2La Paracentric Chromosomal Inversion and Overexpressed Metabolic Genes Enhance Thermotolerance and Pyrethroid Resistance in the Major Malaria Vector Anopheles gambiae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites and Mosquito Populations
2.2. Morphological and Molecular Identification of Mosquito Larvae and Adults
2.3. Initial Assessment of Thermotolerance Profile Using Temperature Gradient
2.4. Impact of Heat Hardening on Pyrethroid Resistance
2.5. Molecular Karyotyping of 2La and 2L+a Inversion Polymorphism
2.6. Transcriptional Profiling of Thermotolerance-Related Genes Using qRT-PCR
2.7. Data Analysis
3. Results
3.1. Distribution and Composition of Anopheles Gambiae Species
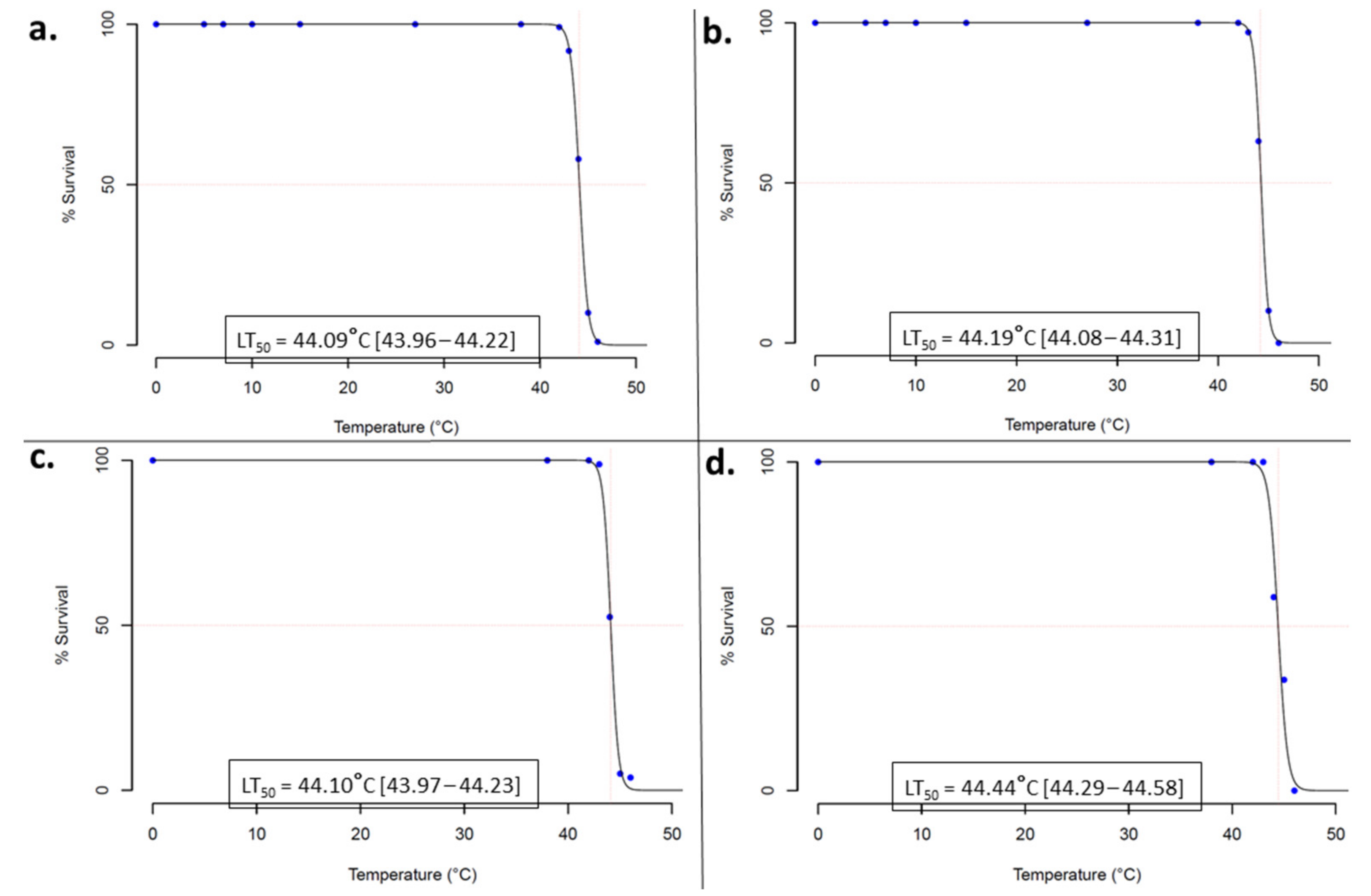
3.2. Thermotolerance Profile and Its Intensity in Anopheles Gambiae s.l. Larvae
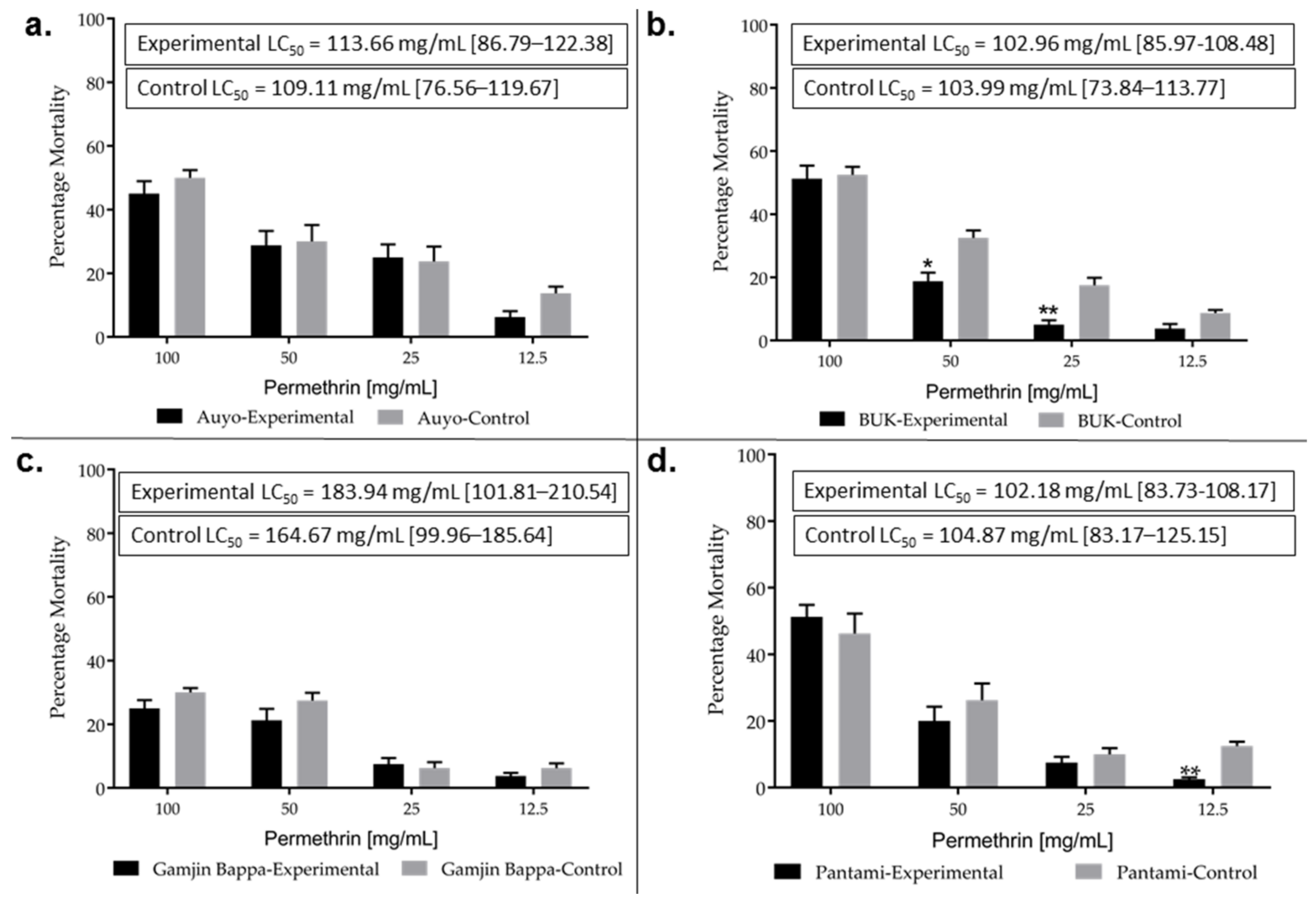
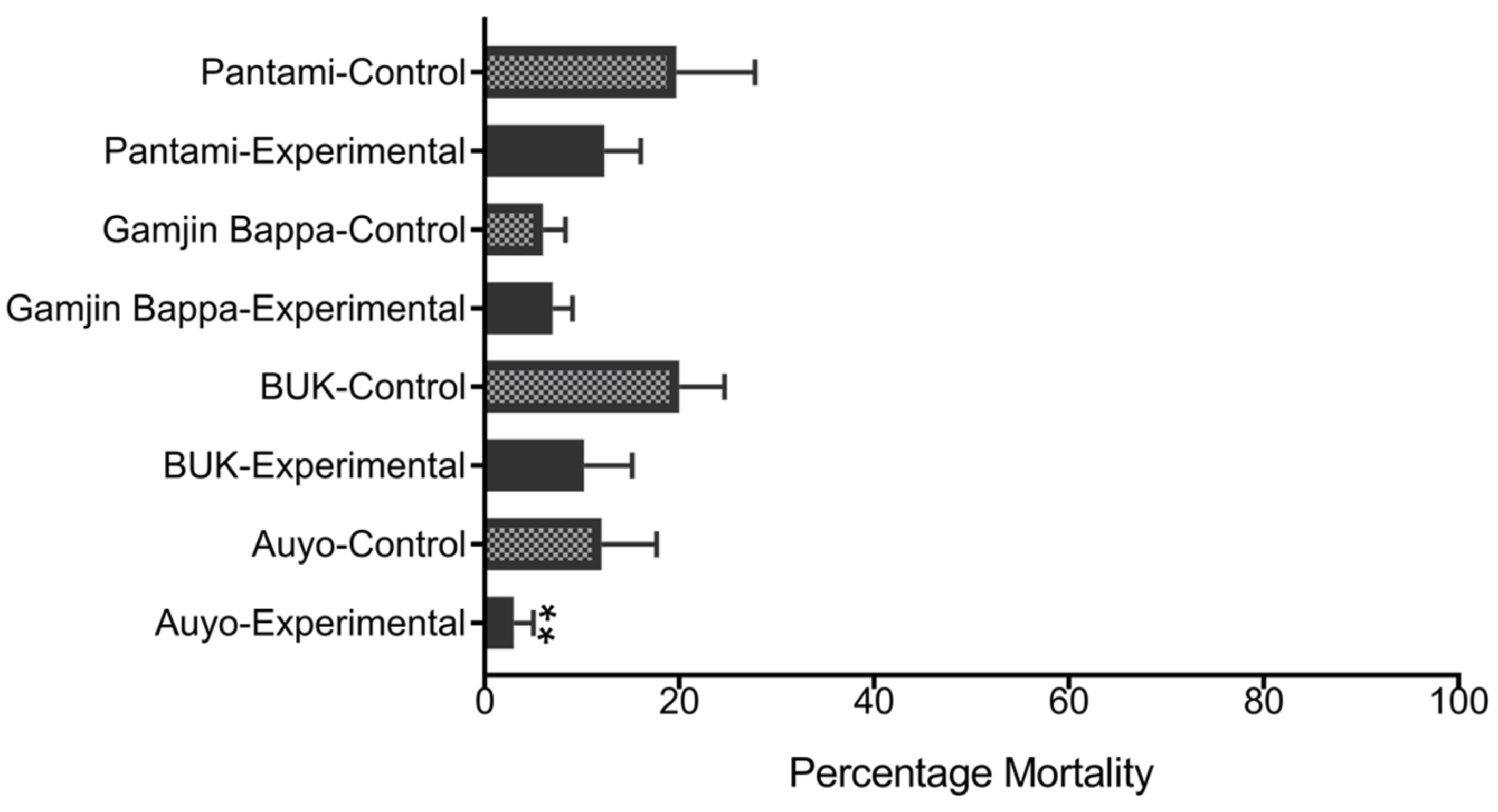
3.3. Impact of Heat Hardening on Pyrethroid Resistance
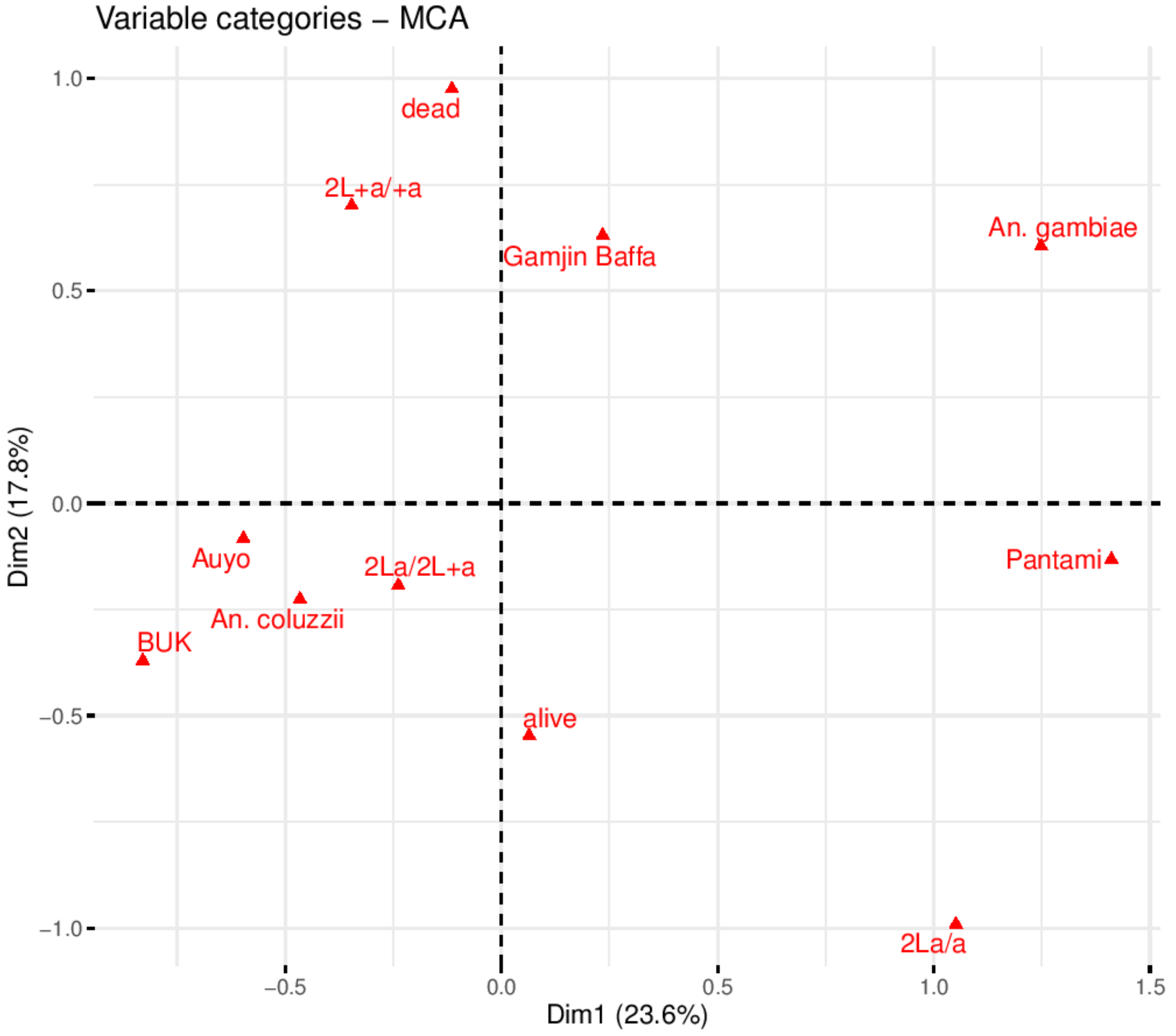
3.4. Role of 2La Chromosomal Inversion on Thermotolerance and Pyrethroid Resistance
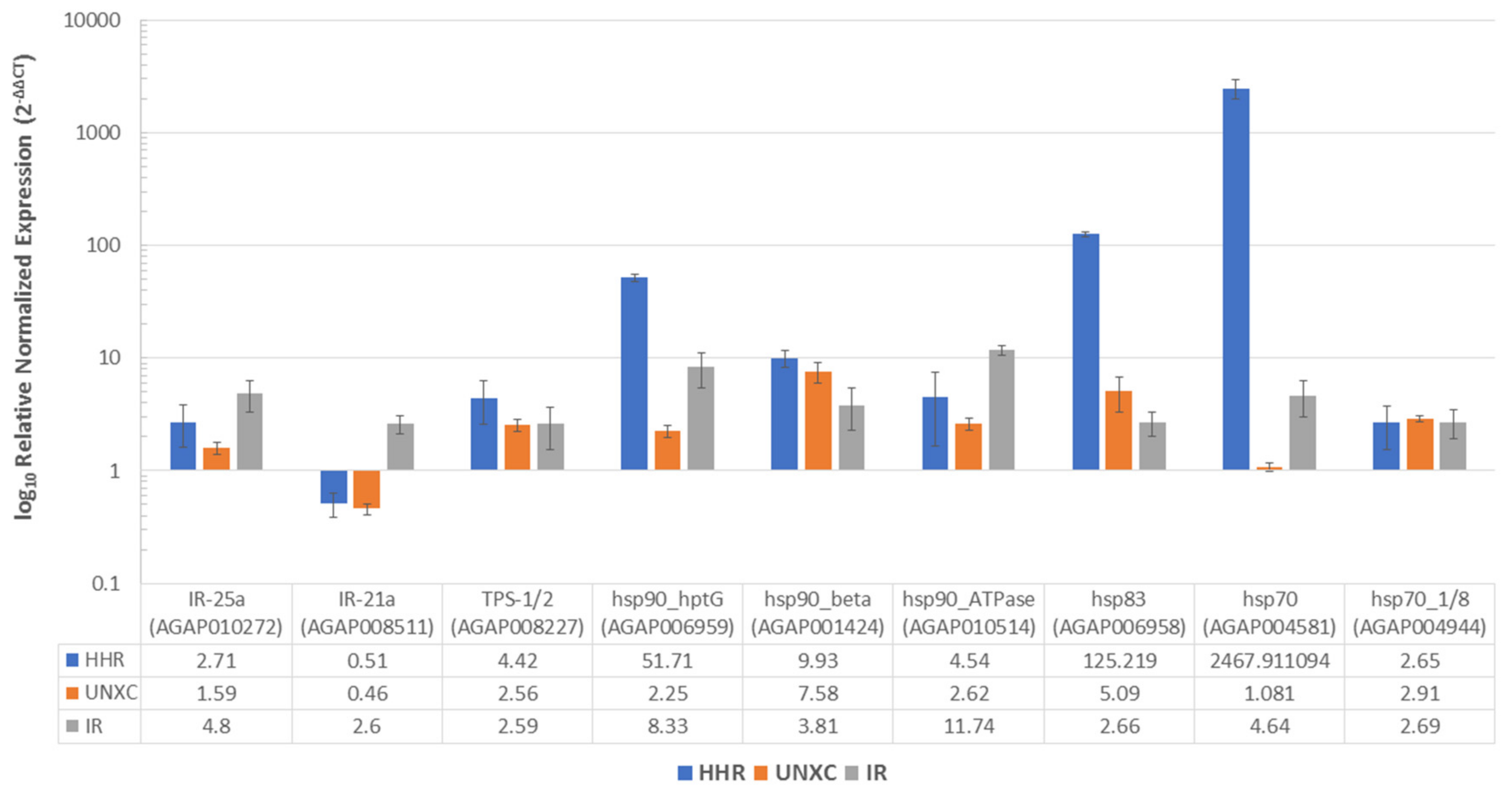
3.5. Impact of Heat Hardening on Expression Profile of Thermotolerance- and Resistance-Associated Genes
4. Discussion
4.1. Evidence of Sympatry between An. coluzzii and An. gambiae s.s. in Northern Nigeria
4.2. Evidence of High Intensity Thermal Stress Tolerance in Anopheles gambiae s.l. Larvae
4.3. Heat Hardening Enhances Insecticides Tolerance in Larvae and Adult Anopheles Gambiae s.l.
4.4. 2La Chromosomal Inversion Enhances Thermotolerance and Permethrin Resistance
4.5. Common Metabolic Resistance Genes Are Associated with Thermotolerance and Permethrin Resistance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hodgson, J.A.; Thomas, C.D.; Oliver, T.H.; Anderson, B.J.; Brereton, T.M.; Crone, E.E. Predicting insect phenology across space and time. Glob. Chang. Biol. 2011, 17, 1289–1300. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Sgro, C. Climate change and evolutionary adaptation. Nat. Cell Biol. 2011, 470, 479–485. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, D.; Ma, A.; Hwang, J.; Bennett, A.; Sturrock, H.; Fan, J.; Zhang, W.; Yang, D.; Feng, X.; et al. Predicting malaria vector distribution under climate change scenarios in China: Challenges for malaria elimination. Sci. Rep. 2016, 6, 20604. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Lippi, C.; Zermoglio, F. Shifting transmission risk for malaria in Africa with climate change: A framework for planning and intervention. Malar. J. 2020, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Semakula, H.M.; Song, G.; Achuu, S.P.; Shen, M.; Chen, J.; Mukwaya, P.I.; Oulu, M.; Mwendwa, P.M.; Abalo, J.; Zhang, S. Prediction of future malaria hotspots under climate change in sub-Saharan Africa. Clim. Chang. 2017, 143, 415–428. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Jacob, D.; Taylor, M.; Bindi, M.; Brown, S.; Camilloni, I.; Diedhiou, A.; Djalante, R.; Ebi, K.L.; Engelbrecht, F.; et al. Impacts of 1.5 °C Global Warming on Natural and Human Systems; IPCC Secretariat: Geneva, Switzerland, 2019. [Google Scholar]
- Rebaudo, F.; Struelens, Q.; Dangles, O. Modelling temperature-dependent development rate and phenology in arthropods: The devRate package for r. Methods Ecol. Evol. 2018, 9, 1144–1150. [Google Scholar] [CrossRef]
- Paaijmans, K.P.; Blanford, S.; Bell, A.S.; Blanford, J.I.; Read, A.F.; Thomas, M.B. Influence of climate on malaria transmission depends on daily temperature variation. Proc. Natl. Acad. Sci. USA 2010, 107, 15135–15139. [Google Scholar] [CrossRef]
- Villena, O.C.; Ryan, S.J.; Murdock, C.C.; Johnson, L.R. Temperature impacts the transmission of malaria parasites by Anopheles gambiae and Anopheles stephensi mosquitoes. bioRxiv 2020. [Google Scholar] [CrossRef]
- Shapiro, L.L.M.; Whitehead, S.A.; Thomas, M.B. Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biol. 2017, 15, e2003489. [Google Scholar] [CrossRef]
- Christiansen-Jucht, C.; E Parham, P.; Saddler, A.; Koella, J.C.; Basañez, M.-G. Temperature during larval development and adult maintenance influences the survival of Anopheles gambiae s.s. Parasites Vectors 2014, 7, 489. [Google Scholar] [CrossRef]
- Huang, J.; Walker, E.D.; Vulule, J.; Miller, J.R. Daily temperature profiles in and around Western Kenyan larval habitats of Anopheles gambiae as related to egg mortality. Malar. J. 2006, 5, 87. [Google Scholar] [CrossRef]
- Hodjati, M.H.; Curtis, C.F. Effects of permethrin at different temperatures on pyrethroid-resistant and susceptible strains of Anopheles. Med Veter- Èntomol. 1999, 13, 415–422. [Google Scholar] [CrossRef]
