Breast Cancer Detection from a Urine Sample by Dog Sniffing: A Preliminary Study for the Development of a New Screening Device, and a Literature Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls
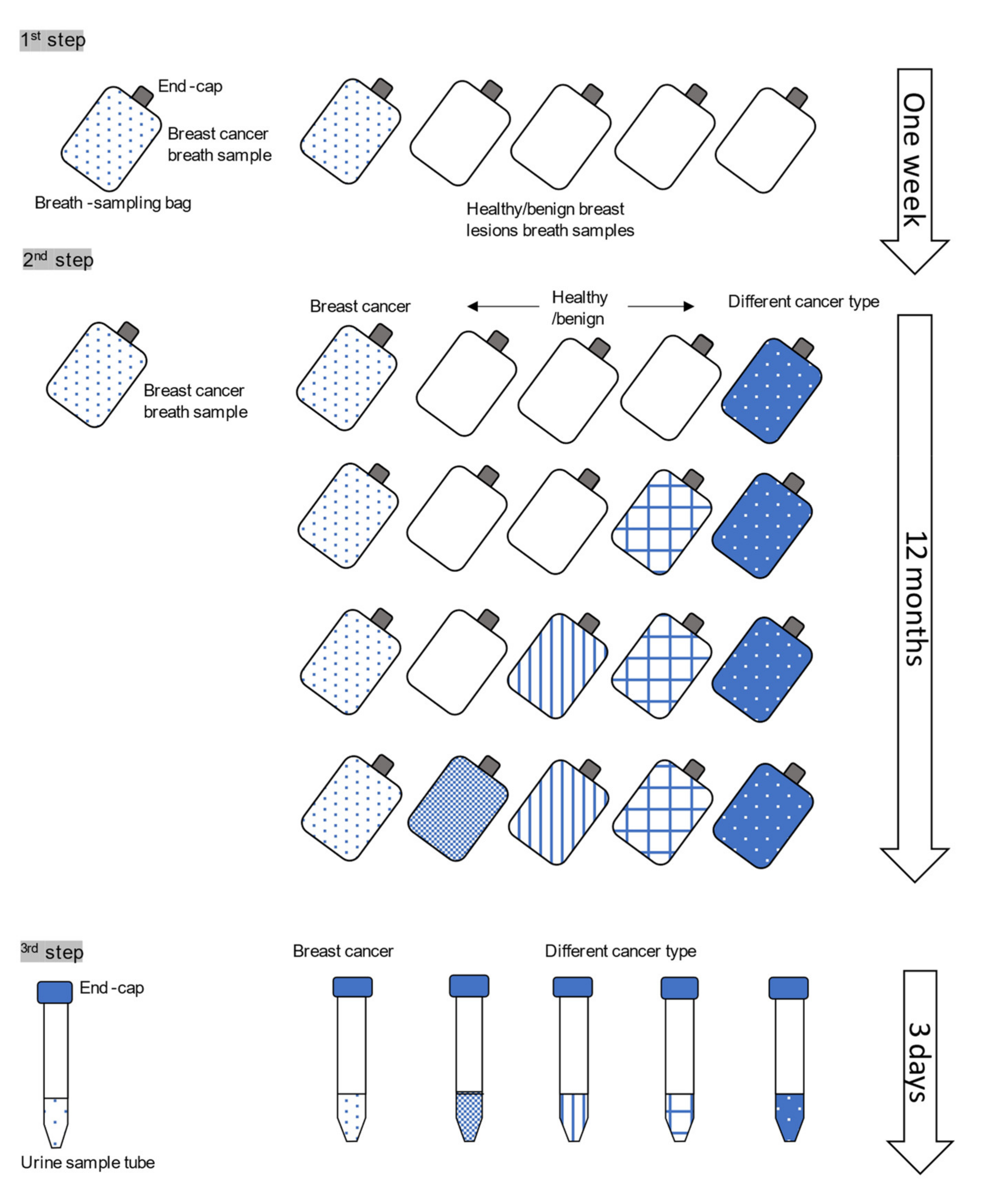
2.2. Urine Sampling
2.3. Dog and Training
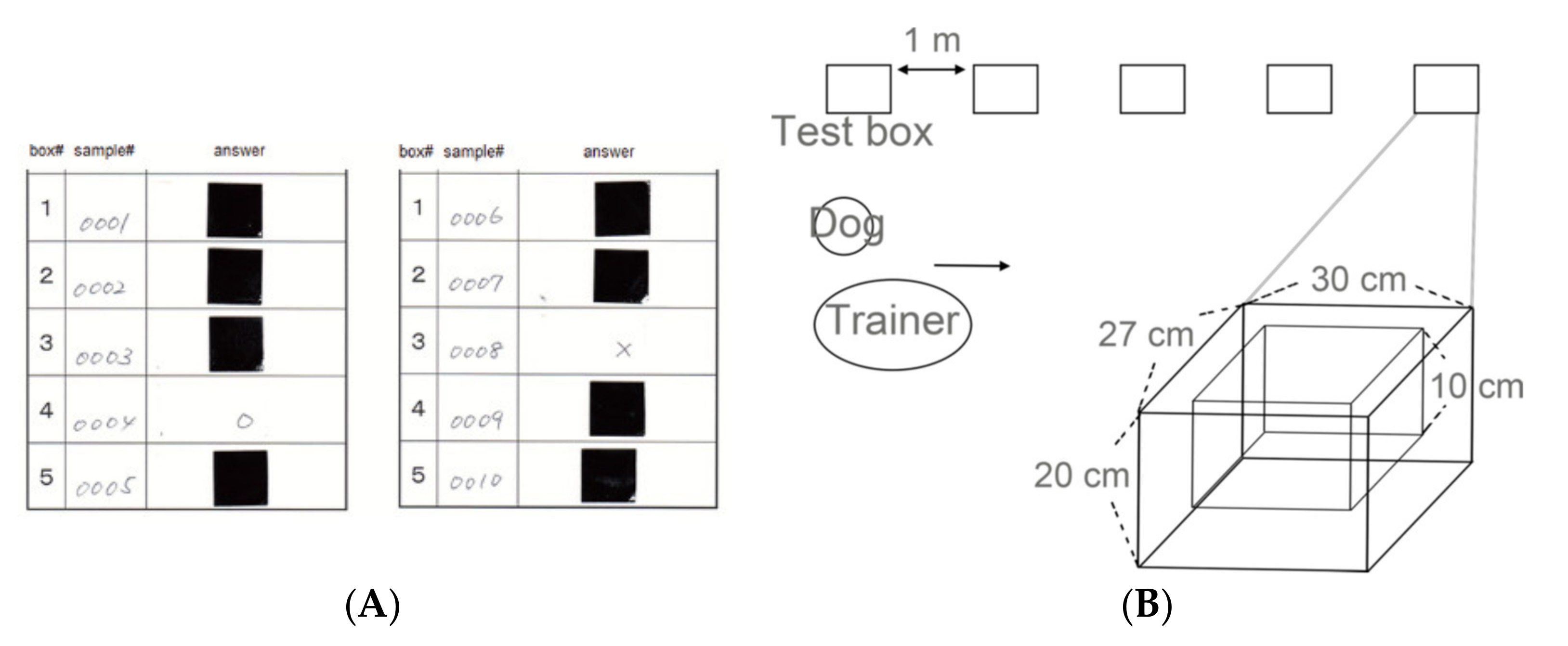
2.4. The Testing Settings
2.4.1. The Test Box
2.4.2. Detection Testing of Urine Samples from Breast Cancer Patients
2.4.3. Evaluation of the Dog’s Response
2.5. Statistical Analysis
2.6. Ethics Approval and Consent to Participate
3. Results
3.1. Patients
3.2. Dog Condition and Round Times before Decision
3.3. Sensitivity and Specificity of the Detection Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, N.; Suzuki, A.; Sobue, T.; Kawai, M.; Yamamoto, S.; Zheng, Y.-F.; Shiono, Y.N.; Saito, H.; Kuriyama, S.; Tohno, E.; et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): A randomised controlled trial. Lancet 2016, 387, 341–348. [Google Scholar] [CrossRef]
- Nelson, H.D.; Tyne, K.; Naik, A.; Bougatsos, C.; Chan, B.K.; Humphrey, L. Screening for breast cancer: An update for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2009, 151, 727–737. [Google Scholar] [CrossRef]
- Njor, S.; Nyström, L.; Moss, S.; Paci, E.; Broeders, M.; Segnan, N.; Lynge, E. Breast cancer mortality in mammographic screening in Europe: A review of incidence-based mortality studies. J. Med. Screen. 2012, 19 (Suppl. S1), 33–41. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M. Mammography screening: A major issue in medicine. Eur. J. Cancer 2018, 90, 34–62. [Google Scholar] [CrossRef]
- Uchida, K.; Ohashi, H.; Kinoshita, S.; Nogi, H.; Kato, K.; Toriumi, Y.; Yamashita, A.; Kamio, M.; Mimoto, R.; Takeyama, H. Breast cancer screening and the changing population pyramid of Japan. Breast Cancer 2015, 22, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, J.; Siersma, V.D. Long-term psychosocial consequences of false-positive screening mammography. Ann. Fam. Med. 2013, 11, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Tohno, E.; Ueno, E.; Watanabe, H. Ultrasound screening of breast cancer. Breast Cancer 2009, 16, 18–22. [Google Scholar] [CrossRef]
- Warner, E. Screening BRCA1 and BRCA2 mutation carriers for breast cancer. Cancers 2018, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Concha, A.R.; Guest, C.M.; Harris, R.; Pike, T.W.; Feugier, A.; Zulch, H.; Mills, D.S. Canine olfactory thresholds to amyl acetate in a biomedical detection scenario. Front. Vet. Sci. 2018, 5, 345. [Google Scholar] [CrossRef]
