Effect of Extender, Storage Time and Temperature on Kinetic Parameters (CASA) on Bull Semen Samples
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
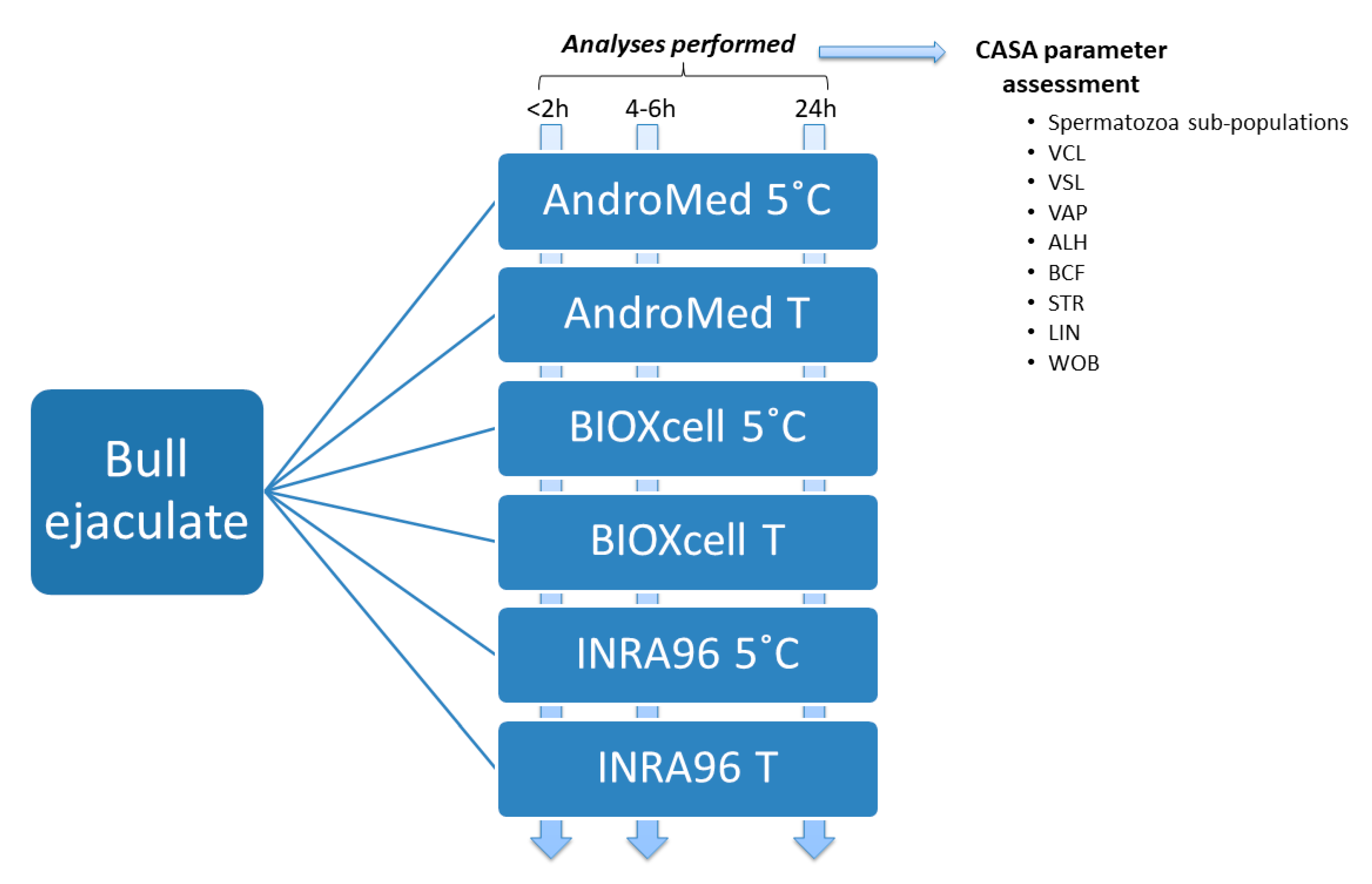
2.1. Animals and Ejaculate Samples
2.2. Computer Assisted Semen Analyses (CASA)
2.3. Statistical Analyses
3. Results
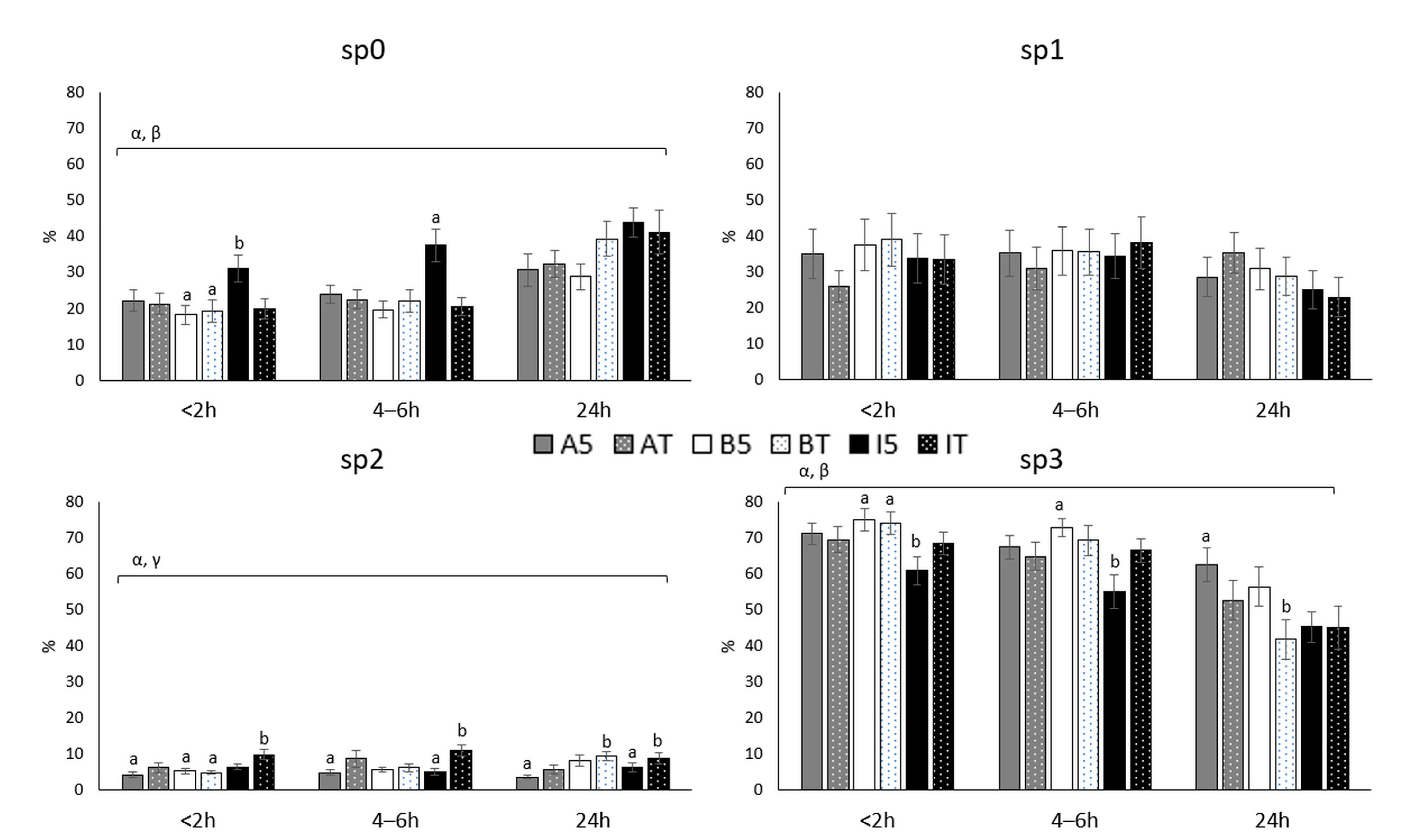
3.1. Sperm Subpopulations
3.2. Kinetic CASA Parameters
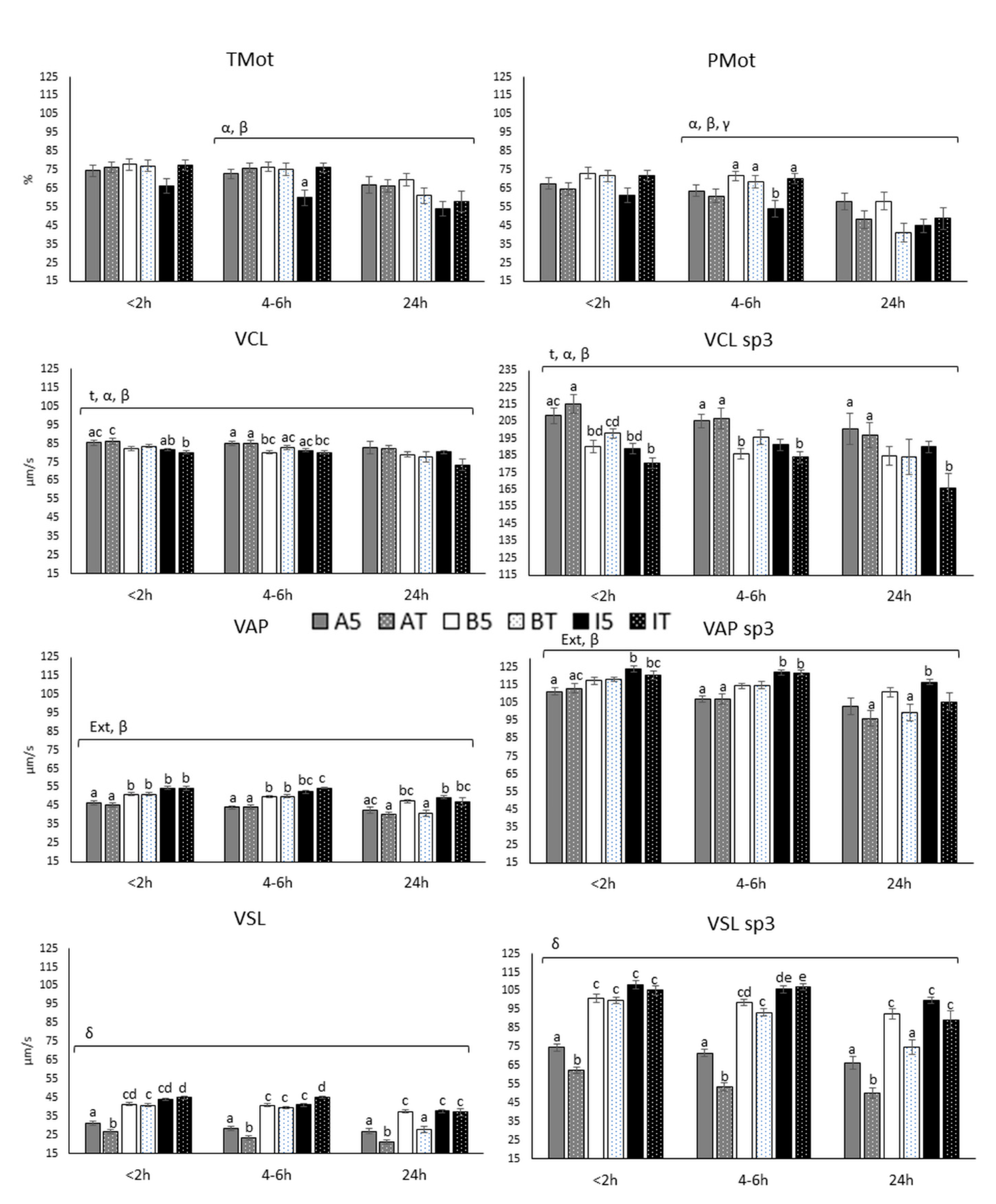
3.2.1. Motility and Velocity Parameters (TMot, PMot, VCL, VAP and VSL)
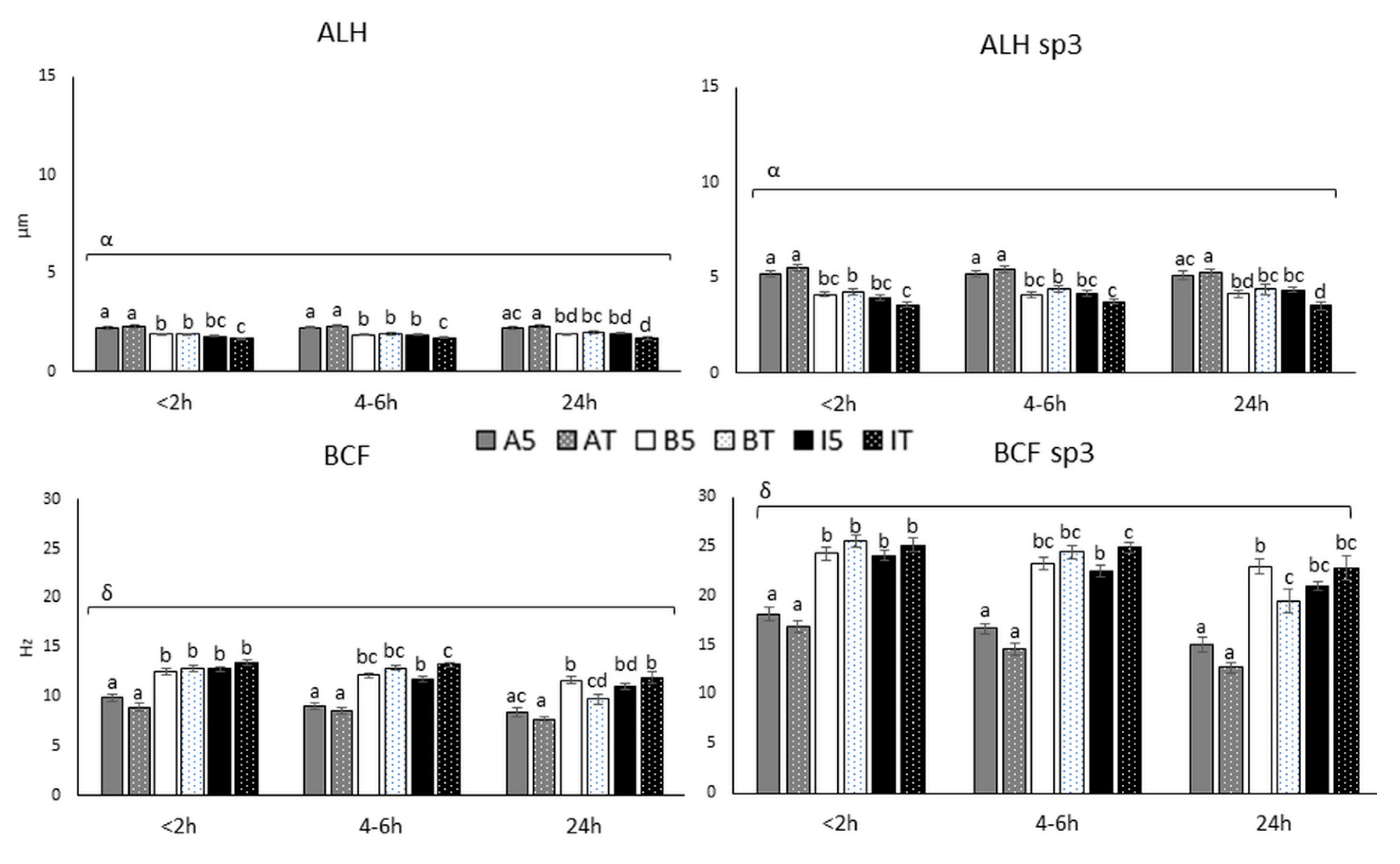
3.2.2. Sperm Movement Parameters (ALH and BCF)
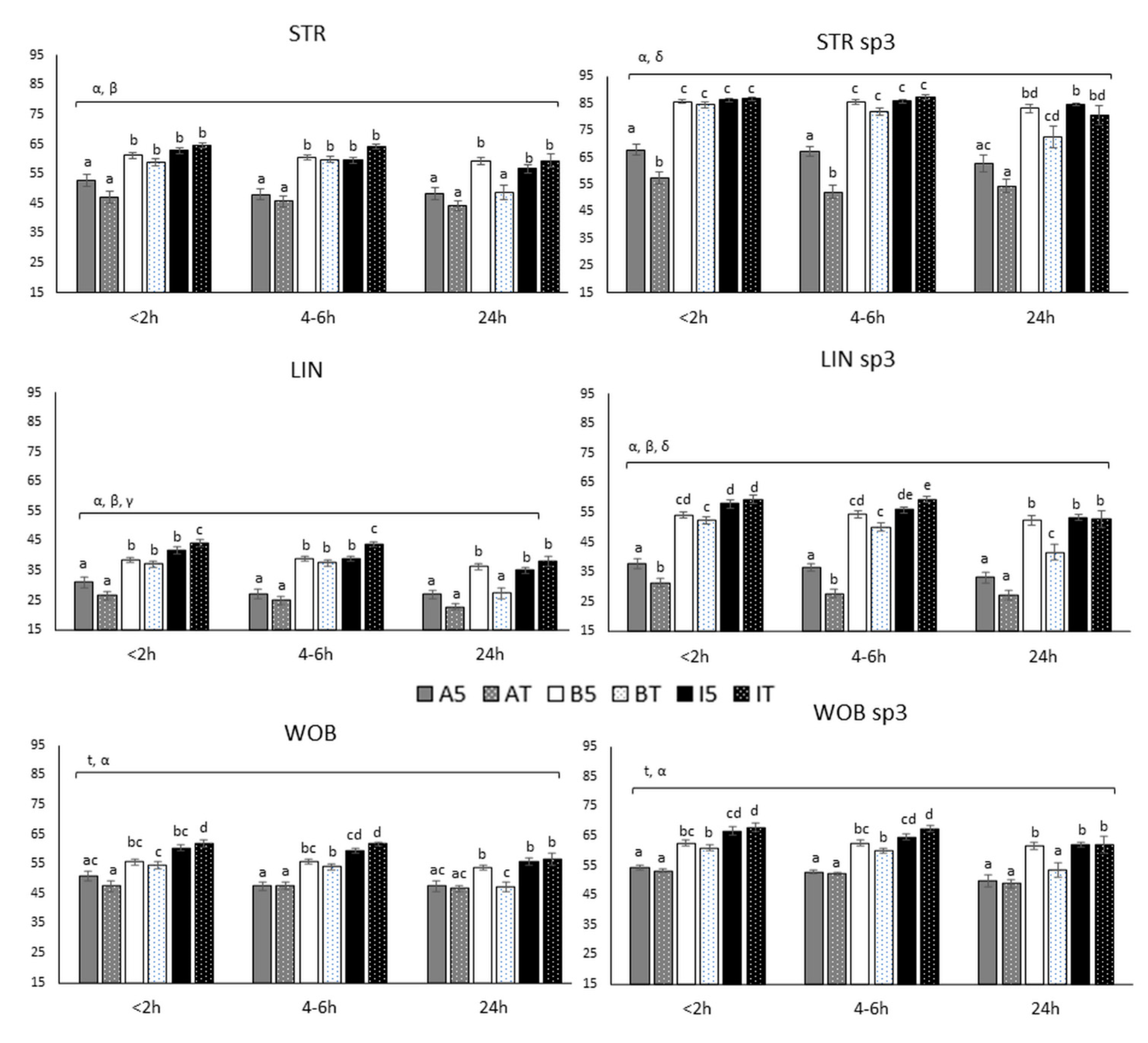
3.2.3. Ratios (STR, LIN and WOB)
3.3. Correlation of Different Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amann, R.P.; Waberski, D. Computer-Assisted Sperm Analysis (CASA): Capabilities and potential developments. Theriogenology 2014, 81, 5–17.e3. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Moreno, A.; Rigau, T.; Rodríguez-Gil, J.E. Multivariate cluster analysis regression procedures as tools to identify motile sperm subpopulations in rabbit semen and to predict semen fertility and litter size. Reprod. Domest. Anim. Zuchthyg. 2007, 42, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, D. Practical Laboratory Andrology; Oxford University Press: Oxford, UK, 1994; ISBN 978-0-19-506595-4. [Google Scholar]
- Boyers, S.P.; Davis, R.O.; Katz, D.F. Automated semen analysis. Curr. Prob. Obstet. Gynecol. Fertil. 1989, 12, 167–200. [Google Scholar]
- Broekhuijse, M.L.W.J.; Soštarić, E.; Feitsma, H.; Gadella, B.M. Additional Value of Computer Assisted Semen Analysis (CASA) compared to conventional motility assessments in pig artificial insemination. Theriogenology 2011, 76, 1473–1486.e1. [Google Scholar] [CrossRef] [PubMed]
- Ibanescu, I.; Siuda, M.; Bollwein, H. Motile sperm subpopulations in bull semen using different clustering approaches—Associations with flow cytometric sperm characteristics and fertility. Anim. Reprod. Sci. 2020, 215, 106329. [Google Scholar] [CrossRef]