- Glunt, K.D.; Oliver, S.V.; Hunt, R.H.; Paaijmans, K.P. The impact of temperature on insecticide toxicity against the malaria vectors Anopheles arabiensis and Anopheles funestus. Malar. J. 2018, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Glunt, K.D.; Paaijmans, K.P.; Read, A.F.; Thomas, M.B. Environmental temperatures significantly change the impact of insecticides measured using WHOPES protocols. Malar. J. 2014, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Wang, Z.; Chung, H. Climate change and the genetics of insecticide resistance. Pest Manag. Sci. 2019, 76, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Ayala, D.; Ullastres, A.; Gonzã¡lez, J. Adaptation through chromosomal inversions in Anopheles. Front. Genet. 2014, 5, 129. [Google Scholar] [CrossRef] [PubMed]
- Dobzhansky, T.; Dobzhansky, T.G. Genetics of the Evolutionary Process; Columbia University Press: New York, NY, USA, 1971; Volume 139. [Google Scholar]
- Kirkpatrick, M. How and Why Chromosome Inversions Evolve. PLoS Biol. 2010, 8, e1000501. [Google Scholar] [CrossRef]
- Cassone, B.J.; Molloy, M.J.; Cheng, C.; Tan, J.C.; Hahn, M.W.; Besansky, N.J. Divergent transcriptional response to thermal stress by Anopheles gambiae larvae carrying alternative arrangements of inversion 2La. Mol. Ecol. 2011, 20, 2567–2580. [Google Scholar] [CrossRef]
- Coluzzi, M.; Sabatini, A.; Petrarca, V.; Di Deco, M. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 483–497. [Google Scholar] [CrossRef]
- Wondji, C.; Simard, F.; Petrarca, V.; Etang, J.; Santolamazza, F.; Della Torre, A.; Fontenille, D. Species and Populations of theAnopheles gambiaeComplex in Cameroon with Special Emphasis on Chromosomal and Molecular Forms ofAnopheles gambiaes.s. J. Med Èntomol. 2005, 42, 998–1005. [Google Scholar] [CrossRef]
- Riehle, M.M.; Bukhari, T.; Gneme, A.; Guelbeogo, W.M.; Coulibaly, B.; Fofana, A.; Pain, A.; Bischoff, E.; Renaud, F.; Beavogui, A.H.; et al. The Anopheles gambiae 2La chromosome inversion is associated with susceptibility to Plasmodium falciparum in Africa. eLife 2017, 6, e25813. [Google Scholar] [CrossRef] [PubMed]
- Fouet, C.; Gray, E.; Besansky, N.J.; Costantini, C. Adaptation to Aridity in the Malaria Mosquito Anopheles gambiae: Chromosomal Inversion Polymorphism and Body Size Influence Resistance to Desiccation. PLOS ONE 2012, 7, e34841. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.M.; Rocca, K.A.C.; Costantini, C.; Besansky, N.J. Inversion 2La is associated with enhanced desiccation resistance in Anopheles gambiae. Malar. J. 2009, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Rocca, K.A.C.; Gray, E.M.; Costantini, C.; Besansky, N.J. 2La chromosomal inversion enhances thermal tolerance of Anopheles gambiae larvae. Malar. J. 2009, 8, 147. [Google Scholar] [CrossRef]
