Integrin Adhesion Complex Organization in Sheep Myometrium Reflects Changing Mechanical Forces during Pregnancy and Postpartum
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Collection
2.2. Cell Culture
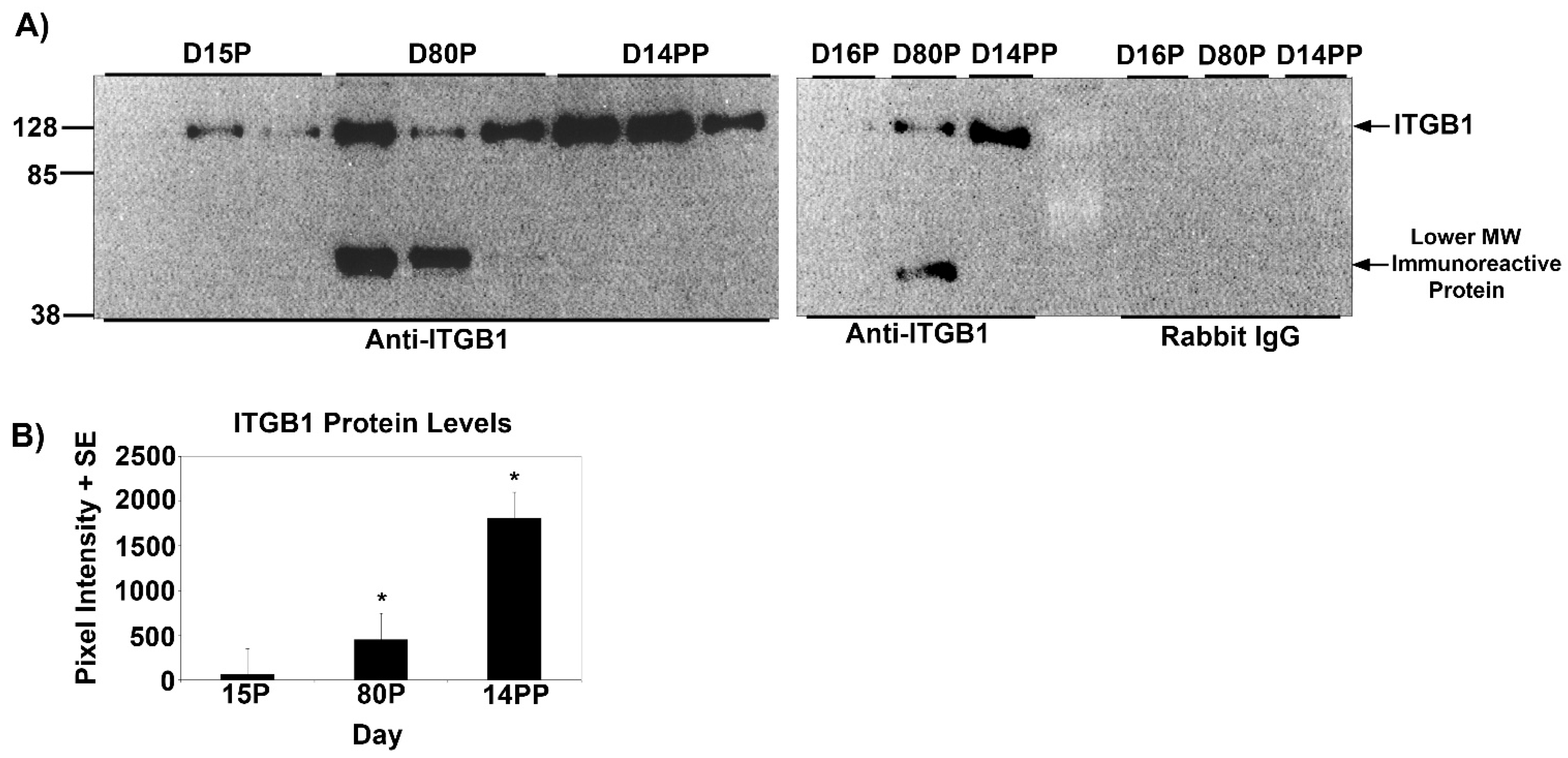
2.3. Western Blot Analyses
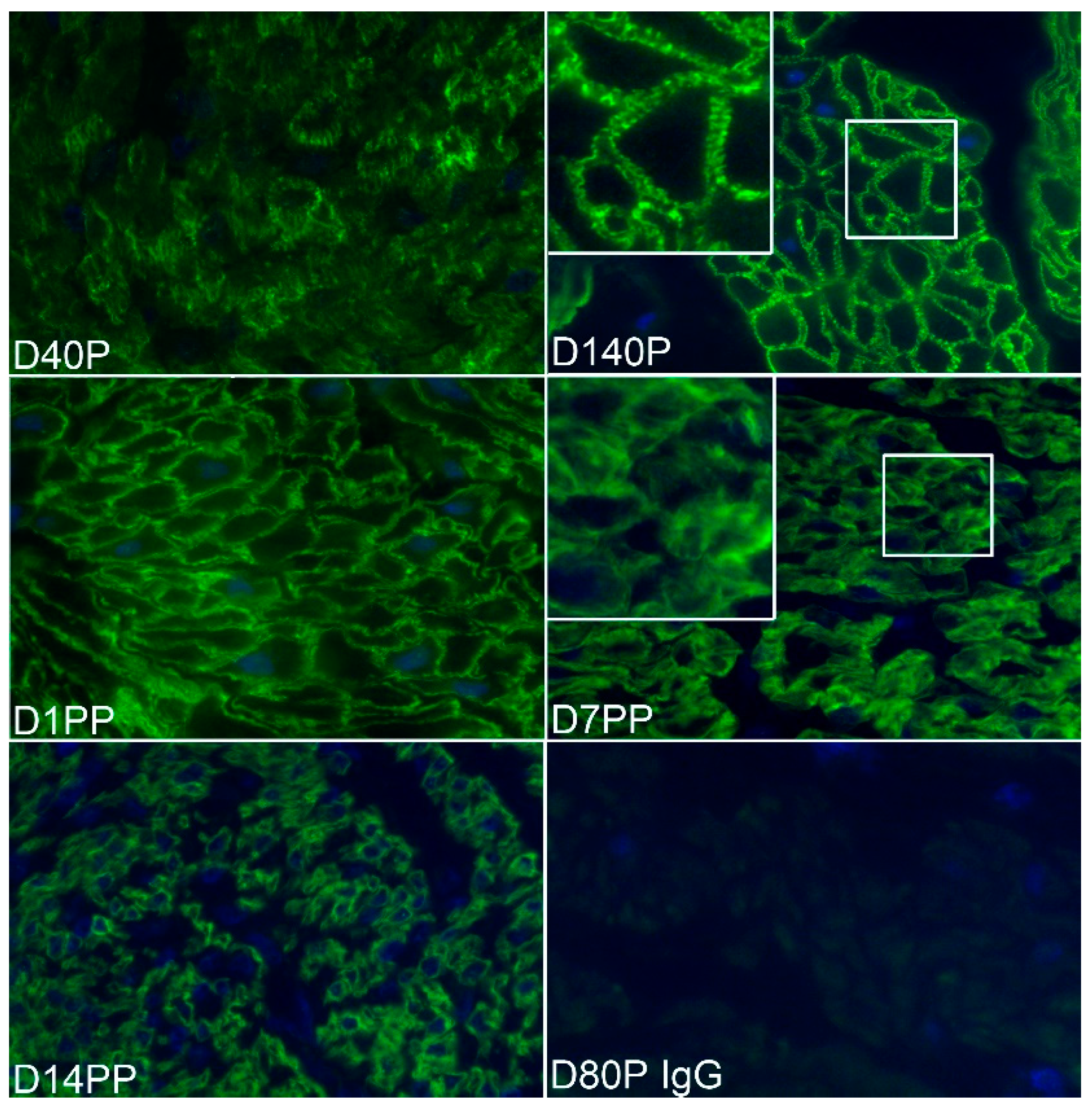
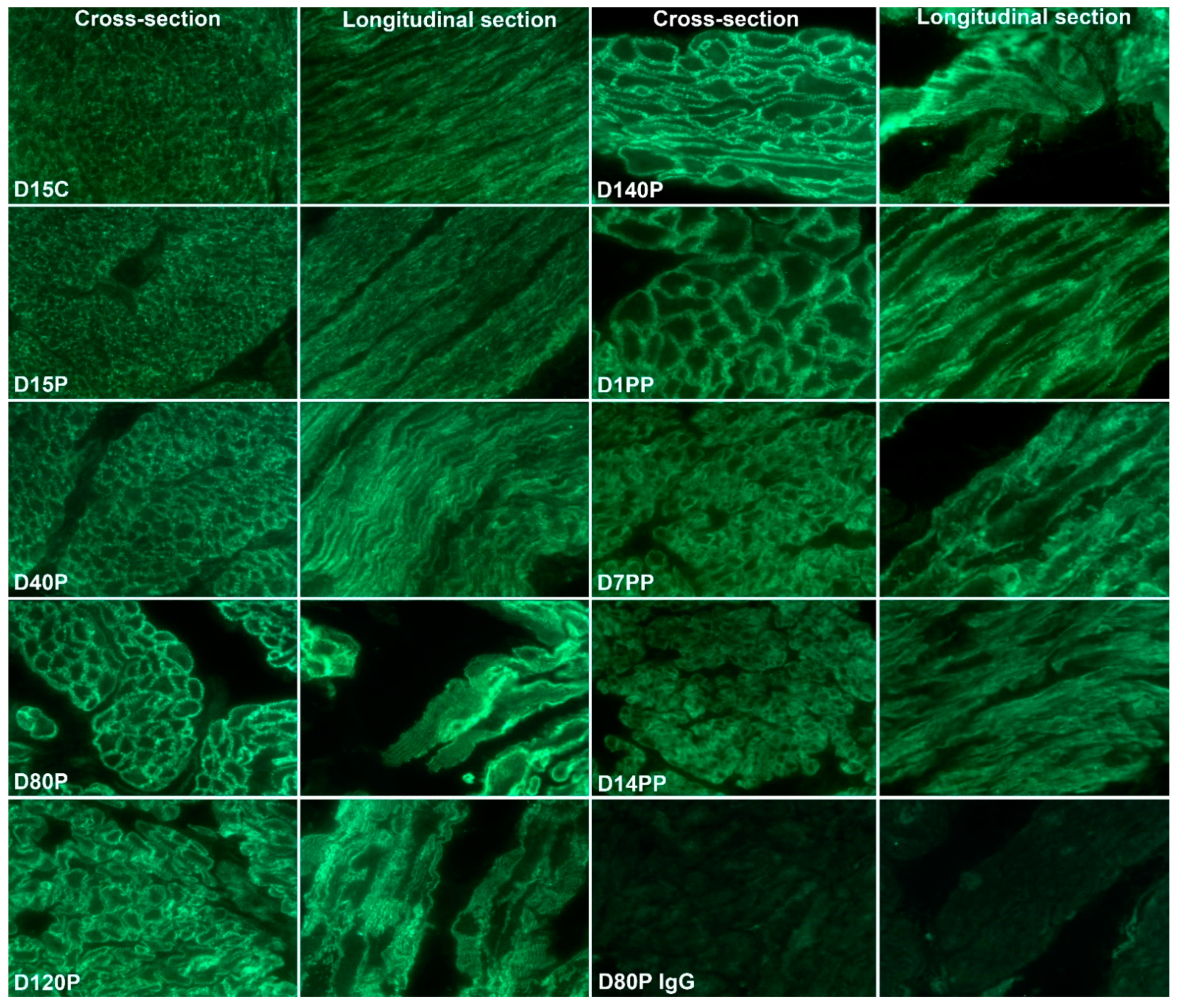
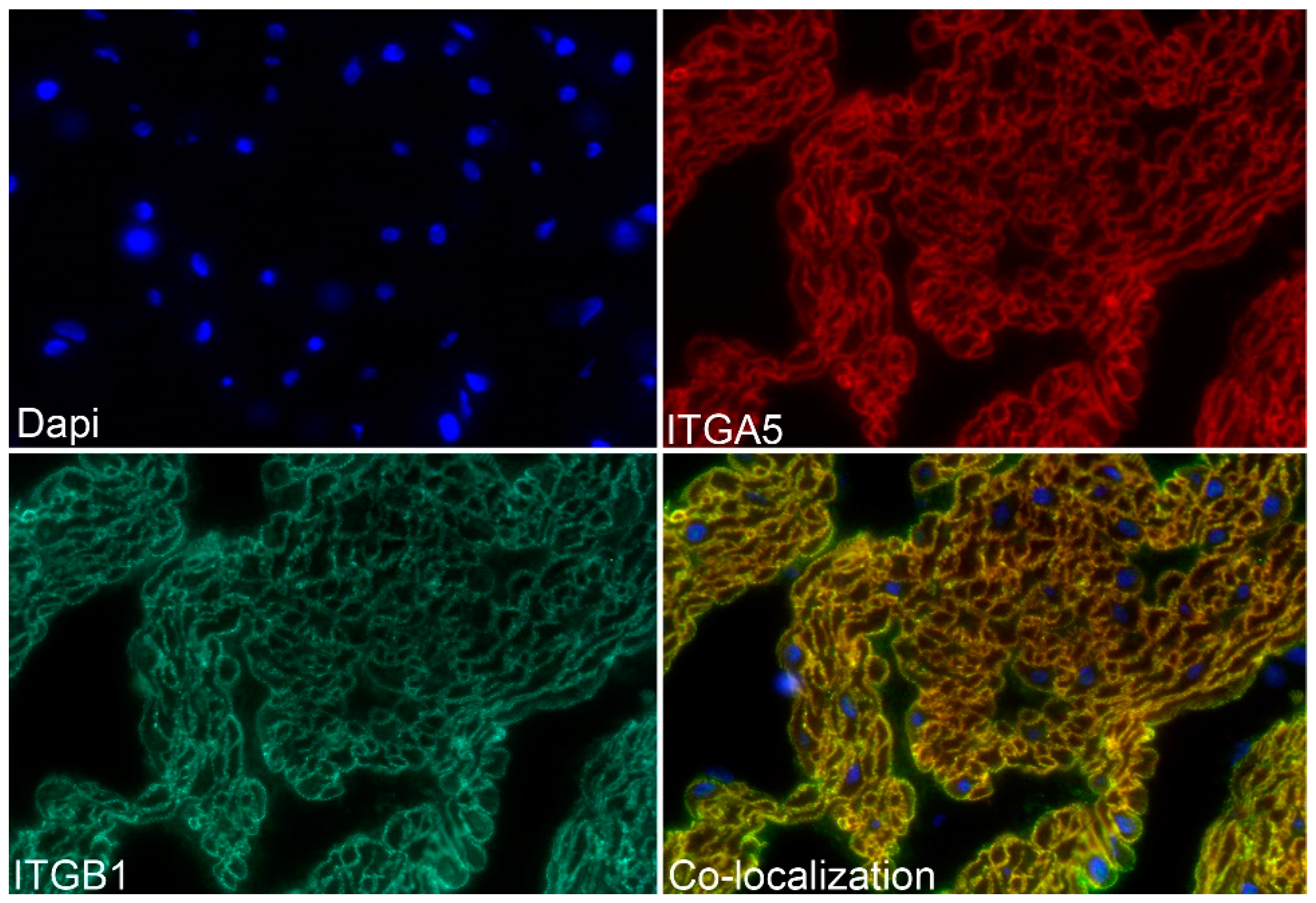
2.4. Immunofluorescence Analyses
2.5. Statistical Analyses
3. Results
3.1. ITGA5 and ITGB1 Proteins Increase in the Myometrium during Mid-Gestation and Are Maintained Postpartum
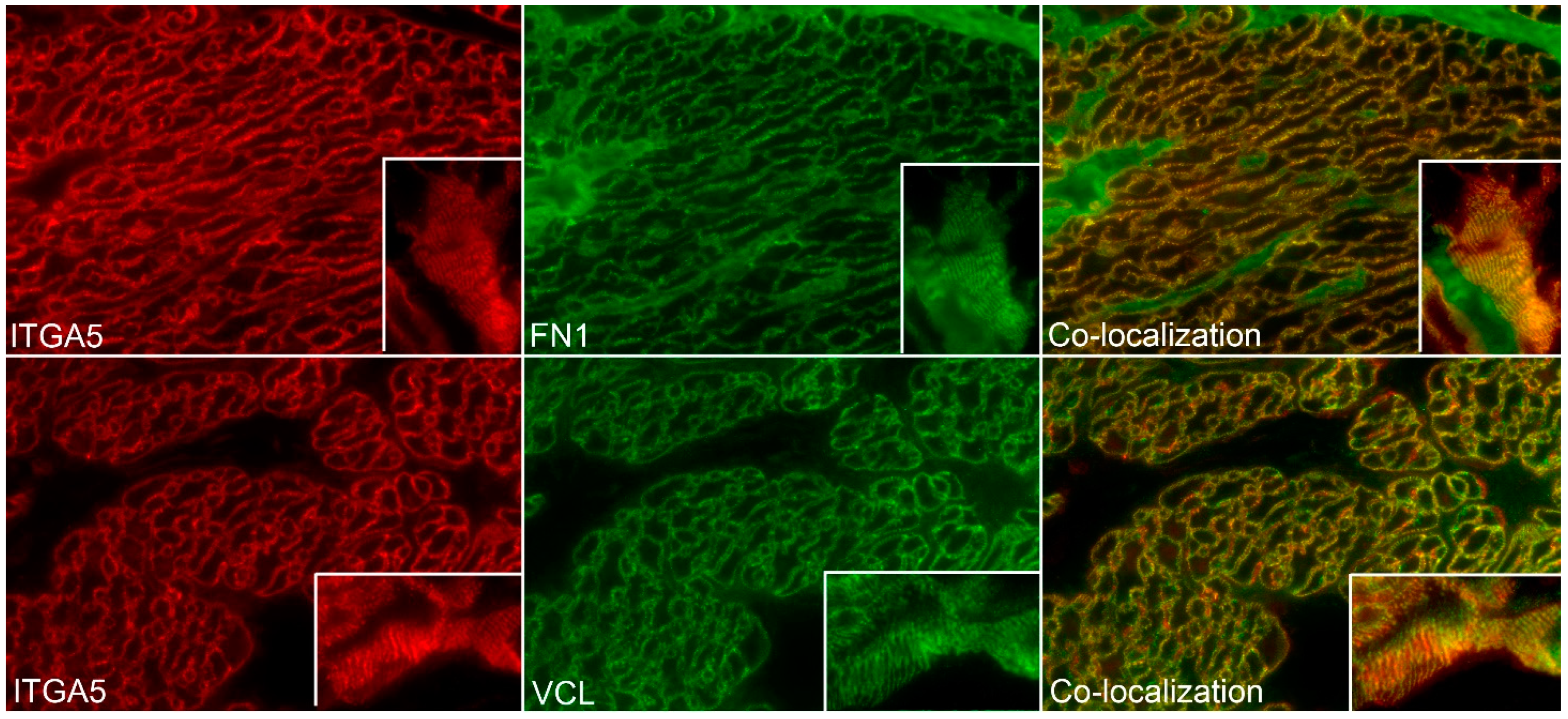
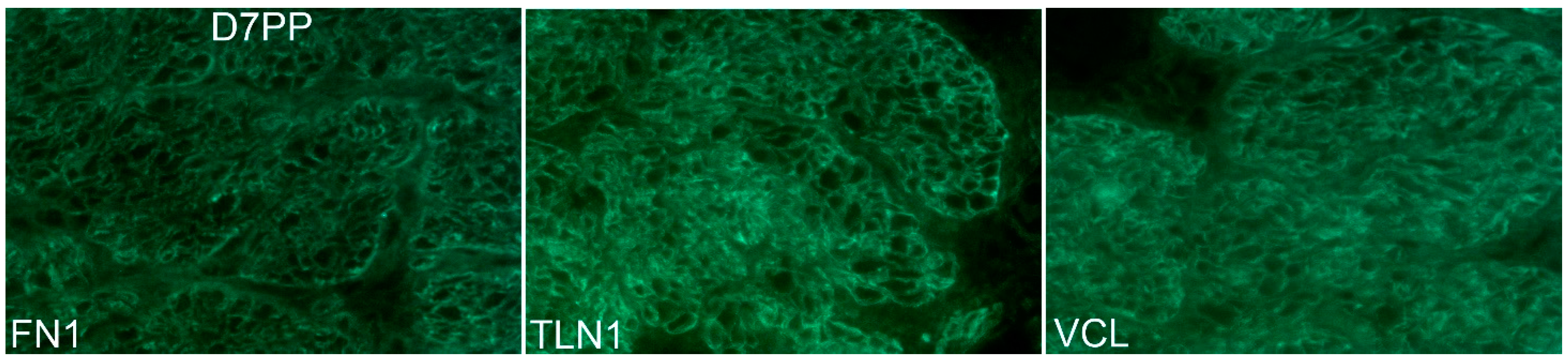
3.2. ITGA5 and ITGB1 Proteins Assemble into Longitudinally Oriented IACs at the Surface of Myometrial Cells during Late Pregnancy That Disperse Postpartum
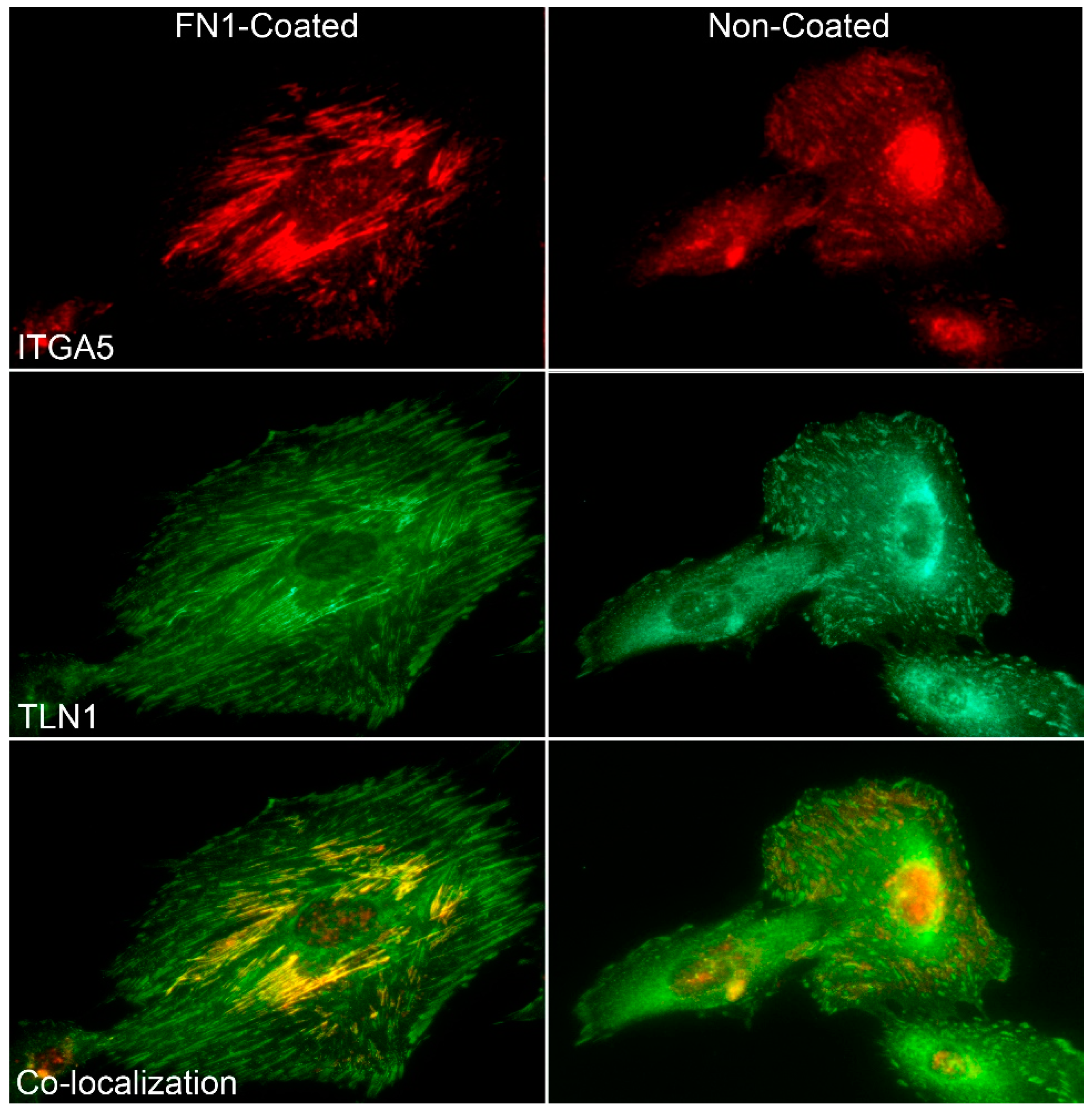
3.3. FN1 Stimulates Activation Of ITGA5, ITGB1, and TLN1 to Rapidly Form IACs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shynlova, O.; Williams, S.J.; Draper, H.; White, B.G.; MacPhee, D.J.; Lye, S.J. Uterine stretch regulates temporal and spatial expression of fibronectin protein and its alpha 5 integrin receptor in myometrium of unilaterally pregnant rats. Biol. Reprod. 2007, 77, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.J.; White, B.G.; MacPhee, D.J. Expression of alpha5 integrin (Itga5) is elevated in the rat myometrium during late pregnancy and labor: Implications for development of a mechanical syncytium. Biol. Reprod. 2005, 72, 1114–1124. [Google Scholar] [CrossRef]