- Henning, H.; Petrunkina, A.M.; Harrison, R.A.P.; Waberski, D.; Henning, H.; Petrunkina, A.M.; Harrison, R.A.P.; Waberski, D. Cluster analysis reveals a binary effect of storage on boar sperm motility function. Reprod. Fertil. Dev. 2014, 26, 623–632. [Google Scholar] [CrossRef]
- Ramió, L.; Rivera, M.M.; Ramírez, A.; Concha, I.I.; Peña, A.; Rigau, T.; Rodríguez-Gil, J.E. Dynamics of motile-sperm subpopulation structure in boar ejaculates subjected to “in vitro” capacitation and further “in vitro” acrosome reaction. Theriogenology 2008, 69, 501–512. [Google Scholar] [CrossRef]
- Flores, E.; Fernández-Novell, J.M.; Peña, A.; Rodríguez-Gil, J.E. The degree of resistance to freezing-thawing is related to specific changes in the structures of motile sperm subpopulations and mitochondrial activity in boar spermatozoa. Theriogenology 2009, 72, 784–797. [Google Scholar] [CrossRef]
- Dorado, J.; Acha, D.; Ortiz, I.; Gálvez, M.J.; Carrasco, J.J.; Díaz, B.; Gómez-Arrones, V.; Calero-Carretero, R.; Hidalgo, M. Relationship between conventional semen characteristics, sperm motility patterns and fertility of andalusian donkeys (Equus Asinus). Anim. Reprod. Sci. 2013, 143, 64–71. [Google Scholar] [CrossRef]
- Yániz, J.L.; Silvestre, M.A.; Santolaria, P.; Soler, C.; Yániz, J.L.; Silvestre, M.A.; Santolaria, P.; Soler, C. CASA-Mot in mammals: An update. Reprod. Fertil. Dev. 2018, 30, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.K.; Trautwein, L.G.C.; Paranzini, C.S.; Perencin, F.M.; Cardoso, G.S.; Martins, M.I.M. Influence of cooling temperature in sperm subpopulations of domestic cats. Anim. Reprod. Sci. 2018, 189, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kathiravan, P.; Kalatharan, J.; Karthikeya, G.; Rengarajan, K.; Kadirvel, G. Objective sperm motion analysis to assess dairy bull fertility using computer-aided system—A review. Reprod. Domest. Anim. 2011, 46, 165–172. [Google Scholar] [CrossRef]