- Brooke, B.; Hunt, R.H.; Coetzee, M. Resistance to dieldrin + fipronil assorts with chromosome inversion 2La in the malaria vector Anopheles gambiae. Med Veter- Èntomol. 2000, 14, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Reidenbach, K.R.; Cheng, C.; Liu, F.; Liu, C.; Besansky, N.J.; Syed, Z. Cuticular differences associated with aridity acclimation in African malaria vectors carrying alternative arrangements of inversion 2La. Parasites Vectors 2014, 7, 176. [Google Scholar] [CrossRef]
- Ayala, D.; Zhang, S.; Chateau, M.; Fouet, C.; Morlais, I.; Costantini, C.; Hahn, M.W.; Besansky, N.J. Association mapping desiccation resistance within chromosomal inversions in the African malaria vector Anopheles gambiae. Mol. Ecol. 2018, 28, 1333–1342. [Google Scholar] [CrossRef]
- VanHook, A.M. Understanding hygrosensation: How flies sense changes in humidity. Sci. Signal. 2016, 9, ec127. [Google Scholar] [CrossRef]
- Enjin, A.; Zaharieva, E.E.; Frank, D.D.; Mansourian, S.; Suh, G.S.; Gallio, M.; Stensmyr, M.C. Humidity Sensing in Drosophila. Curr. Biol. 2016, 26, 1352–1358. [Google Scholar] [CrossRef]
- White, B.J.; Hahn, M.W.; Pombi, M.; Cassone, B.J.; Lobo, N.F.; Simard, F.; Besansky, N.J. Localization of Candidate Regions Maintaining a Common Polymorphic Inversion (2La) in Anopheles gambiae. PLoS Genet. 2007, 3, e217. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Mukhtar, M.M.; Datti, J.A.; Irving, H.; Kusimo, M.O.; Tchapga, W.; Lawal, N.; Sambo, F.I.; Wondji, C.S. Temporal escalation of Pyrethroid Resistance in the major malaria vector Anopheles coluzzii from Sahelo-Sudanian Region of northern Nigeria. Sci. Rep. 2019, 9, 7395. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Mukhtar, M.M.; Irving, H.; Labbo, R.; Kusimo, M.O.; Mahamadou, I.; Wondji, C.S. High Plasmodium infection and multiple insecticide resistance in a major malaria vector Anopheles coluzzii from Sahel of Niger Republic. Malar. J. 2019, 18, 181. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; A Manu, Y.; Tukur, Z.; Irving, H.; Wondji, C.S. High frequency of kdr L1014F is associated with pyrethroid resistance in Anopheles coluzzii in Sudan savannah of northern Nigeria. BMC Infect. Dis. 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gillies, M.; Coetzee, M. A supplement to anophelinae of Africa south of Sahara (Afro-tropical region). Publ. South Afr. Inst. Med. Res. 1987, 55, 1–143. [Google Scholar]
- Livak, K.J. Organization and Mapping of a Sequence on the Drosophila Melanogaster X and Y Chromosomes That Is Transcribed during Spermatogenesis. Genetics 1984, 107, 611–634. [Google Scholar] [CrossRef] [PubMed]