- Burridge, K.; Chrzanowska-Wodnicka, M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 1996, 12, 463–518. [Google Scholar] [CrossRef]
- Humphries, J.D.; Chastney, M.R.; Askari, J.A.; Humphries, M.J. Signal transduction via integrin adhesion complexes. Curr. Opin. Cell Biol. 2019, 56, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Challis, J.R.G.; Matthews, S.G.; Gibb, W.; Lye, S.J. Endocrine and paracrine regulation of birth at term and preterm. Endocr. Rev. 2000, 21, 514–550. [Google Scholar] [PubMed]
- Shynlova, O.; Nadeem, L.; Zhang, J.; Dunk, C.; Lye, S. Myometrial activation: Novel concepts underlying labor. Placenta 2020, 92, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.M.; Harkness, R.D. Collagen formation and changes in cell population in the rat’s uterus after distension with wax. Q. J. Exp. Physiol. Cogn. Med. Sci. 1968, 53, 33–42. [Google Scholar] [CrossRef]
- Goldspink, D.F.; Douglas, A.J. Protein turnover in gravid and nongravid horns of uterus in pregnant rats. Am. J. Physiol. 1988, 254 Pt 1, E549–E554. [Google Scholar] [CrossRef]
- Csapo, A.; Erdos, T.; de Mattos, C.R.; Gramss, E.; Moscowitz, C. Stretch-induced uterine growth, protein synthesis and function. Nature 1965, 207, 1378–1379. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, M.A.; Modzelewska, K.; Kwong, L.; Keely, P.J. Focal adhesion regulation of cell behavior. Biochim. Biophys. Acta 2004, 1692, 103–119. [Google Scholar] [CrossRef]
