Author Contributions
Conceptualization, L.B.R., I.R. and M.d.M.C.; methodology, M.d.M.C. and L.B.R.; software, I.R.; validation, M.d.M.C. and I.G.-C.; investigation, M.d.M.C. and I.G.-C.; writing—original draft preparation, I.G.-C. and M.d.M.C.; writing—review and editing, E.C., I.R., L.B.R., F.C. and L.C.D.; supervision, E.C., F.C., I.R., M.d.M.C. and L.C.D.; project administration, I.R. and M.d.M.C.; funding acquisition, I.R. and M.d.M.C. All authors have read and agreed to the published version of the manuscript.
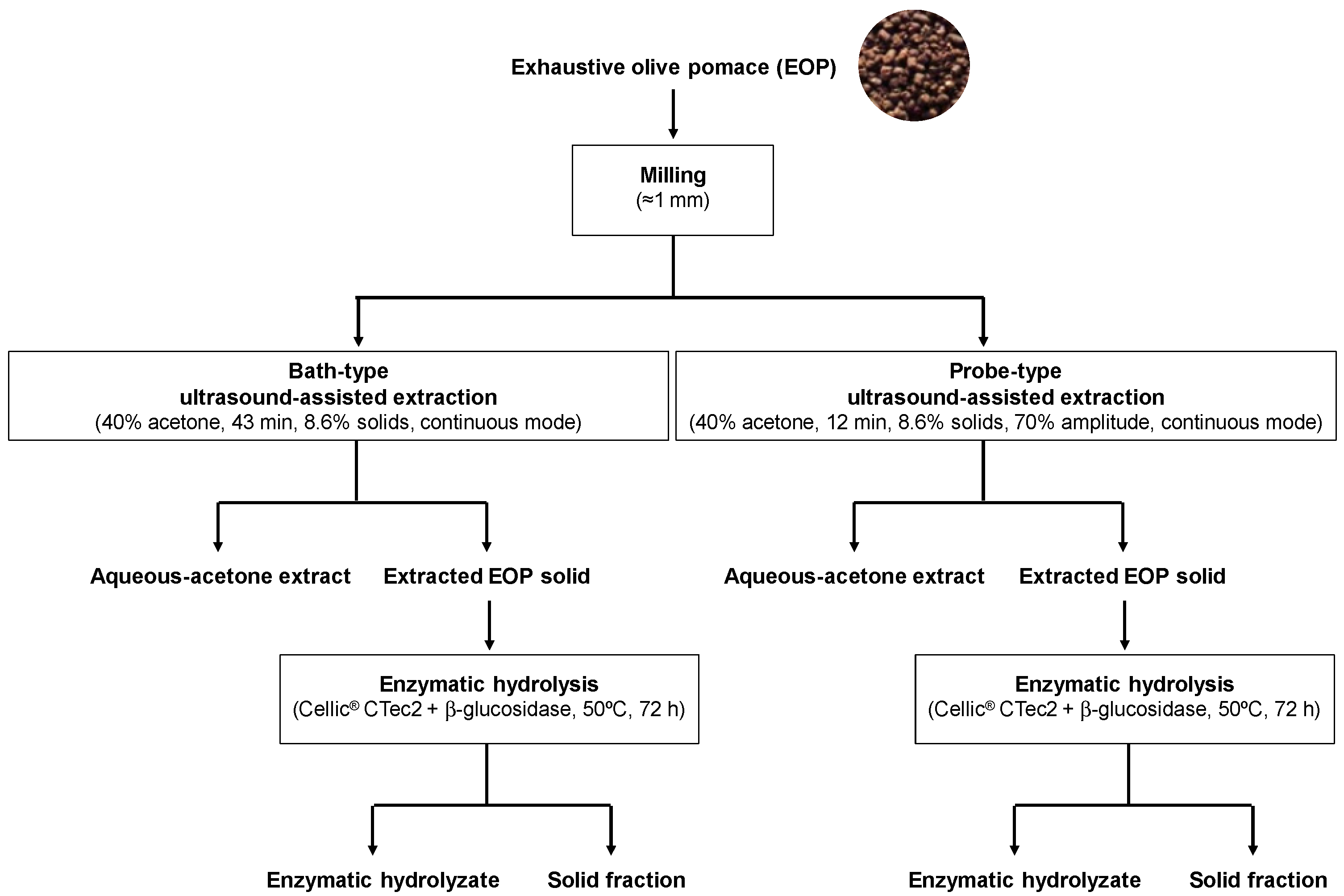
Figure 1.
