of the International Bull Fertility Conference—Theory to Practice, Westport, Ireland, 27–30 May 2018; pp. 1–66. [Google Scholar]
- Kathiravan, P.; Kalatharan, J.; Karthikeya, G.; Rengarajan, K.; Kadirvel, G. Objective Sperm Motion Analysis to Assess Dairy Bull Fertility Using Computer-Aided System—A Review. Reprod. Domest. Anim. 2011, 46, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Batellier, F.; Vidament, M.; Fauquant, J.; Duchamp, G.; Arnaud, G.; Yvon, J.M.; Magistrini, M. Advances in cooled semen technology. Anim. Reprod. Sci. 2001, 68, 181–190. [Google Scholar] [CrossRef]
- Fitzpatrick, L.A.; Fordyce, G.; McGowan, M.R.; Bertram, J.D.; Doogan, V.J.; De Faveri, J.; Miller, R.G.; Holroyd, R.G. Bull selection and use in northern Australia. Anim. Reprod. Sci. 2002, 71, 39–49. [Google Scholar] [CrossRef]
- Holroyd, R.G.; Doogan, V.J.; De Faveri, J.; Fordyce, G.; McGowan, M.R.; Bertram, J.D.; Vankan, D.M.; Fitzpatrick, L.A.; Jayawardhana, G.A.; Miller, R.G. Bull selection and use in northern Australia. 4. Calf output and predictors of fertility of bulls in multiple-sire herds. Anim. Reprod. Sci. 2002, 71, 67–79. [Google Scholar] [CrossRef]
- Farrell, P.B.; Presicce, G.A.; Brockett, C.C.; Foote, R.H. Quantification of bull sperm characteristics measured by computer-assisted sperm analysis (CASA) and the relationship to fertility. Theriogenology 1998, 49, 871–879. [Google Scholar] [CrossRef]
- Christensen, P.; No, P.B.B.; Lehn-Jensen, H. The Relationship between Semen Quality and the Nonreturn Rate of Bulls. Reprod. Domest. Anim. 1999, 34, 503–507. [Google Scholar] [CrossRef]
- García-Paloma, J.A.; Pérez Garnelo, S.S.; Montoya Monsalve, G.; Astiz Blanco, S. Aptitud reproductiva en toros de monta natural. II. Valoración física, colecta y valoración seminal. Bol. ANEMBE 2017, 115, 17–36. [Google Scholar]
- Reist-Marti, S.B.; Abdulai, A.; Simianer, H. Conservation programmes for African cattle: Design, cost and benefits. J. Anim. Breed. Genet. 2005, 122, 95–109. [Google Scholar] [CrossRef]
- Murphy, E.M.; Murphy, C.; O’Meara, C.; Dunne, G.; Eivers, B.; Lonergan, P.; Fair, S. A comparison of semen diluents on the in vitro and in vivo fertility of liquid bull semen. J. Dairy Sci. 2017, 100, 1541–1554. [Google Scholar] [CrossRef]
- Murphy, E.M.; O’ Meara, C.; Eivers, B.; Lonergan, P.; Fair, S. Optimizing storage temperature of liquid bovine semen diluted in INRA96. J. Dairy Sci. 2018, 101, 5549–5558. [Google Scholar] [CrossRef]
- Landaeta-Hernández, A.J.; Yelich, J.V.; Lemaster, J.W.; Fields, M.J.; Tran, T.; Chase, C.C.; Rae, D.O.; Chenoweth, P.J. Environmental, genetic and social factors affecting the expression of estrus in beef cows. Theriogenology 2002, 57, 1357–1370. [Google Scholar] [CrossRef]
- Layek, S.S.; Mohanty, T.K.; Kumaresan, A.; Parks, J.E. Cryopreservation of bull semen: Evolution from egg yolk based to soybean based extenders. Anim. Reprod. Sci. 2016, 172, 1–9. [Google Scholar] [CrossRef]
- Papa, P.M. Effect of glycerol on the viability and fertility of cooled bovine semen. Theriogenology 2015, 83, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.M.O.; Forell, F.; Oliveira, A.T.D.; Rodrigues, J.L. Current status of sperm cryopreservation: Why isn’t it better? Theriogenology 2002, 57, 327–344. [Google Scholar] [CrossRef]
- van Wagtendonk-de Leeuw, A.M.; Haring, R.M.; Kaal-Lansbergen, L.M.T.E.; den Daas, J.H.G. Fertility results using bovine semen cryopreserved with extenders based on egg yolk and soy bean extract. Theriogenology 2000, 54, 57–67. [Google Scholar] [CrossRef]
- Lonergan, P. Review: Historical and futuristic developments in bovine semen technology. Animal 2018, 12, s4–s18. [Google Scholar] [CrossRef]
- AndroMed® | Minitube. Available online: https://www.minitube.com/catalog/es/andromed-p4722/ (accessed on 28 June 2021).