- Pickel, D.; Manucy, G.P.; Walker, D.B.; Hall, S.B.; Walker, J.C. Evidence for canine olfactory detection of melanoma. Appl. Anim. Behav. Sci. 2004, 89, 107–116. [Google Scholar] [CrossRef]
- Elliker, K.R.; Williams, H.C. Detection of skin cancer odours using dogs: A step forward in melanoma detection training and research methodologies. Br. J. Dermatol. 2016, 175, 851–852. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.M. Olfactory detection of human bladder cancer by dogs: Proof of principle study. BMJ Br. Med. J. 2004, 329, 712. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, M.; Jezierski, T.; Broffman, M.; Hubbard, A.; Turner, K.; Janecki, T. Diagnostic accuracy of canine scent detection in early- and late-stage lung and breast cancers. Integr. Cancer Ther. 2006, 5, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, R.; Boedeker, E.; Friedrich, U.; Sagert, J.; Dippon, J.; Friedel, G.; Walles, T. Canine scent detection in the diagnosis of lung cancer: Revisiting a puzzling phenomenon. Eur. Respir. J. 2012, 39, 669–676. [Google Scholar] [CrossRef]
- Schallschmidt, K.; Becker, R.; Zwaka, H.; Menzel, R.; Johnen, D.; Fischer-Tenhagen, C.; Rolff, J.; Nehls, I. In vitro cultured lung cancer cells are not suitable for animal-based breath biomarker detection. J. Breath Res. 2015, 9. [Google Scholar] [CrossRef]
- Hackner, K.; Errhalt, P.; Mueller, M.R.; Speiser, M.; Marzluf, B.A.; Schulheim, A.; Schenk, P.; Bilek, J.; Doll, T. Canine scent detection for the diagnosis of lung cancer in a screening-like situation. J. Breath Res. 2016, 10. [Google Scholar] [CrossRef]
- Junqueira, H.; Quinn, T.A.; Biringer, R.; Hussein, M.; Smeriglio, C.; Barrueto, L.; Finizio, J.; Huang, X.Y.M. Accuracy of Canine Scent Detection of Non-Small Cell Lung Cancer in Blood Serum. J. Am. Osteopath. Assoc. 2019, 119, 413. [Google Scholar] [CrossRef]
- Seo, I.S.; Lee, H.G.; Koo, B.; Koh, C.S.; Park, H.Y.; Im, C.; Shin, H.C. Cross detection for odor of metabolic waste between breast and colorectal cancer using canine olfaction. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.T.; Schatz, C.B.; Myers, L.J.; Kosty, M.; Gonczy, C.; Kroener, J.; Tran, M.; Kurtzhals, P.; Heath, S.; Koziol, J.A.; et al. The use of canines in the detection of human cancers. J. Altern. Complementary Med. 2008, 14, 61–67. [Google Scholar] [CrossRef]