Scheme summarizing the procedures used and samples obtained in this work.
Figure 1.
Scheme summarizing the procedures used and samples obtained in this work.
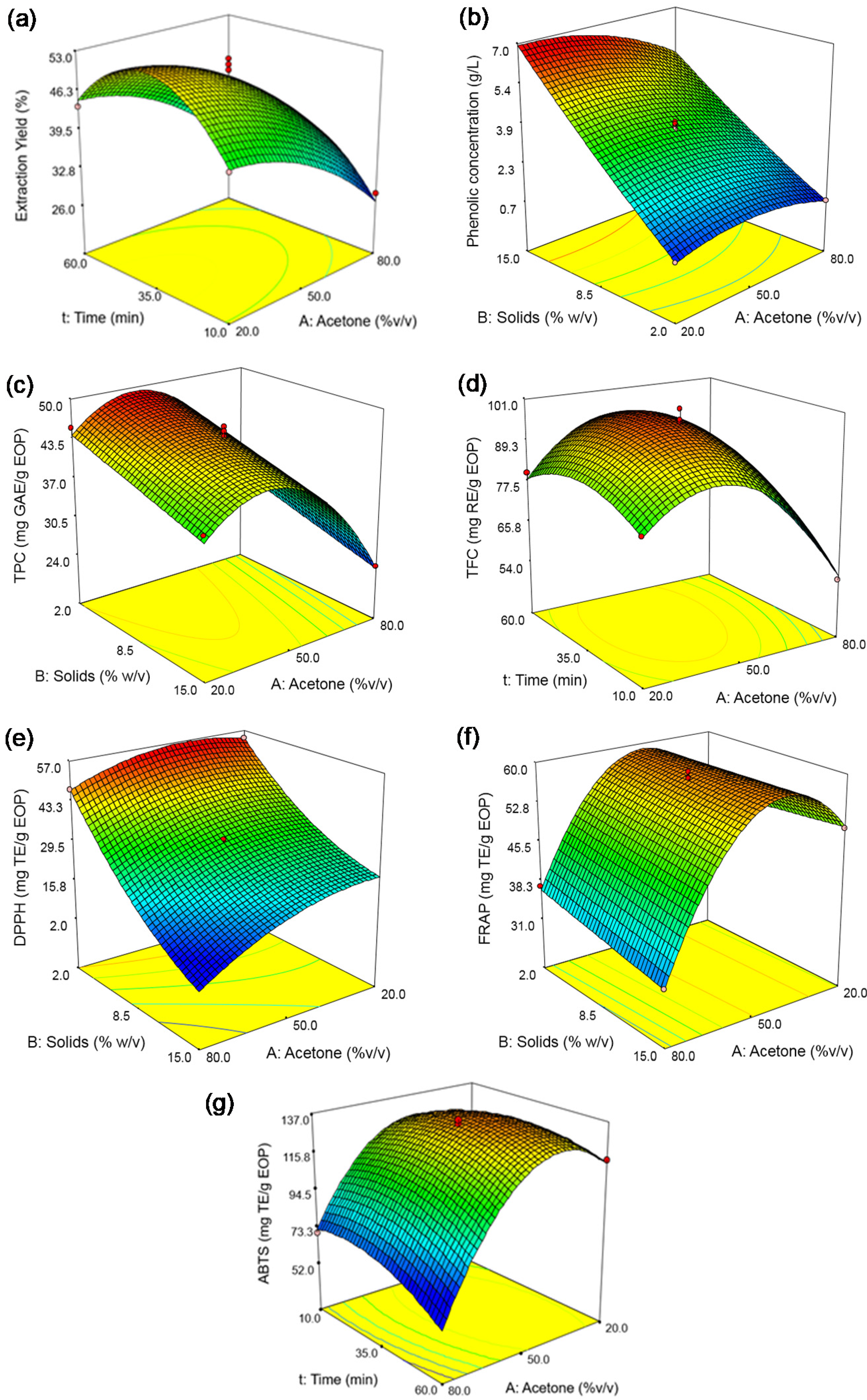
Figure 2.