- Santolamazza, F.; Mancini, E.; Simard, F.; Qi, Y.; Tu, Z.; Della Torre, A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar. J. 2008, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Benedict, M.Q.; Cockburn, A.; A Seawright, J. Heat-shock mortality and induced thermotolerance in larvae of the mosquito Anopheles albimanus. J. Am. Mosq. Control. Assoc. 1991, 7, 547–550. [Google Scholar]
- Oliver, S.V.; Brooke, B.D. The effect of elevated temperatures on the life history and insecticide resistance phenotype of the major malaria vector Anopheles arabiensis (Diptera: Culicidae). Malar. J. 2017, 16, 73. [Google Scholar] [CrossRef]
- WHO. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; World Health Organization: Geneva, Switzerland, 2005; pp. 1–41. [Google Scholar]
- WHO. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes; World Health Organization: Geneva, Switzerland, 2013; ISBN 978 92 4 150515 4 4. [Google Scholar]
- White, B.J.; Besansky, N.J.; Grushko, O.; Della Torre, A.; Sharakhov, I.; Brengues, C.; Kayondo, J.K.; Santolamazza, F.; Simard, F.; Pombi, M.; et al. Molecular Karyotyping of the 2La Inversion in Anopheles Gambiae. Am. J. Trop. Med. Hyg. 2007, 76, 334–339. [Google Scholar] [CrossRef]
- Sharakhov, I.V.; White, B.J.; Kayondo, J.; Lobo, N.F.; Santolamazza, F.; della Torre, A.; Simard, F.; Collins, F.H.; Besansky, N.J. Breakpoint structure reveals the unique origin of an interspecific chromosomal inversion (2La) in the Anopheles gambiae complex. Proc. Natl. Acad. Sci. USA 2006, 103, 6258–6262. [Google Scholar] [CrossRef]
- Dahlgaard, J.; Loeschcke, V.; Michalak, P.; Justesen, J. Induced thermotolerance and associated expression of the heat-shock protein Hsp70 in adultDrosophila melanogaster. Funct. Ecol. 1998, 12, 786–793. [Google Scholar] [CrossRef]
- Adedeji, E.O.; Ogunlana, O.O.; Fatumo, S.; Beder, T.; Ajamma, Y.; Koenig, R.; Adebiyi, E. Anopheles metabolic proteins in malaria transmission, prevention and control: A review. Parasites Vectors 2020, 13, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.B.; Lopez-Martinez, G.; Patrick, K.R.; Phillips, Z.P.; Krause, T.B.; Denlinger, D.L. Drinking a hot blood meal elicits a protective heat shock response in mosquitoes. Proc. Natl. Acad. Sci. USA 2011, 108, 8026–8029. [Google Scholar] [CrossRef]
- Greppi, C.; Laursen, W.J.; Budelli, G.; Chang, E.C.; Daniels, A.M.; van Giesen, L.; Smidler, A.L.; Catteruccia, F.; Garrity, P.A. Mosquito heat seeking is driven by an ancestral cooling receptor. Science 2020, 367, 681–684. [Google Scholar] [CrossRef]