- Cornu, J.N.; Cancel-Tassin, G.; Ondet, V.; Girardet, C.; Cussenot, O. Olfactory detection of prostate cancer by dogs sniffing urine: A step forward in early diagnosis. Eur. Urol. 2011, 59, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Elliker, K.R.; Sommerville, B.A.; Broom, D.M.; Neal, D.E.; Armstrong, S.; Williams, H.C. Key considerations for the experimental training and evaluation of cancer odour detection dogs: Lessons learnt from a double-blind, controlled trial of prostate cancer detection. BMC Urol. 2014, 14, 22. [Google Scholar] [CrossRef]
- Horvath, G.; Jarverud, G.A.; Jarverud, S.; Horvath, I. Human ovarian carcinomas detected by specific odor. Integr. Cancer Ther. 2008, 7, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Andersson, H.; Paulsson, G. Characteristic odour in the blood reveals ovarian carcinoma. BMC Cancer 2010, 10, 643. [Google Scholar] [CrossRef]
- Horvath, G.; Andersson, H.; Nemes, S. Cancer odor in the blood of ovarian cancer patients: A retrospective study of detection by dogs during treatment, 3 and 6 months afterward. BMC Cancer 2013, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, H.; Kohnoe, S.; Yamazato, T.; Satoh, Y.; Morizono, G.; Shikata, K.; Morita, M.; Watanabe, A.; Morita, M.; Kakeji, Y.; et al. Colorectal cancer screening with odour material by canine scent detection. Gut 2011, 60, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Kitiyakara, T.; Redmond, S.; Unwanatham, N.; Rattanasiri, S.; Thakkinstian, A.; Tangtawee, P.; Mingphruedhi, S.; Sobhonslidsuk, A.; Intaraprasong, P.; Kositchaiwat, C. The detection of hepatocellular carcinoma (HCC) from patients’ breath using canine scent detection: A proof-of-concept study. J. Breath Res. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Flores, H.; Apresa-García, T.; Garay-Villar, Ó.; Sánchez-Pérez, A.; Flores-Villegas, D.; Bandera-Calderón, A.; García-Palacios, R.; Rojas-Sánchez, T.; Romero-Morelos, P.; Sánchez-Albor, V.; et al. A non-invasive tool for detecting cervical cancer odor by trained scent dogs. BMC Cancer 2017, 17, 79. [Google Scholar] [CrossRef]
- Yamamoto, A.; Kamoi, S.; Kurose, K.; Ito, M.; Takeshita, T.; Kure, S.; Sakamoto, K.; Sato, Y.; Miyashita, M. The Trained Sniffer Dog Could Accurately Detect the Urine Samples from the Patients with Cervical Cancer, and Even Cervical Intraepithelial Neoplasia Grade 3: A Pilot Study. Cancers 2020, 12, 3291. [Google Scholar] [CrossRef]
- Jezierski, T.; Walczak, M.; Ligor, T.; Rudnicka, J.; Buszewski, B. Study of the art: Canine olfaction used for cancer detection on the basis of breath odour. Perspectives and limitations. J. Breath Res. 2015, 9. [Google Scholar] [CrossRef]