Response surfaces obtained by the Box–Behnken design for exhausted olive pomace: (a) extraction yield, (b) phenolic concentration, (c) total phenolic content (TPC), (d) total flavonoid content (TFC), (e) DPPH assay, (f) FRAP assay, and (g) ABTS assay. The solid loading was fixed at 8.5% in plots (a,d,g), and the time at 35 min in plots (b,c,e,f).
Figure 2.
Response surfaces obtained by the Box–Behnken design for exhausted olive pomace: (a) extraction yield, (b) phenolic concentration, (c) total phenolic content (TPC), (d) total flavonoid content (TFC), (e) DPPH assay, (f) FRAP assay, and (g) ABTS assay. The solid loading was fixed at 8.5% in plots (a,d,g), and the time at 35 min in plots (b,c,e,f).
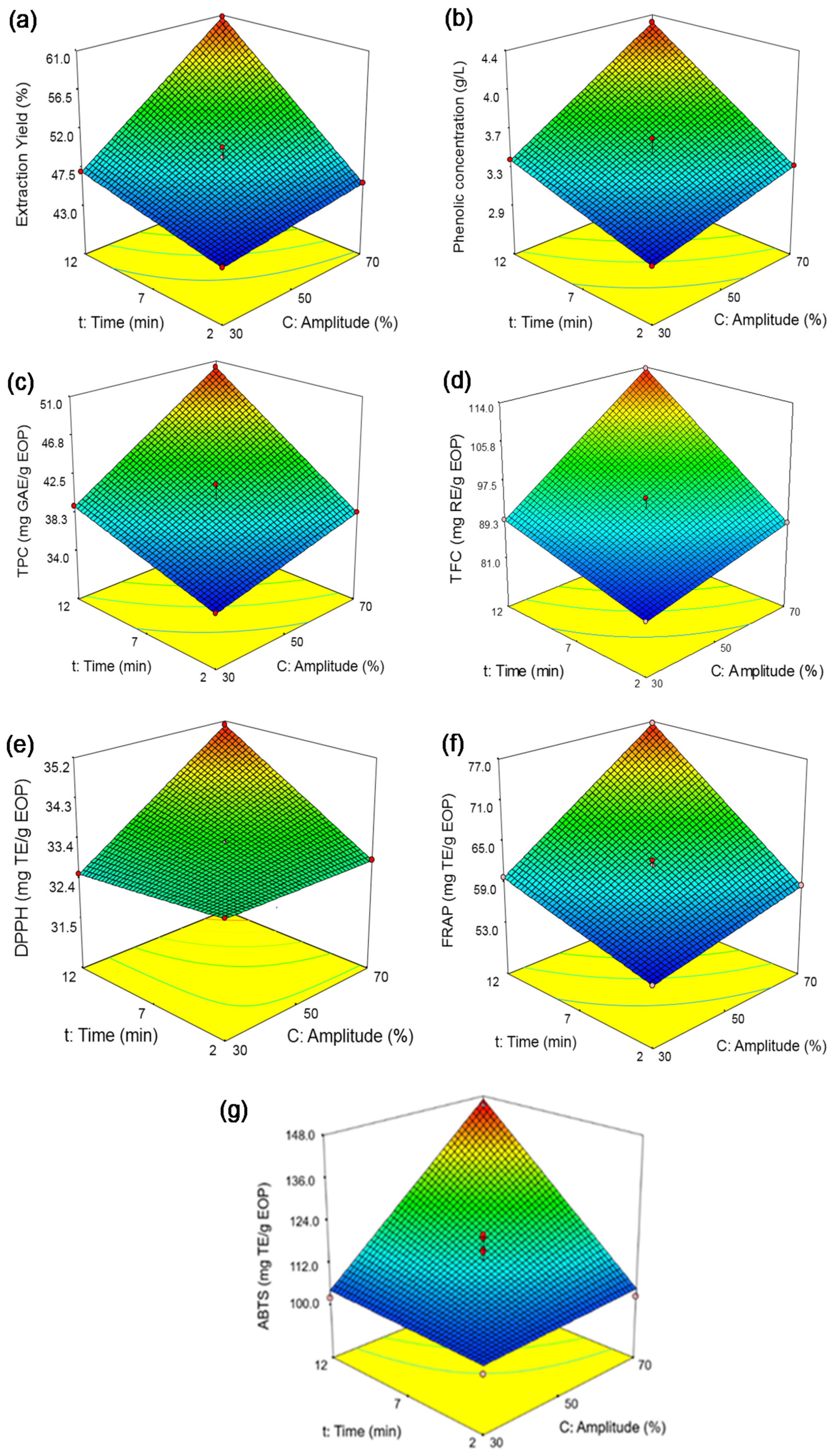
Figure 3.