- Amann, R.P.; DeJarnette, J.M. Impact of genomic selection of ai dairy sires on their likely utilization and methods to estimate fertility: A paradigm shift. Theriogenology 2012, 77, 795–817. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, A.; Malama, E.; Witschi, U.; Leiding, C.; Siuda, M.; Janett, F.; Bollwein, H. Effects of an extension of the equilibration period up to 96 hours on the characteristics of cryopreserved bull semen. Theriogenology 2017, 89, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Leite, T.G.; do Vale Filho, V.R.; de Arruda, R.P.; de Andrade, A.F.C.; Emerick, L.L.; Zaffalon, F.G.; Martins, J.A.M.; Andrade, V.J. de Effects of extender and equilibration time on post-thaw motility and membrane integrity of cryopreserved gyr bull semen evaluated by CASA and flow cytometry. Anim. Reprod. Sci. 2010, 120, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Víquez, L.; Barquero, V.; Soler, C.; Roldan, E.R.S.; Valverde, A. Kinematic sub-populations in bull spermatozoa: A comparison of classical and bayesian approaches. Biology 2020, 9, 138. [Google Scholar] [CrossRef]
- Fernandez-Novo, A.; Santos-Lopez, S.; Barrajon-Masa, C.; Mozas, P.; de Mercado, E.; Caceres, E.; Garrafa, A.; Gonzalez-Martin, J.V.; Perez-Villalobos, N.; Oliet, A.; et al. Effects of Extender Type, Storage Time, and Temperature on Bull Semen Parameters. Biology 2021, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.G.; Hasler, J.F. A 100-Year Review: Reproductive technologies in dairy science. J. Dairy Sci. 2017, 100, 10314–10331. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.W. Welfare aspects of theriogenology: Investigating alternatives to electroejaculation of bulls. Theriogenology 2005, 64, 469–479. [Google Scholar] [CrossRef]
- Jiménez-Rabadán, P.; Ramón, M.; García-Álvarez, O.; Maroto-Morales, A.; del Olmo, E.; Pérez-Guzmán, M.D.; Bisbal, A.; Fernández-Santos, M.R.; Garde, J.J.; Soler, A.J. Effect of semen collection method (artificial vagina vs. electroejaculation), extender and centrifugation on post-thaw sperm quality of blanca-celtibérica buck ejaculates. Anim. Reprod. Sci. 2012, 132, 88–95. [Google Scholar] [CrossRef]
- Morrell, J.M.; Valeanu, A.S.; Lundeheim, N.; Johannisson, A. Sperm quality in frozen beef and dairy bull semen. Acta Vet. Scand. 2018, 60, 41. [Google Scholar] [CrossRef]