- Williams, H.; Pembroke, A. Sniffer dogs in the melanoma clinic? Lancet 1989, 333, 734. [Google Scholar] [CrossRef]
- Church, J.; Williams, H. Another sniffer dog for the clinic? Lancet 2001, 358, 930. [Google Scholar] [CrossRef]
- McCulloch, M.; Turner, K.; Broffman, M. Lung cancer detection by canine scent: Will there be a lab in the lab? Eur. Respir. J. 2012, 39, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.L.; Browne, C.M.; Schoon, A.; Cox, C.; Poling, A. Animal olfactory detection of human diseases: Guidelines and systematic review. J. Vet. Behav. 2017, 20, 59–73. [Google Scholar] [CrossRef]
- Boedeker, E.; Friedel, G.; Walles, T. Sniffer dogs as part of a bimodal bionic research approach to develop a lung cancer screening. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 511–515. [Google Scholar] [CrossRef]
- Gika, H.G.; Theodoridis, G.A.; Wilson, I.D. Liquid chromatography and ultra-performance liquid chromatography-mass spectrometry fingerprinting of human urine: Sample stability under different handling and storage conditions for metabonomics studies. J. Chromatogr. A 2008, 1189, 314–322. [Google Scholar] [CrossRef]
- Amundsen, T.; Sundstrom, S.; Buvik, T.; Gederaas, O.A.; Haaverstad, R. Can dogs smell lung cancer? First study using exhaled breath and urine screening in unselected patients with suspected lung cancer. Acta. Oncol. 2014, 53, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Shirasu, M.; Touhara, K. The scent of disease: Volatile organic compounds of the human body related to disease and disorder. J. Biochem. 2011, 150, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Moreira, N.; Pinto, J.; Pires-Luis, A.S.; Henrique, R.; Jeronimo, C.; Bastos, M.L.; Gil, A.M.; Carvalho, M.; Guedes de Pinho, P. GC-MS metabolomics-based approach for the identification of a potential VOC-biomarker panel in the urine of renal cell carcinoma patients. J. Cell Mol. Med. 2017, 21, 2092–2105. [Google Scholar] [CrossRef]
- Woollam, M.; Teli, M.; Angarita-Rivera, P.; Liu, S.; Siegel, A.P.; Yokota, H.; Agarwal, M. Detection of volatile organic compounds (VOCS) in urine via gas chromatography-mass spectrometry QTOF to differentiate between localized and metastatic models of breast cancer. Sci. Rep. 2019, 9, 2526. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Ditkoff, B.A.; Fisher, P.; Greenberg, J.; Gunawardena, R.; Kwon, C.S.; Rahbari-Oskoui, F.; Wong, C. Volatile markers of breast cancer in the breath. Breast J. 2003, 9, 184–191. [Google Scholar] [CrossRef]