- BioXCell. Available online: https://www.imv-technologies.es/producto/bioxcell (accessed on 28 June 2021).
- IMV Technologies INRA 96. Available online: https://www.imv-technologies.es/producto/inra (accessed on 28 June 2021).
- Tamuli, M.K.; Watson, P.F. Use of a simple staining technique to distinguish acrosomal changes in the live sperm sub-population. Anim. Reprod. Sci. 1994, 35, 247–254. [Google Scholar] [CrossRef]
- Pintado, B.; de la Fuente, J.; Roldan, E.R. Permeability of boar and bull spermatozoa to the nucleic acid stains propidium iodide or Hoechst 33258, or to eosin: Accuracy in the assessment of cell viability. J. Reprod. Fertil. 2000, 118, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, R.; Shannon, P. Storage of bovine semen in liquid and frozen state. Anim. Reprod. Sci. 2000, 62, 23–53. [Google Scholar] [CrossRef]
- Jiménez-Rabadán, P.; Ramón, M.; García-Álvarez, O.; Maroto-Morales, A.; del Olmo, E.; Pérez-Guzmán, M.D.; Bisbal, A.; Fernández-Santos, M.R.; Garde, J.J.; Soler, A.J. Effect of semen collection method (artificial vagina vs. electroejaculation), extender and centrifugation on post-thaw sperm quality of Blanca-Celtibérica buck ejaculates. Anim. Reprod. Sci. 2012, 132, 88–95. [Google Scholar] [CrossRef]
- Muiño, R.; Peña, A.I.; Rodríguez, A.; Tamargo, C.; Hidalgo, C.O. Effects of cryopreservation on the motile sperm subpopulations in semen from Asturiana de los Valles bulls. Theriogenology 2009, 72, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Aires, V.A.; Hinsch, K.-D.; Mueller-Schloesser, F.; Bogner, K.; Mueller-Schloesser, S.; Hinsch, E. In vitro and in vivo comparison of egg yolk-based and soybean lecithin-based extenders for cryopreservation of bovine semen. Theriogenology 2003, 60, 269–279. [Google Scholar] [CrossRef]
- Thun, R.; Hurtado, M.; Janett, F. Comparison of Biociphos-Plus® and TRIS-egg yolk extender for cryopreservation of bull semen. Theriogenology 2002, 57, 1087–1094. [Google Scholar] [CrossRef]
- Crespilho, A.M.; Nichi, M.; Guasti, P.N.; Freitas-Dell’Aqua, C.P.; Sá Filho, M.F.; Maziero, R.R.; Dell’Aqua, J.A.; Papa, F.O. Sperm fertility and viability following 48h of refrigeration: Evaluation of different extenders for the preservation of bull semen in liquid state. Anim. Reprod. Sci. 2014, 146, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P. The effect of diluents containing glycine, and glycine and glycerol, on the fertility of diluted bovine semen. N. Z. J. Agric. Res. 1964, 7, 357–363. [Google Scholar] [CrossRef]
- Rehman, F.; Zhao, C.; Shah, M.A.; Qureshi, M.S.; Wang, X. Semen Extenders and Artificial Insemination in Ruminants. Available online: http://thesciencepublishers.com/veterinaria/files/201308142-RV%20(1).pdf (accessed on 28 April 2021).