- Bompart, D.; García-Molina, A.; Valverde, A.; Caldeira, C.; Yániz, J.; Núñez de Murga, M.; Soler, C. CASA-mot technology: How results are affected by the frame rate and counting chamber. Reprod. Fertil. Dev. 2018, 30, 810–819. [Google Scholar] [CrossRef]
- Yeste, M.; Bonet, S.; Rodríguez-Gil, J.E.; Álamo, M.M.R.D. Evaluation of sperm motility with CASA-mot: Which factors may influence our measurements? Reprod. Fertil. Dev. 2018, 30, 789–798. [Google Scholar] [CrossRef]
- Hoflack, G.; Opsomer, G.; Rijsselaere, T.; Soom, A.V.; Maes, D.; Kruif, A.D.; Duchateau, L. Comparison of computer-assisted sperm motility analysis parameters in semen from Belgian Blue and Holstein–Friesian bulls. Reprod. Domest. Anim. 2007, 42, 153–161. [Google Scholar] [CrossRef]
- Muiño, R.; Tamargo, C.; Hidalgo, C.O.; Peña, A.I. Identification of sperm subpopulations with defined motility characteristics in ejaculates from Holstein Bulls: Effects of cryopreservation and between-bull variation. Anim. Reprod. Sci. 2008, 109, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, J.W.; Adams, C.E. Mechanisms of selective fertilization in the rabbit: Sperm transport and viability. J. Reprod. Fertil. 1971, 26, 219–231. [Google Scholar] [CrossRef][Green Version]
- Nadir, S.; Saacke, R.G.; Bame, J.; Mullins, J.; Degelos, S. Effect of freezing semen and dosage of sperm on number of accessory sperm, fertility, and embryo quality in artificially inseminated cattle. J. Anim. Sci. 1993, 71, 199–204. [Google Scholar] [CrossRef]
- Murphy, E.M.; O’ Meara, C.; Eivers, B.; Lonergan, P.; Fair, S. Optimizing storage temperature of liquid bovine semen diluted in INRA96. J. Dairy Sci. 2018, 101, 5549–5558. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, R.; Shannon, P. Storage of bovine semen in liquid and frozen state. Anim. Reprod. Sci. 2000, 62, 23–53. [Google Scholar] [CrossRef]
- Muiño, R.; Peña, A.I.; Rodríguez, A.; Tamargo, C.; Hidalgo, C.O. Effects of cryopreservation on the motile sperm subpopulations in semen from Asturiana de Los Valles bulls. Theriogenology 2009, 72, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Á.; Polichronopoulos, T.; Gáspárdy, A.; Solti, L.; Cseh, S. Correlation between bull fertility and sperm