- Phillips, M.; Gleeson, K.; Hughes, J.M.; Greenberg, J.; Cataneo, R.N.; Baker, L.; McVay, W.P. Volatile organic compounds in breath as markers of lung cancer: A cross-sectional study. Lancet 1999, 353, 1930–1933. [Google Scholar] [CrossRef]
- Phillips, M.; Bauer, T.L.; Pass, H.I. A volatile biomarker in breath predicts lung cancer and pulmonary nodules. J. Breath Res. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Jobu, K.; Sun, C.; Yoshioka, S.; Yokota, J.; Onogawa, M.; Kawada, C.; Inoue, K.; Shuin, T.; Sendo, T.; Miyamura, M. Metabolomics study on the biochemical profiles of odor elements in urine of human with bladder cancer. Biol. Pharm. Bull. 2012, 35, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Trefz, P.; Bergmann, A.; Steffens, M.; Ziller, M.; Miekisch, W.; Schubert, J.S.; Kohler, H.; Reinhold, P. Physiological variability in volatile organic compounds (VOCs) in exhaled breath and released from faeces due to nutrition and somatic growth in a standardized caprine animal model. J. Breath Res. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Pleil, J.; Giese, R. Integrating exhaled breath diagnostics by disease-sniffing dogs with instrumental laboratory analysis. J. Breath Res. 2017, 11. [Google Scholar] [CrossRef]
- Sohn, E. Better cancer screening in resource-poor nations. Nature 2020, 579, S17–S19. [Google Scholar] [CrossRef]
| Diagnosis | Number |
|---|---|
| Gastric cancer | 38 (26.0%) |
| Cervical cancer | 36 (24.7%) |
| HSIL | 21 (14.4%) |
| Endometrial cancer | 17 (12%) |
| Ovarian cancer | 16 (11.0%) |
| Colorectal cancer | 7 (4.8%) |
| Peritoneal cancer | 3 (2.0%) |
| Uterine sarcoma | 2 (1.4%) |
| Esophageal cancer | 1 (0.7%) |
| Endometrial stromal sarcoma | 1 (0.7%) |
| Vulvar cancer | 1(0.7%) |
| Liposarcoma | 1 (0.7%) |
| Metastatic adrenal carcinoma | 1 (0.7%) |
| LSIL | 1 (0.7%) |
| Total | 146 |
| Box 1 | Box 2 | Box 3 | Box 4 | Box 5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test -Run | Diagnosis | Age | Stage | Diagnosis | Age | Stage | Diagnosis | Age | Stage | Diagnosis | Age | Stage | Diagnosis | Age | Stage |
| 1 | HSIL | 31 | NA | GC | 41 | IB | HSIL | 36 | NA | BC | 33 | 0 | HSIL | 35 | NA |
| 2 | EmC | 71 | IB | GC | 77 | IA | BC | 74 | I | GC | 70 | IA | GC | 70 | IA |
| 3 | GC | 60 | IB | CC | 51 | IIB | CC | 53 | IIB | CRC | 63 | II | BC | 56 | IIA |
| 4 | CC | 85 | IIIB | CRC | 71 | IIIB | CRC | 68 | IIIB | BC | 64 | IIIB | EmC | 57 | IIIA |
| 5 | GC | 74 | IB | EC | 59 | IA | HSIL | 50 | NA | HSIL | 47 | NA | BC | 60 | 0 |