Response surfaces obtained by the Box–Behnken design for exhausted olive pomace: (a) extraction yield, (b) phenolic concentration, (c) total phenolic content (TPC), (d) total flavonoid content (TFC), (e) DPPH assay, (f) FRAP assay, and (g) ABTS assay.
Figure 3.
Response surfaces obtained by the Box–Behnken design for exhausted olive pomace: (a) extraction yield, (b) phenolic concentration, (c) total phenolic content (TPC), (d) total flavonoid content (TFC), (e) DPPH assay, (f) FRAP assay, and (g) ABTS assay.
Figure 4.
Electropherograms at 200 nm of the water–acetone extract obtained by (a) bath- and (b) probe-type ultrasound-assisted extraction of exhausted olive pomace in the best obtained conditions.
Figure 4.
Electropherograms at 200 nm of the water–acetone extract obtained by (a) bath- and (b) probe-type ultrasound-assisted extraction of exhausted olive pomace in the best obtained conditions.
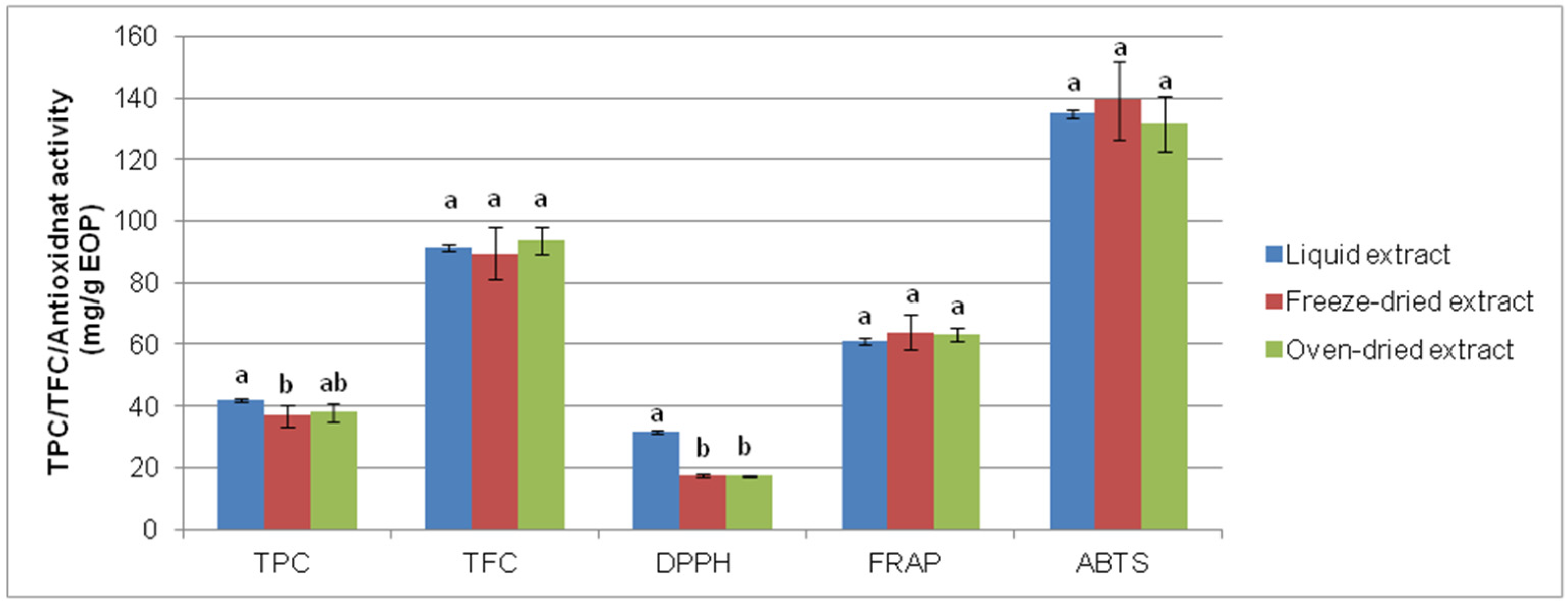
Figure 5.