- Krzyzosiak, J.; Molan, P.; McGowan, L.; Vishwanath, R. Effect of sperm number and oxygenation state of the storage media on in vitro fertility of bovine sperm stored at ambient temperature. Theriogenology 2001, 55, 1401–1415. [Google Scholar] [CrossRef]
- Murphy, E.M.; Eivers, B.; O’Meara, C.M.; Lonergan, P.; Fair, S. Effect of storage temperature, nitrogen gassing and sperm concentration on the in vitro semen quality and in vivo fertility of liquid bull semen stored in INRA96. Theriogenology 2018, 108, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Al Naib, A.; Ward, F.; Kelly, A.K.; Wade, M.; Marti, J.I.; Lonergan, P. Effect of duration of storage at ambient temperature on fertilizing ability and mucus penetration ability of fresh bovine sperm. Theriogenology 2011, 76, 1070–1075. [Google Scholar] [CrossRef]
- Kumaresan, A.; Johannisson, A.; Al-Essawe, E.M.; Morrell, J.M. Sperm viability, reactive oxygen species, and DNA fragmentation index combined can discriminate between above- and below-average fertility bulls. J. Dairy Sci. 2017, 100, 5824–5836. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.; Boelling, D.; Pedersen, K.M.; Korsgaard, I.R.; Jensen, J. Relationship between sperm viability as determined by flow cytometry and nonreturn rate of dairy bulls. J. Androl. 2005, 26, 98–106. [Google Scholar]
- Christensen, P.; Labouriau, R.; Birck, A.; Boe-Hansen, G.B.; Pedersen, J.; Borchersen, S. Relationship among seminal quality measures and field fertility of young dairy bulls using low-dose inseminations. J. Dairy Sci. 2011, 94, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Jimenez, S.; Rivera del Alamo, M.M.; Álvarez-Rodríguez, M.; Hidalgo, C.O.; Peña, A.I.; Muiño, R.; Rodríguez-Gil, J.E.; Mogas, T. In vitro assessment of egg yolk-, soya bean lecithin- and liposome-based extenders for cryopreservation of dairy bull semen. Anim. Reprod. Sci. 2020, 215, 106315. [Google Scholar] [CrossRef]
- Stradaioli, G.; Noro, T.; Sylla, L.; Monaci, M. Decrease in glutathione (GSH) content in bovine sperm after cryopreservation: Comparison between two extenders. Theriogenology 2007, 67, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, T.; Lymberopoulos, A.; Theodosiadou, E. Association of soybean-based extenders with field fertility of stored ram (Ovis aries) semen: A randomized double-blind parallel group design. Theriogenology 2013, 79, 517–527. [Google Scholar] [CrossRef]