| 6 | CRC | 70 | I | EmC | 70 | IIIC | BC | 75 | I | Metastatic adrenal cancer | 80 | IV | CC | 85 | IIIB |
| 7 | OC | 51 | IIIC | GC | 51 | IA | BC | 49 | IIA | HSIL | 46 | NA | EmC | 44 | IB |
| 8 | GC | 57 | IA | OC(rec) | 58 | NA | PerC | 61 | IIIC | GC | 60 | IIA | BC | 62 | IIIB |
| 9 | HSIL | 26 | NA | HSIL | 27 | NA | Liposarcoma | 39 | NA | BC | 28 | I | GC | 41 | IB |
| 10 | HSIL | 44 | NA | US | 41 | IV | GC | 57 | IIA | CC | 54 | IIIB | BC | 50 | 0 |
| 11 | HSIL | 44 | NA | ESS | 41 | IV | GC | 57 | IIA | CC | 54 | IIIB | BC | 50 | 0 |
| 12 | EmC | 70 | IB | GC | 71 | IA | CC | 74 | IB | BC | 69 | I | CC(rec) | 69 | NA |
| 13 | HSIL | 30 | NA | HSIL | 36 | NA | BC | 42 | I | GC | 55 | IV | CC | 34 | IA |
| 14 | CC | 61 | IB | OC (rec) | 65 | NA | BC | 56 | I | EmC | 79 | IV | GC | 75 | IA |
| 15 | GC | 64 | IA | BC | 59 | I | CC | 55 | IVB | CC | 57 | IA | PerC (rec) | 65 | NA |
| 16 | HSIL | 39 | NA | LSIL | 44 | NA | OC | 61 | IA | BC | 47 | IIA | HSIL | 39 | NA |
| 17 | HSIL | 18 | NA | CC | 82 | IIIB | BC | 38 | IIIA | CC | 36 | IA | HSIL | 38 | NA |
| 18 | HSIL | 44 | NA | BC | 47 | IIA | HSIL | 47 | NA | CC | 41 | IA | CC | 44 | IIIB |
| 19 | OC(rec) | 56 | NA | CC | 57 | IA | OC | 61 | IA | CC | 61 | IB | BC | 59 | I |
| 20 | BC | 67 | IIA | CC | 65 | IV | CC | 75 | IVB | CC | 62 | IB | GC | 63 | IA |
| 21 | Vulvar cancer | 78 | I | CC | 85 | IIIB | EmC | 82 | IV | BC | 84 | I | GC | 77 | IA |
| 22 | healthy | 39 | NA | EmC | 49 | IB | BC | 48 | I | GC | 46 | IA | HSIL | 42 | NA |
| 23 | GC | 64 | IA | BC | 64 | I | CC | 62 | IB | Healthy | 27 | NA | OC | 61 | IA |
| 24 | Healthy | 35 | NA | GC | 41 | IB | OC | 45 | IIIC | CC | 38 | IIIB | BC | 46 | I |
| 25 | OC | 50 | IC | Healthy | 39 | NA | GC | 51 | IA | BC | 48 | I | GC | 55 | IA |
| 26 | Healthy | 78 | NA | CC | 34 | IA | HSIL | 33 | NA | BC | 34 | I | HSIL | 35 | NA |
| 27 | Healthy | 56 | NA | GC | 77 | IA | BC | 67 | IIA | GC | 71 | IA | CRC | 70 | IV |
| 28 | CC | 51 | IIb | GC | 51 | IA | CC | 47 | CIS | BC | 49 | IIA | EmC | 49 | IB |
| 29 | GC | 70 | IA | EmC | 70 | IIIc | Healthy | 57 | NA | CRC | 68 | IIIB | BC | 69 | IIA |
| 30 | CC | 44 | IIIB | Healthy | 41 | NA | Uterine sarcoma | 41 | NA | BC | 44 | IIA | CC | 42 | IIIb |
| 31 | EmC | 71 | IB | BC | 71 | IIA | OC | 88 | IV | Healthy | 66 | NA | OC | 85 | IIIC |
| 32 | Healthy | 51 | NA | EmC | 70 | IB | BC | 69 | I | GC | 70 | IA | EmC | 63 | IC |
| 33 | CC | 62 | IB | OC | 61 | IA | Healthy | 53 | NA | BC | 61 | I | GC | 62 | IA |