- Robinson, E.E.; Foty, R.A.; Corbett, S.A. Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion. Mol. Biol. Cell 2004, 15, 973–981. [Google Scholar] [CrossRef]
- Vogel, V. Mechanotransduction involving multimodular proteins: Converting force into biochemical signals. Annu. Rev. Biophys. Biomol. Struct. 2006, 35, 459–488. [Google Scholar] [CrossRef]
- Hynes, R.O. Fibronectins; Springer: New York, NY, USA, 1990. [Google Scholar]
- Wierzbicka-Patynowski, I.; Schwarzbauer, J.E. The ins and outs of fibronectin matrix assembly. J. Cell Sci. 2003, 116 Pt 16, 3269–3276. [Google Scholar] [CrossRef]
- Sastry, S.K.; Burridge, K. Focal adhesions: A nexus for intracellular signaling and cytoskeletal dynamics. Exp. Cell Res. 2000, 261, 25–36. [Google Scholar] [CrossRef]
- Larsen, M.; Artym, V.V.; Green, J.A.; Yamada, K.M. The matrix reorganized: Extracellular matrix remodeling and integrin signaling. Curr. Opin. Cell Biol. 2006, 18, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Katz, B.Z.; Zamir, E.; Bershadsky, A.; Kam, Z.; Yamada, K.M.; Geiger, B. Physical state of the extracellular matrix regulates the structure and molecular composition of cell-matrix adhesions. Mol. Biol. Cell 2000, 11, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, C.G.; Yamada, K.M.; Sheetz, M.P. The relationship between force and focal complex development. J. Cell Biol. 2002, 159, 695–705. [Google Scholar] [CrossRef]