Comparison of freeze-drying and oven-drying of exhausted olive pomace-derived extracts obtained by bath-type ultrasound-assisted extraction: total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity determined by DPPH, ferric-reducing power (FRAP), and ABTS assays. Data represent the average value and standard deviation (n = 5). For each parameter, bars accompanied by different letters indicate significant differences (p < 0.05).
Figure 5.
Comparison of freeze-drying and oven-drying of exhausted olive pomace-derived extracts obtained by bath-type ultrasound-assisted extraction: total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity determined by DPPH, ferric-reducing power (FRAP), and ABTS assays. Data represent the average value and standard deviation (n = 5). For each parameter, bars accompanied by different letters indicate significant differences (p < 0.05).
Table 1.
Uncoded and coded values of the factors studied by a Box–Behnken design and a two-level factorial design using bath (B)- and probe (P)-type ultrasound-assisted extraction (UAE), respectively.
Table 1.
Uncoded and coded values of the factors studied by a Box–Behnken design and a two-level factorial design using bath (B)- and probe (P)-type ultrasound-assisted extraction (UAE), respectively.
| Independent Variable | Nomenclature | Units | B-UAE Values | P-UAE Values |
|---|
| (−1) | 0 | (+1) | (−1) | 0 | (+1) |
|---|
| Acetone concentration | A | %, v/v | 20 | 50 | 80 | - | - | - |
| Extraction time | t | min | 10 | 35 | 60 | 2 | 7 | 12 |
| Solid loading | B | % | 2 | 8.5 | 15 | - | - | - |
| Amplitude | C | % | - | - | - | 30 | 50 | 70 1 |
Table 2.
Comparison of the application of milling and ultrasound in the extraction of exhausted olive pomace (EOP): Extraction yield (%), total phenolic content (TPC) (mg gallic acid equivalents/g EOP), total flavonoid content (TFC) (mg rutin equivalents/g EOP), and antioxidant activity (DPPH, FRAP, and ABTS) (mg Trolox equivalents/g EOP). Data represent the average value and standard deviation (n ≥ 3).
Table 2.
Comparison of the application of milling and ultrasound in the extraction of exhausted olive pomace (EOP): Extraction yield (%), total phenolic content (TPC) (mg gallic acid equivalents/g EOP), total flavonoid content (TFC) (mg rutin equivalents/g EOP), and antioxidant activity (DPPH, FRAP, and ABTS) (mg Trolox equivalents/g EOP). Data represent the average value and standard deviation (n ≥ 3).
| | Extraction Yield | TPC | TFC | DPPH | FRAP | ABTS |
|---|
| Pelletized EOP |
| Control 1 | 16.41 ± 1.32 c | 11.49 ± 0.11 d | 29.35 ± 0.08 d | 6.30 ± 0.01 d | 14.42 ± 0.21 d | 31.01 ± 1.02 d |
| UAE | 23.41 ± 1.29 b | 18.05 ± 0.80 c | 47.13 ± 1.60 c | 8.43 ± 0.05 c | 25.90 ± 1.35 c | 52.04 ± 2.57 c |
| Milled EOP |
| Control 1 | 43.97 ± 2.18 a | 36.69 ± 0.32 b | 83.08 ± 1.00 b | 31.19 ± 0.10 b | 47.59 ± 0.58 b | 115.61 ± 0.84 b |
| UAE | 46.79 ± 3.63 a | 44.59 ± 1.46 a | 96.39 ± 2.37 a | 34.16 ± 0.33 a | 59.27 ± 2.29 a | 127.08 ± 4.15 a |
Table 3.