| 34 | Healthy | 50 | NA | BC | 56 | I | OC(rec) | 56 | NA | GC | 55 | IA | CC | 57 | IA |
| 35 | GC | 77 | IA | Healthy | 60 | NA | EmC | 77 | IC | GC | 75 | IA | BC | 75 | I |
| 36 | BC | 59 | I | CC | 61 | IB | Healthy | 50 | NA | EmC(rec) | 58 | NA | GC | 57 | IA |
| 37 | GC | 57 | IIA | Healthy | 57 | NA | CC | 53 | IIB | CC | 51 | IIB | BC | 56 | IIA |
| 38 | Healthy | 56 | NA | CRC | 63 | II | BC | 64 | IIA | GC | 60 | IIA | CC | 53 | IIA |
| 39 | Healthy | 45 | NA | OC | 49 | IA | GC | 41 | IB | BC | 45 | I | EmC | 49 | IB |
| 40 | GC | 64 | IA | OC | 68 | IA | BC | 64 | I | OC | 56 | IA | Healthy | 60 | NA |
| Reference | Cancer Type | Material | Numbers of the Tested Cases | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Pickel, D.P., 2004 [11] | Malignant melanoma | tumor | 7 | 82% | 100% |
| Willis, 2004 [13] | bladder cancer | urine | 36 108 cancer negative | 41% | ND |
| McCulloch, M., 2006 [14] | lung cancer (LC), breast cancer (BC) | breath | LC: 55 BC: 31 83 healthy | LC: 99% | LC: 99% |
| BC: 88% | BC: 98% | ||||
| Gordon, R.T., 2008 [20] | BC, prostate cancer (PC) | breath | BC: 18 PC: 33 | ND “no better than chance” | ND |
| Horvath, G., 2008 [23] | ovarian cancer (OC) | tumor tissue | 31 control(fat/muscle/normal ovary) | 100%, | 97.50% |
| Horvath, G., 2010 [24] | OC | tumor tissue (T), blood (Bl) | 40 controls (4 uterine corpus cancer, 2 uterine cervical cancer, 2 vulvar cancer, and healthy) | T: 100%, Bl: 100% | T: 95% Bl: 98% |
| Cornu, J.N., 2010 [21] | OC | urine | 33 | 91% | 91% |
| 33 healthy | |||||
| Ehmann, R., 2012 [15] | LC | breath | 60, 110 healthy/50 COPD | 71% | 93% |
| Sonoda, H., 2011 [26] | CRC | breath (Br), stool (Stl) | Br: 33/132 healthy Stl: 37/148 healthy | Br: 91% Stl: 97% | Br:99% Stl: 99% |
| Horvath, G., 2013 [25] | OC | blood | 42 210 healthy | 97% | 99–100% |
| Elliker, K.R., 2014 [22] | PC | urine | 16 48 controls (healthy/hyperplasia) | 13–25% | 71% |
| Schallschmidt, K., 2015 [16] | LC | head space gas of cell culture | 10–20% | 40–50% | |
| Hackner, K., 2016 [17] | LC | breath | 29 93 without LC | Positive predictive values 30.9% Negative predictive value 84.0% | |
| Kitiyakara, T., 2017 [27] | HCC | breath | 37 healthy | 78% | ND |
| Guerrero-Flores, H., CC, 2017 [28] | CC | smear | 50 30 healthy controls | 92.80% | 99.10% |
| Seo, I.S., 2018 [19] | BC + CRC | cell culture liquid | >90% | <90% | |
| Junqueira, H., 2019 [18] | NCSLC | blood serum | ND healthy | 96.70% | 97.50% |
| Checkpoints | Methodological Recommendations | |