- Eddinger, T.J.; Schiebout, J.D.; Swartz, D.R. Adherens junction-associated protein distribution differs in smooth muscle tissue and acutely isolated cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G684–G697. [Google Scholar] [CrossRef] [PubMed]
- Gabella, G. Structural apparatus for force transmission in smooth muscles. Physiol. Rev. 1984, 64, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Burkin, H.R.; Rice, M.; Sarathy, A.; Thompson, S.; Singer, C.A.; Buxton, I.L. Integrin upregulation and localization to focal adhesion sites in pregnant human myometrium. Reprod. Sci. 2013, 20, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, R.C.; Burghardt, J.R.; Taylor, J.D., 2nd; Reeder, A.T.; Nguen, B.T.; Spencer, T.E.; Bayless, K.J.; Johnson, G.A. Enhanced focal adhesion assembly reflects increased mechanosensation and mechanotransduction at maternal-conceptus interface and uterine wall during ovine pregnancy. Reproduction 2009, 137, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Monga, M.; Ku, C.Y.; Dodge, K.; Sanborn, B.M. Oxytocin-stimulated responses in a pregnant human immortalized myometrial cell line. Biol. Reprod. 1996, 55, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Joyce, M.M.; White, F.J.; Burghardt, R.C.; Muniz, J.J.; Spencer, T.E.; Bazer, F.W.; Johnson, G.A. Interferon stimulated gene 15 conjugates to endometrial cytosolic proteins and is expressed at the uterine-placental interface throughout pregnancy in sheep. Endocrinology 2005, 146, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.A.; Bazer, F.W.; Jaeger, L.A.; Ka, H.; Garlow, J.E.; Pfarrer, C.; Spencer, T.E.; Burghardt, R.C. Muc-1, integrin, and osteopontin expression during the implantation cascade in sheep. Biol. Reprod. 2001, 65, 820–828. [Google Scholar] [CrossRef]