- Lloyd, R.E.; Fazeli, A.; Watson, P.F.; Holt, W.V. The oviducal protein, heat-shock 70-kDa protein 8, improves the long-term survival of ram spermatozoa during storage at 17 °C in a commercial extender. Reprod. Fertil. Dev. 2012, 24, 543. [Google Scholar] [CrossRef] [PubMed]
- Lymberopoulos, A.G.; Khalifa, T.A.A. Sperm Chromatin Stability During In Vitro Manipulation of Beef Bull Semen. Reprod. Domest. Anim. 2010, 45, 307–314. [Google Scholar] [CrossRef]
- Tarig, A.A.; Wahid, H.; Rosnina, Y.; Yimer, N.; Goh, Y.M.; Baiee, F.H.; Khumran, A.M.; Salman, H.; Ebrahimi, M. Effect of different concentrations of egg yolk and virgin coconut oil in Tris-based extenders on chilled and frozen-thawed bull semen. Anim. Reprod. Sci. 2017, 182, 21–27. [Google Scholar] [CrossRef]
- de Paz, P.; Esteso, M.C.; Alvarez, M.; Mata, M.; Chamorro, C.A.; Anel, L. Development of extender based on soybean lecithin for its application in liquid ram semen. Theriogenology 2010, 74, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Valeanu, A.S.; Lundeheim, N.; Johannisson, A. Sperm quality in frozen beef and dairy bull semen. Acta Vet. Scand. 2018, 60, 41. [Google Scholar] [CrossRef]
- Sannat, C.; Nair, A.; Sahu, S.B.; Sahasrabudhe, S.A.; Kumar, A.; Gupta, A.K.; Shende, R.K. Critical sources of bacterial contamination and adoption of standard sanitary protocol during semen collection and processing in Semen Station. Vet. World 2015, 8, 631–635. [Google Scholar] [CrossRef]
- Sannat, C.; Nair, A.; Sahu, S.B.; Sahasrabudhe, S.A.; Kumar, A.; Gupta, A.K.; Shende, R.K. Effect of species, breed, and age on bacterial load in bovine and bubaline semen. Vet. World 2015, 8, 461–466. [Google Scholar] [CrossRef]
- Smole, I.; Thomann, A.; Frey, J.; Perreten, V. Repression of common bull sperm flora and in vitro impairment of sperm motility with Pseudomonas aeruginosa introduced by contaminated lubricant. Reprod. Domest. Anim. 2010, 45, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Reda, A.A.; Almaw, G.; Abreha, S.; Tadeg, W.; Tadesse, B. Bacteriospermia and Sperm Quality of Cryopreserved Bull Semen Used in Artificial Insemination of Cows in South Wollo Zone, Ethiopia. Vet. Med. Int. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Goularte, K.L.; Voloski, F.L.S.; Redú, J.F.M.; Ferreira, C.E.R.; Vieira, A.D.; Duval, E.H.; Mondadori, R.G.; Lucia, T. Antibiotic resistance in microorganisms isolated in a bull semen stud. Reprod. Domest. Anim. 2020, 55, 318–324. [Google Scholar] [CrossRef]
- Patel, H.V.; Patel, R.K.; Chauhan, J.B. Biochemical properties of microbial load in frozen semen of cattle. Wayamba J. Anim. Sci. 2011, 3, 117–121. [Google Scholar]
- Gloria, A.; Contri, A.; Wegher, L.; Vignola, G.; Dellamaria, D.; Carluccio, A. The effects of antibiotic additions to extenders on fresh and frozen–thawed bull semen. Anim. Reprod. Sci. 2014, 150, 15–23. [Google Scholar] [CrossRef]
- Love, C.C. Semen Collection Techniques. Vet. Clin. N. Am. Equine Pract. 1992, 8, 111–128. [Google Scholar] [CrossRef]
- Contri, A.; Gloria, A.; Robbe, D.; Valorz, C.; Wegher, L.; Carluccio, A. Kinematic study on the effect of pH on bull sperm function. Anim. Reprod. Sci. 2013, 136, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Norman, C.; Johnson, C.E.; Porterfield, I.D.; Dunbar, R.S. Effect of pH on the Life-Span and Metabolism of Bovine Sperm Kept at Room Temperatures. J. Dairy Sci. 1958, 41, 1803–1812. [Google Scholar] [CrossRef]
- Gillan, L.; Kroetsch, T.; Chis Maxwell, W.M.; Evans, G. Assessment of in vitro sperm characteristics in relation to fertility in dairy bulls. Anim. Reprod. Sci. 2008, 103, 201–214. [Google Scholar] [CrossRef]
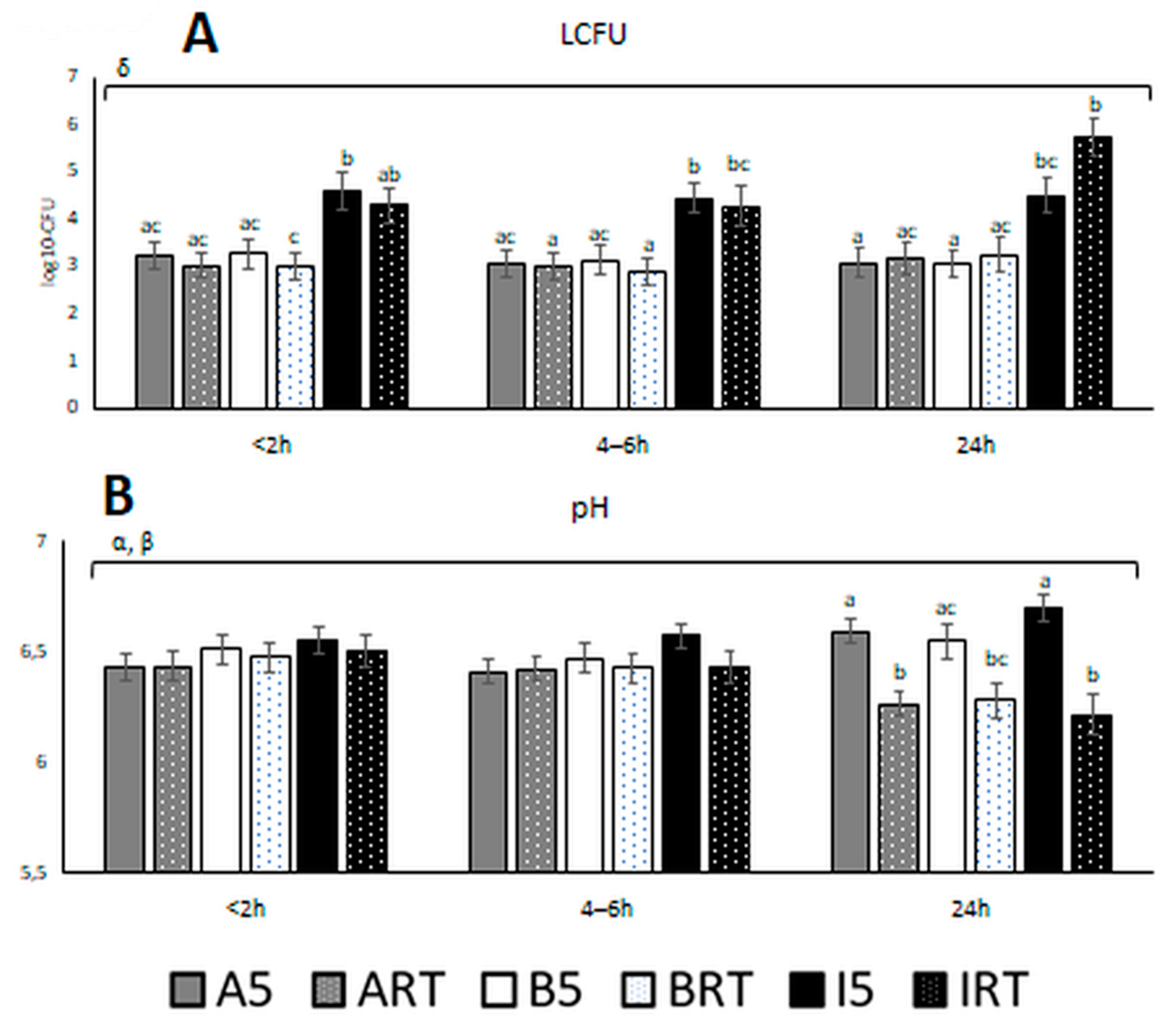
| Extender | <2 h | |
| 5 °C | Room Temperature | |
| AndroMed® | 34,005 a,c ± 68,899 | 19,923 a,c ± 46,652 |
| BIOXcell® | 145,451 a,c ± 597,538 | 121,654 c ± 558,367 |
| INRA96® | 25,466,834 b ± 82,941,641 | 21,824,412 a, b ± 73,257,122 |
| Extender | 4–6 h | |
| 5 °C | Room Temperature | |
| AndroMed® | 23,906 a,c ± 42,218 | 19,366 a ± 56,645 |
| BIOXcell® | 160,113 a,c ± 676,803 | 31,612 a ± 104,373 |
| INRA96® | 5,692,810 b ± 24,774,913 | 26,230,196 b, c ± 99,887,451 |
| Extender | 24 h | |
| 5 °C | Room Temperature | |
| AndroMed® | 60,572 a ± 205,292 | 71,884 a, c ± 151,384 |