cell velocity parameters generated by Computer-Assisted Semen Analysis. Acta Vet. Hung. 2015, 63, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Defoin, L.; Granados, A.; Donnay, I. Analysing motility parameters on fresh bull semen could help to predict resistance to freezing: A preliminary study. Reprod. Domest. Anim. 2008, 43, 606–611. [Google Scholar] [CrossRef]
- Farrell, P.B.; Presicce, G.A.; Brockett, C.C.; Foote, R.H. Quantification of bull sperm Characteristics Measured by Computer-Assisted Sperm Analysis (CASA) and the relationship to fertility. Theriogenology 1998, 49, 871–879. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Honparkhe, M.; Kaur, S.; Kaur, H.; Ghuman, S.P.S.; Brar, P.S. Comparison of in vitro and in vivo fertilizing potential of buffalo bull semen frozen in egg yolk-, soya bean lecithin- and liposome-based extenders. Reprod. Domest. Anim. 2018, 53, 195–202. [Google Scholar] [CrossRef]
- Murphy, E.M.; O’Meara, C.; Eivers, B.; Lonergan, P.; Fair, S. Comparison of plant- and egg yolk-based semen diluents on in vitro sperm kinematics and in vivo fertility of frozen-thawed bull semen. Anim. Reprod. Sci. 2018, 191, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Umair, M.; Iqbal, S. Comparison of tris egg yolk-based, Triladyl® and Optixell® extender on post-thaw quality, kinematics and in vivo fertility of Nili Ravi buffalo (Bubalus Bubalis) bull spermatozoa. Andrologia 2018, 50, e13063. [Google Scholar] [CrossRef] [PubMed]
- Camus, A.; González, A.; Abdelfattah, E.M. Novel protein-free semen medium improves fertility potential of frozen bovine sperm. Anim. Reprod. Sci. 2016, 169, 108. [Google Scholar] [CrossRef]
- Röpke, T.; Oldenhof, H.; Leiding, C.; Sieme, H.; Bollwein, H.; Wolkers, W.F. Liposomes for cryopreservation of bovine sperm. Theriogenology 2011, 76, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Saini, M.; Kumar, D.; Balhara, A.K.; Yadav, S.P.; Singh, P.; Yadav, P.S. Liposome-based semen extender is suitable alternative to egg yolk-based extender for cryopreservation of buffalo (Bubalus Bubalis) semen. Anim. Reprod. Sci. 2015, 159, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Jimenez, S.; Rivera del Alamo, M.M.; Álvarez-Rodríguez, M.; Hidalgo, C.O.; Peña, A.I.; Muiño, R.; Rodríguez-Gil, J.E.; Mogas, T. In vitro assessment of egg yolk-, soya bean lecithin- and liposome-based extenders for cryopreservation of dairy bull semen. Anim. Reprod. Sci. 2020, 215, 106315. [Google Scholar] [CrossRef]
- Hyakutake, T.; Suzuki, H.; Yamamoto, S. Effect of viscosity on motion characteristics of bovine sperm. J. Aero Aqua Bio-Mech. 2015, 4, 63–70. [Google Scholar] [CrossRef][Green Version]
- Tung, C.; Lin, C.; Harvey, B.; Fiore, A.G.; Ardon, F.; Wu, M.; Suarez, S.S. Fluid viscoelasticity promotes collective swimming of sperm. Sci. Rep. 2017, 7, 3152. [Google Scholar] [CrossRef]
- Kumar Yata, V.; Kumar Gangwar, D.; Sharma, V.; Kumar Dubey, S.; Kumar Yadav, S.; Choudhary, S.; Kumar, S.; Kumar Mohanty, T.; Kumar Mohanty, A. Semen analysis and sperm characteristics of Karan Fries cattle. Anim. Reprod. Sci. 2020, 212, 106250. [Google Scholar] [CrossRef]
- Tarig, A.A.; Wahid, H.; Rosnina, Y.; Yimer, N.; Goh, Y.M.; Baiee, F.H.; Khumran, A.M.; Salman, H.; Assi, M.A.; Ebrahimi, M. Effect of different concentrations of soybean lecithin and virgin coconut oil in tris-based extender on the quality of chilled and frozen-thawed bull semen. Vet. World 2017, 10, 672–678. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tardif, A.L.; Farrell, P.B.; Trouern-Trend, V.; Foote, R.H. Computer-Assisted Sperm Analysis for assessing initial semen quality and changes during storage at 5 °C. J. Dairy Sci. 1997, 80, 1606–1612. [Google Scholar] [CrossRef]
- Dias, E.A.R.; Campanholi, S.P.; Rossi, G.F.; Freitas Dell’Aqua, C.dP.; Dell’Aqua, J.A.; Papa, F.O.; Zorzetto, M.F.; de Paz, C.C.P.; Oliveira, L.Z.; Mercadante, M.E.Z.; et al. Evaluation of cooling and freezing systems of bovine semen. Anim. Reprod. Sci. 2018, 195, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.M.O.; Forell, F.; Oliveira, A.T.D.; Rodrigues, J.L. Current status of sperm cryopreservation: Why isn’t it better? Theriogenology 2002, 57, 327–344. [Google Scholar] [CrossRef]