- Muniz, J.J.; Joyce, M.M.; Taylor, J.D., 2nd; Burghardt, J.R.; Burghardt, R.C.; Johnson, G.A. Glycosylation dependent cell adhesion molecule 1-like protein and L-selectin expression in sheep interplacentomal and placentomal endometrium. Reproduction 2006, 131, 751–761. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanchanawong, P.; Shtengel, G.; Pasaera, A.M.; Ramko, E.V.B.; Davidson, M.W.; Hess, H.F.; Waterman, C. Nanoscale architecture of integrin-based cell adhesions. Nature 2010, 468, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Bershadsky, A.; Kozlov, M.; Geiger, B. Adhesion-mediated mechanosensitivity: A time to experiment, and a time to theorize. Curr. Opin. Cell Biol. 2006, 18, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Labouesse, M. Signalling through mechanical inputs: A coordinated process. J. Cell Sci. 2012, 125 Pt 13, 3039–3049. [Google Scholar] [CrossRef]
- Chen, C.S. Mechanotransduction—A field pulling together? J. Cell Sci. 2008, 121 Pt 20, 3285–3292. [Google Scholar] [CrossRef]
- Ono, M.; Maruyama, T.; Masuda, H.; Kajitani, T.; Nagashima, T.; Arase, T.; Ito, M.; Ohta, K.; Uchida, H.; Asada, H.; et al. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 18700–18705. [Google Scholar] [CrossRef] [PubMed]