- Lü, X.; Han, S.-C.; Li, Z.-G.; Li, L.-Y.; Li, J. Gene Characterization and Enzymatic Activities Related to Trehalose Metabolism of In Vitro Reared Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) under Sustained Cold Stress. Insects 2020, 11, 767. [Google Scholar] [CrossRef]
- Riveron, J.M.; Ibrahim, S.S.; Chanda, E.; Mzilahowa, T.; Cuamba, N.; Irving, H.; Barnes, K.G.; Ndula, M.; Wondji, C.S. The highly polymorphic CYP6M7 cytochrome P450 gene partners with the directionally selected CYP6P9a and CYP6P9b genes to expand the pyrethroid resistance front in the malaria vector Anopheles funestus in Africa. BMC Genom. 2014, 15, 1–19. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Fadel, A.N.; Ibrahim, S.S.; Tchouakui, M.; Terence, E.; Wondji, M.J.; Tchoupo, M.; Wanji, S.; Wondji, C.S. A combination of metabolic resistance and high frequency of the 1014F kdr mutation is driving pyrethroid resistance in Anopheles coluzzii population from Guinea savanna of Cameroon. Parasites Vectors 2019, 12, 263. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Fadel, A.N.; Tchouakui, M.; Terence, E.; Wondji, M.J.; Tchoupo, M.; Kérah-Hinzoumbé, C.; Wanji, S.; Wondji, C.S. High insecticide resistance in the major malaria vector Anopheles coluzzii in Chad Republic. Infect. Dis. Poverty 2019, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- E Sinka, M.; Bangs, M.J.; Manguin, S.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Kabaria, C.W.; et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasites Vectors 2010, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Onyabe, D.Y.; E Conn, J. The distribution of two major malaria vectors, Anopheles gambiae and Anopheles arabiensis, in Nigeria. Memórias do Instituto Oswaldo Cruz 2001, 96, 1081–1084. [Google Scholar] [CrossRef]
- Ranson, H.; Abdallah, H.; Badolo, A.; Guelbeogo, W.M.; Kerah-Hinzoumbe, C.; Yangalbe-Kalnone, E.; Sagnon, N.; Simard, F.; Coetzee, M. Insecticide resistance in Anopheles gambiae: Data from the first year of a multi-country study highlight the extent of the problem. Malar. J. 2009, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Gimonneau, G.; Bouyer, J.; Morand, S.; Besansky, N.J.; Diabate, A.; Simard, F. A behavioral mechanism underlying ecological divergence in the malaria mosquito Anopheles gambiae. Behav. Ecol. 2010, 21, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Dao, A.; Yaro, A.S.; Diallo, M.; Timbiné, S.; Huestis, D.L.; Kassogué, Y.; Traoré, A.I.; Sanogo, Z.L.; Samaké, D.; Lehmann, T. Signatures