- Christensen, P.; No, P.B.B.; Lehn-Jensen, H. The Relationship between semen quality and the nonreturn rate of bulls. Reprod. Domest. Anim. 1999, 34, 503–507. [Google Scholar] [CrossRef]
- Gliozzi, T.M.; Turri, F.; Manes, S.; Cassinelli, C.; Pizzi, F. The Combination of kinetic and flow cytometric semen parameters as a tool to predict fertility in cryopreserved bull semen. Animal 2017, 11, 1975–1982. [Google Scholar] [CrossRef]
| PMotT < 2 | PMotT4 | PMotT24 | Pob3T < 2 | Pob3T4 | Pob3T24 | |
|---|---|---|---|---|---|---|
| TMotT < 2 | 0.947 | 0.707 | 0.481 | 0.922 | 0.689 | 0.444 |
| TMotT4 | 0.756 | 0.909 | 0.517 | 0.759 | 0.870 | 0.482 |
| TMotT24 | 0.519 | 0.604 | 0.855 | 0.570 | 0.687 | 0.810 |
| PMotT < 2 | 0.914 | 0.674 | 0.413 | |||
| PMotT4 | 0.700 | 0.898 | 0.456 | |||
| PMotT24 | 0.532 | 0.619 | 0.964 |
| VAPT0 | VAPT4 | VAPT24 | ALHT0 | ALHT4 | ALHT24 | LINT0 | LINT4 | LINT24 | BCFT0 | BCFT4 | BCFT24 | STRT0 | STRT4 | STRT24 | WOBT0 | WOBT4 | WOBT24 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VCLT0 | 0.715 | 0.52 | 0.461 | −0.438 | ||||||||||||||
| VCLT4 | 0.467 | 0.714 | 0.593 | −0.491 | −0.479 | −0.527 | −0.440 | |||||||||||
| VCLT24 | 0.724 | 0.87 | ||||||||||||||||
| VSLT0 | 0.834 | 0.558 | −0.782 | −0.754 | −0.561 | 0.913 | 0.717 | 0.476 | 0.903 | 0.687 | 0.491 | 0.798 | 0.706 | 0.786 | 0.613 | |||
| VSLT4 | 0.577 | 0.835 | −0.691 | −0.77 | −0.473 | 0.667 | 0.943 | 0.588 | 0.706 | 0.879 | 0.602 | 0.634 | 0.897 | 0.518 | 0.541 | 0.814 | 0.424 | |
| VSLT24 | 0.470 | 0.569 | 0.82 | −0.407 | −0.417 | 0.431 | 0.527 | 0.914 | 0.429 | 0.465 | 0.912 | 0.482 | 0.881 | 0.501 | 0.799 | |||
| VAPT0 | 0.489 | −0.532 | −0.533 | 0.797 | 0.511 | 0.764 | 0.463 | 0.412 | 0.527 | 0.482 | 0.727 | 0.469 | ||||||
| VAPT4 | 0.461 | −0.579 | −0.534 | 0.455 | 0.791 | 0.492 | 0.45 | 0.733 | 0.539 | 0.666 | 0.411 | 0.841 | 0.432 | |||||
| VAPT24 | 0.688 | 0.739 | 0.653 | 0.806 | ||||||||||||||
| ALHT0 | 0.822 | 0.609 | −0.724 | −0.669 | −0.714 | −0.708 | −0.403 | −0.522 | −0.592 | −0.493 | −0.664 | |||||||
| ALHT4 | 0.658 | −0.726 | −0.778 | −0.432 | −0.726 | −0.797 | −0.427 | −0.675 | −0.725 | −0.628 | −0.735 | |||||||