- Norwitz, E.R.; Lye, S.J. Biology of Parturition. In Creasy and Resnik’s Maternal-Fetal Medicine: Princinples and Practice; Creasy, R.K., Resnik, R., Iams, J.D., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2009; pp. 70–85. [Google Scholar]
- Shynlova, O.; Mitchell, J.A.; Tsampalieros, A.; Langille, B.L.; Lye, S.J. Progesterone and gravidity differentially regulate expression of extracellular matrix components in the pregnant rat myometrium. Biol. Reprod. 2004, 70, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Hytonen, V.P.; Vogel, V. How force might activate talin’s vinculin binding sites: SMD reveals a structural mechanism. PLoS Comput. Biol. 2008, 4, e24–e39. [Google Scholar] [CrossRef] [PubMed]
- Mykuliak, V.V.; Haining, A.W.M.; von Essen, M.; Del Río Hernández, A.; Hytönen, V.P. Mechanical unfolding reveals stable 3-helix intermediates in talin and α-catenin. PLoS Comput. Biol. 2018, 14, e1006126–e1006145. [Google Scholar] [CrossRef] [PubMed]
- MacPhee, D.J.; Mostachfi, H.; Han, R.; Lye, S.J.; Post, M.; Caniggia, I. Focal adhesion kinase is a key mediator of human trophoblast development. Lab. Investig. 2001, 81, 1469–1483. [Google Scholar] [CrossRef]
- Seong, J.; Tajik, A.; Sun, J.; Guan, J.L.; Humphries, M.J.; Craig, S.E.; Shekaran, A.; Garcia, A.J.; Lu, S.; Lin, M.Z.; et al. Distinct biophysical mechanisms of focal adhesion kinase mechanoactivation by different extracellular matrix proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 19372–19377. [Google Scholar] [CrossRef]