| BIOXcell® | 32,566 a ± 83,193 | 101,347 a,c ± 201,840 |
| INRA96® | 13,386,748 b,c ± 49,276,426 | 181,508,58 b ± 488,516,056 |
| Parameters | logCFU-T4 | logCFU-T24 | PMot-T < 2 | PMot-T4 | PMot-T24 | SpV-T < 2 | SpV-T4 | SpV-T24 | SpM-T < 2 | SpM-T4 | SpM-T24 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TMot-T < 2 | 0.947 | 0.707 | 0.481 | 0.607 | 0.688 | 0.605 | 0.524 | 0.518 | 0.472 | ||
| TMot-T4 | 0.909 | 0.517 | 0.69 | 0.62 | 0.518 | 0.485 | |||||
| TMot-T24 | 0.855 | 0.602 | |||||||||
| PMot-T < 2 | 0.512 | 0.622 | 0.544 | 0.533 | 0.538 | 0.472 | |||||
| PMot-T4 | 0.569 | 0.496 | 0.489 | 0.448 | |||||||
| PMot-T24 | 0.467 | ||||||||||
| logCFU-T < 2 | −0.42 | −0.401 | |||||||||
| logCFU-T4 | −0.405 | ||||||||||
| LAcI-T < 2 | 0.433 | 0.411 | |||||||||
| LAcI-T4 | 0.405 | 0.428 | |||||||||
| LAcI-T24 | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Novo, A.; Santos-Lopez, S.; Barrajon-Masa, C.; Mozas, P.; de Mercado, E.; Caceres, E.; Garrafa, A.; Gonzalez-Martin, J.V.; Perez-Villalobos, N.; Oliet, A.; et al. Effects of Extender Type, Storage Time, and Temperature on Bull Semen Parameters. Biology 2021, 10, 630. https://doi.org/10.3390/biology10070630
Fernandez-Novo A, Santos-Lopez S, Barrajon-Masa C, Mozas P, de Mercado E, Caceres E, Garrafa A, Gonzalez-Martin JV, Perez-Villalobos N, Oliet A, et al. Effects of Extender Type, Storage Time, and Temperature on Bull Semen Parameters. Biology. 2021; 10(7):630. https://doi.org/10.3390/biology10070630
Chicago/Turabian StyleFernandez-Novo, Aitor, Sergio Santos-Lopez, Clara Barrajon-Masa, Patricia Mozas, Eduardo de Mercado, Elisa Caceres, Aizic Garrafa, Juan Vicente Gonzalez-Martin, Natividad Perez-Villalobos, Agustin Oliet, and et al. 2021. "Effects of Extender Type, Storage Time, and Temperature on Bull Semen Parameters" Biology 10, no. 7: 630. https://doi.org/10.3390/biology10070630
APA StyleFernandez-Novo, A., Santos-Lopez, S., Barrajon-Masa, C., Mozas, P., de Mercado, E., Caceres, E., Garrafa, A., Gonzalez-Martin, J. V., Perez-Villalobos, N., Oliet, A., Astiz, S., & Perez-Garnelo, S. S. (2021). Effects of Extender Type, Storage Time, and Temperature on Bull Semen Parameters. Biology, 10(7), 630. https://doi.org/10.3390/biology10070630