of aestivation and migration in Sahelian malaria mosquito populations. Nat. Cell Biol. 2014, 516, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, T.; Weetman, D.; Huestis, D.L.; Yaro, A.S.; Kassogue, Y.; Diallo, M.; Donnelly, M.J.; Dao, A. Tracing the origin of the early wet-seasonAnopheles coluzziiin the Sahel. Evol. Appl. 2017, 10, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Huestis, D.L.; Artis, M.L.; Armbruster, P.A.; Lehmann, T. Photoperiodic responses of Sahelian malaria mosquitoes Anopheles coluzzii and An. arabiensis. Parasites Vectors 2017, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Morange, M.; Bensaude, O. Protein denaturation during heat shock and related stress. Escherichia coli beta-galactosidase and Photinus pyralis luciferase inactivation in mouse cells. J. Biol. Chem. 1989, 264, 10487–10492. [Google Scholar] [CrossRef]
- Glunt, K.D.; Blanford, J.I.; Paaijmans, K.P. Chemicals, Climate, and Control: Increasing the Effectiveness of Malaria Vector Control Tools by Considering Relevant Temperatures. PLOS Pathog. 2013, 9, e1003602. [Google Scholar] [CrossRef]
- Matoke-Muhia, D.; Gimnig, J.E.; Kamau, L.; Shililu, J.; Bayoh, M.N.; Walker, E.D. Decline in frequency of the 2La chromosomal inversion in Anopheles gambiae (s.s.) in Western Kenya: correlation with increase in ownership of insecticide-treated bed nets. Parasites Vectors 2016, 9, 334. [Google Scholar] [CrossRef]
- Labbé, P.; Berticat, C.; Berthomieu, A.; Unal, S.; Bernard, C.; Weill, M.; Lenormand, T. Forty Years of Erratic Insecticide Resistance Evolution in the Mosquito Culex pipiens. PLoS Genet. 2007, 3, e205. [Google Scholar] [CrossRef]
- Nigatu, W.; Curtis, C.F.; Lulu, M. Test for association of DDT resistance with inversion polymorphism in Anopheles arabiensis from Ethiopia. J. Am. Mosq. Control. Assoc. 1995, 11, 238–240. [Google Scholar]
- Brooke, B.; Hunt, R.H.; Chandre, F.; Carnevale, P.; Coetzee, M. Stable Chromosomal Inversion Polymorphisms and Insecticide Resistance in the Malaria Vector MosquitoAnopheles gambiae (Diptera: Culicidae). J. Med Èntomol. 2002, 39, 568–573. [Google Scholar] [CrossRef]
- Love, R.R.; Steele, A.M.; Coulibaly, M.B.; Traore, S.F.; Emrich, S.J.; Fontaine, M.; Besansky, N.J. Chromosomal inversions and ecotypic differentiation inAnopheles gambiae: the perspective from whole-genome sequencing. Mol. Ecol. 2016, 25, 5889–5906. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Sørensen, J.G.; Loeschcke, V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 2003, 28, 175–216. [Google Scholar] [CrossRef]