- Nicolas, A.; Geiger, B.; Safran, S.A. Cell mechanosensitivity controls the anisotropy of focal adhesions. Proc. Natl. Acad. Sci. USA 2004, 101, 12520–12525. [Google Scholar] [CrossRef]
- Macphee, D.J.; Lye, S.J. Focal adhesion signaling in the rat myometrium is abruptly terminated with the onset of labor. Endocrinology 2000, 141, 274–283. [Google Scholar] [CrossRef][Green Version]
- Johnson, G.A.; Burghardt, R.C.; Joyce, M.M.; Spencer, T.E.; Bazer, F.W.; Pfarrer, C.; Gray, C.A. Osteopontin expression in uterine stroma indicates a decidualization-like differentiation during ovine pregnancy. Biol. Reprod. 2003, 68, 1951–1958. [Google Scholar] [CrossRef]
- Seo, H.; Li, X.; Wu, G.; Bazer, F.W.; Burghardt, R.C.; Bayless, K.J.; Johnson, G.A. Mechanotransduction drives morphogenesis to develop folding during placental development in pigs. Placenta 2020, 90, 62–70. [Google Scholar] [CrossRef]
- Frank, J.W.; Seo, H.; Burghardt, R.C.; Bayless, K.J.; Johnson, G.A. ITGAV (alpha v integrins) bind SPP1 (osteopontin) to support trophoblast cell adhesion. Reproduction 2017, 153, 695–706. [Google Scholar] [CrossRef]
- Erikson, D.W.; Burghardt, R.C.; Bayless, K.J.; Johnson, G.A. Secreted phosphoprotein 1 (SPP1, osteopontin) binds to integrin alpha v beta 6 on porcine trophectoderm cells and integrin alpha v beta 3 on uterine luminal epithelial cells, and promotes trophectoderm cell adhesion and migration. Biol. Reprod. 