| ALHT24 | −0.571 | −0.465 | −0.568 | −0.473 | −0.543 | −0.473 | −0.492 | |||||||||||
| LINT0 | 0.646 | 0.451 | 0.846 | 0.595 | 0.414 | 0.931 | 0.636 | 0.926 | 0.587 | |||||||||
| LINT4 | 0.537 | 0.658 | 0.874 | 0.527 | 0.610 | 0.957 | 0.470 | 0.530 | 0.903 | |||||||||
| LINT24 | 0.430 | 0.480 | 0.895 | 0.430 | 0.508 | 0.960 | 0.412 | 0.505 | 0.879 | |||||||||
| BCFT0 | 0.726 | 0.481 | 0.808 | 0.674 | 0.776 | 0.556 | ||||||||||||
| BCFT4 | 0.533 | 0.561 | 0.843 | 0.422 | 0.471 | 0.785 | ||||||||||||
| BCFT24 | 0.491 | 0.873 | 0.494 | 0.811 | ||||||||||||||
| STRT0 | 0.640 | 0.809 | 0.507 | |||||||||||||||
| STRT4 | 0.469 | 0.523 | 0.791 | |||||||||||||||
| STRT24 | 0.419 | 0.821 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Novo, A.; Santos-Lopez, S.; Barrajon-Masa, C.; Mozas, P.; de Mercado, E.; Caceres, E.; Garrafa, A.; Gonzalez-Martin, J.V.; Perez-Villalobos, N.; Oliet, A.; et al. Effect of Extender, Storage Time and Temperature on Kinetic Parameters (CASA) on Bull Semen Samples. Biology 2021, 10, 806. https://doi.org/10.3390/biology10080806
Fernandez-Novo A, Santos-Lopez S, Barrajon-Masa C, Mozas P, de Mercado E, Caceres E, Garrafa A, Gonzalez-Martin JV, Perez-Villalobos N, Oliet A, et al. Effect of Extender, Storage Time and Temperature on Kinetic Parameters (CASA) on Bull Semen Samples. Biology. 2021; 10(8):806. https://doi.org/10.3390/biology10080806
Chicago/Turabian StyleFernandez-Novo, Aitor, Sergio Santos-Lopez, Clara Barrajon-Masa, Patricia Mozas, Eduardo de Mercado, Elisa Caceres, Aizic Garrafa, Juan V. Gonzalez-Martin, Natividad Perez-Villalobos, Agustín Oliet, and et al. 2021. "Effect of Extender, Storage Time and Temperature on Kinetic Parameters (CASA) on Bull Semen Samples" Biology 10, no. 8: 806. https://doi.org/10.3390/biology10080806
APA StyleFernandez-Novo, A., Santos-Lopez, S., Barrajon-Masa, C., Mozas, P., de Mercado, E., Caceres, E., Garrafa, A., Gonzalez-Martin, J. V., Perez-Villalobos, N., Oliet, A., Astiz, S., & Perez-Garnelo, S. S. (2021). Effect of Extender, Storage Time and Temperature on Kinetic Parameters (CASA) on Bull Semen Samples. Biology, 10(8), 806. https://doi.org/10.3390/biology10080806