- Loeschcke, V.; Krebs, R.A.; Barker, J.S.F. Genetic variation for resistance and acclimation to high temperature stress in Drosophila buzzatii. Biol. J. Linn. Soc. 1994, 52, 83–92. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. HEAT-SHOCK PROTEINS, MOLECULAR CHAPERONES, AND THE STRESS RESPONSE: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Spring, J.H.; Robichaux, S.R.; Hamlin, J.A. The role of aquaporins in excretion in insects. J. Exp. Biol. 2009, 212, 358–362. [Google Scholar] [CrossRef]
- Thompson, S.N. Trehalose—the insect ‘blood’sugar. Adv. Insect Phys. 2003, 31, 205–285. [Google Scholar]

| Sampling Site | 44 °C (Larvae) | 100 mg/mL Permethrin (Larvae) | 0.75% Permethrin (Adults) | |||
|---|---|---|---|---|---|---|
| An. coluzzii | An. gambiae s.s. | An. coluzzii | An. gambiae s.s. | An. coluzzii | An. gambiae s.s. | |
| Auyo | 19 | 4 | 14 | 3 | 25 | 3 |
| BUK | 25 | 2 | 18 | 2 | 31 | 1 |
| G/Bappa | 8 | 11 | 10 | 7 | 17 | 11 |
| Pantami | 18 | 12 | 16 | 14 | 10 | 16 |
| Sub-total | 69 (70.4%) | 29 (29.6%) | 58 (69%) | 26 (31%) | 83 (72.8%) | 31 (27.2%) |
| Total | 98 | 84 | 114 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, S.S.; Mukhtar, M.M.; Muhammad, A.; Wondji, C.S. 2La Paracentric Chromosomal Inversion and Overexpressed Metabolic Genes Enhance Thermotolerance and Pyrethroid Resistance in the Major Malaria Vector Anopheles gambiae. Biology 2021, 10, 518. https://doi.org/10.3390/biology10060518
Ibrahim SS, Mukhtar MM, Muhammad A, Wondji CS. 2La Paracentric Chromosomal Inversion and Overexpressed Metabolic Genes Enhance Thermotolerance and Pyrethroid Resistance in the Major Malaria Vector Anopheles gambiae. Biology. 2021; 10(6):518. https://doi.org/10.3390/biology10060518
Chicago/Turabian StyleIbrahim, Sulaiman S., Muhammad M. Mukhtar, Abdullahi Muhammad, and Charles S. Wondji. 2021. "2La Paracentric Chromosomal Inversion and Overexpressed Metabolic Genes Enhance Thermotolerance and Pyrethroid Resistance in the Major Malaria Vector Anopheles gambiae" Biology 10, no. 6: 518. https://doi.org/10.3390/biology10060518
APA StyleIbrahim, S. S., Mukhtar, M. M., Muhammad, A., & Wondji, C. S. (2021). 2La Paracentric Chromosomal Inversion and Overexpressed Metabolic Genes Enhance Thermotolerance and Pyrethroid Resistance in the Major Malaria Vector Anopheles gambiae. Biology, 10(6), 518. https://doi.org/10.3390/biology10060518