2009, 81, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Erikson, D.W.; Burghardt, R.C.; Spencer, T.E.; Wu, G.; Bayless, K.J.; Johnson, G.A.; Bazer, F.W. Secreted phosphoprotein 1 binds integrins to initiate multiple cell signaling pathways, including FRAP1/mTOR, to support attachment and force-generated migration of trophectoderm cells. Matrix Biol. 2010, 29, 369–382. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McLendon, B.A.; Kramer, A.C.; Seo, H.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A. Integrin Adhesion Complex Organization in Sheep Myometrium Reflects Changing Mechanical Forces during Pregnancy and Postpartum. Biology 2021, 10, 508. https://doi.org/10.3390/biology10060508
McLendon BA, Kramer AC, Seo H, Bazer FW, Burghardt RC, Johnson GA. Integrin Adhesion Complex Organization in Sheep Myometrium Reflects Changing Mechanical Forces during Pregnancy and Postpartum. Biology. 2021; 10(6):508. https://doi.org/10.3390/biology10060508
Chicago/Turabian StyleMcLendon, Bryan A., Avery C. Kramer, Heewon Seo, Fuller W. Bazer, Robert C. Burghardt, and Gregory A. Johnson. 2021. "Integrin Adhesion Complex Organization in Sheep Myometrium Reflects Changing Mechanical Forces during Pregnancy and Postpartum" Biology 10, no. 6: 508. https://doi.org/10.3390/biology10060508
APA StyleMcLendon, B. A., Kramer, A. C., Seo, H., Bazer, F. W., Burghardt, R. C., & Johnson, G. A. (2021). Integrin Adhesion Complex Organization in Sheep Myometrium Reflects Changing Mechanical Forces during Pregnancy and Postpartum. Biology, 10(6), 508. https://doi.org/10.3390/biology10060508