|---|---|---|
| Dogs | Breed | German Shepherd, Labrador Retriever. |
| Samples | Sampling tube | It should be simple and handy to be used by sample donors without training. |
| Storage time | Not determined. | |
| Sample collection | Collection in the same location. A large numbers/varieties of the samples. | |
| Control samples | They are comparable to positive samples except for disease status | |
| Training conditions | Reinforcement/ reward | Intermittent reinforcements |
| Sample arrangement | Odor line-up/circle | |
| Positive/Negative ratio | It should reflect the disease prevalence. Positive sample prevalence reflecting the prevalence of disease in operating setting. | |
| Testing conditions | Sample sources different from source used in training should be used. | |
| The dog, trainer, and experimenter are blind to the status of all samples (“Double-blinded”). | ||
| Accurate knowledge of sample status. | ||
| Sufficient large number of sample sources | ||
| Operation conditions | Ongoing training should be performed. Training conditions cannot be discriminable from operational conditions. | |
| Regular evaluation of performance with another diagnostic tool. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kure, S.; Iida, S.; Yamada, M.; Takei, H.; Yamashita, N.; Sato, Y.; Miyashita, M. Breast Cancer Detection from a Urine Sample by Dog Sniffing: A Preliminary Study for the Development of a New Screening Device, and a Literature Review. Biology 2021, 10, 517. https://doi.org/10.3390/biology10060517
Kure S, Iida S, Yamada M, Takei H, Yamashita N, Sato Y, Miyashita M. Breast Cancer Detection from a Urine Sample by Dog Sniffing: A Preliminary Study for the Development of a New Screening Device, and a Literature Review. Biology. 2021; 10(6):517. https://doi.org/10.3390/biology10060517
Chicago/Turabian StyleKure, Shoko, Shinya Iida, Marina Yamada, Hiroyuki Takei, Naoyuki Yamashita, Yuji Sato, and Masao Miyashita. 2021. "Breast Cancer Detection from a Urine Sample by Dog Sniffing: A Preliminary Study for the Development of a New Screening Device, and a Literature Review" Biology 10, no. 6: 517. https://doi.org/10.3390/biology10060517
APA StyleKure, S., Iida, S., Yamada, M., Takei, H., Yamashita, N., Sato, Y., & Miyashita, M. (2021). Breast Cancer Detection from a Urine Sample by Dog Sniffing: A Preliminary Study for the Development of a New Screening Device, and a Literature Review. Biology, 10(6), 517. https://doi.org/10.3390/biology10060517