Box–Behnken experimental design in terms of actual and coded factors applied to the bath-type ultrasound-assisted extraction of exhausted olive pomace (EOP) and experimental values of the response variables: yield (%), phenolic concentration (PC) (g gallic acid equivalents/L), total phenolic content (TPC) (mg gallic acid equivalents/g EOP), total flavonoid content (TFC) (mg rutin equivalents/g EOP), and antioxidant activity (DPPH, FRAP, and ABTS) (mg Trolox equivalents/g EOP).
Table 3.
Box–Behnken experimental design in terms of actual and coded factors applied to the bath-type ultrasound-assisted extraction of exhausted olive pomace (EOP) and experimental values of the response variables: yield (%), phenolic concentration (PC) (g gallic acid equivalents/L), total phenolic content (TPC) (mg gallic acid equivalents/g EOP), total flavonoid content (TFC) (mg rutin equivalents/g EOP), and antioxidant activity (DPPH, FRAP, and ABTS) (mg Trolox equivalents/g EOP).
| Run | A 1 | t 1 | B 1 | T 2 | Yield | PC | TPC | TFC | DPPH | FRAP | ABTS |
|---|
| 1 | 50 (0) | 35 (0) | 8.5 (0) | 39 | 50.72 | 3.94 | 46.34 | 96.36 | 32.46 | 63.18 | 136.02 |
| 2 | 20 (−1) | 60 (1) | 8.5 (0) | 41 | 43.54 | 3.44 | 40.44 | 79.94 | 30.87 | 51.53 | 116.62 |
| 3 | 50 (0) | 35 (0) | 8.5 (0) | 40 | 45.38 | 3.69 | 43.46 | 94.32 | 32.09 | 58.33 | 130.60 |
| 4 | 50 (0) | 10 (−1) | 15 (1) | 31 | 43.87 | 5.78 | 38.53 | 86.96 | 17.31 | 55.06 | 119.19 |
| 5 | 50 (0) | 10 (−1) | 2 (−1) | 32 | 42.87 | 0.96 | 48.05 | 84.95 | 56.74 | 49.71 | 144.78 |
| 6 | 20 (−1) | 35 (0) | 2 (−1) | 39 | 46.38 | 0.91 | 45.32 | 87.02 | 55.54 | 48.88 | 137.02 |
| 7 | 80 (1) | 35 (0) | 15 (1) | 40 | 26.84 | 3.65 | 24.34 | 61.65 | 10.49 | 31.57 | 69.69 |
| 8 | 20 (−1) | 10 (−1) | 8.5 (0) | 31 | 41.65 | 3.25 | 38.27 | 77.71 | 33.08 | 47.79 | 100.62 |
| 9 | 80 (1) | 10 (−1) | 8.5 (0) | 32 | 28.12 | 2.05 | 24.09 | 54.88 | 18.74 | 28.96 | 69.90 |
| 10 | 20 (−1) | 35 (0) | 15 (1) | 39 | 43.65 | 5.68 | 37.83 | 80.23 | 9.99 | 50.58 | 110.55 |
| 11 | 50 (0) | 35 (0) | 8.5 (0) | 40 | 46.47 | 3.64 | 42.81 | 94.27 | 30.98 | 58.20 | 129.85 |
| 12 | 80 (1) | 35 (0) | 2 (−1) | 39 | 58.62 | 0.74 | 36.98 | 71.80 | 48.93 | 37.16 | 90.98 |
| 13 | 50 (0) | 60 (1) | 15 (1) | 45 | 46.65 | 6.52 | 43.45 | 93.92 | 16.48 | 58.85 | 119.33 |
| 14 | 50 (0) | 35 (0) | 8.5 (0) | 40 | 51.65 | 3.81 | 44.77 | 100.04 | 31.91 | 59.33 | 133.37 |
| 15 | 50 (0) | 60 (1) | 2 (−1) | 45 | 47.48 | 0.98 | 49.03 | 92.16 | 55.28 | 52.71 | 134.88 |
| 16 | 50 (0) | 35 (0) | 8.5 (0) | 40 | 49.74 | 3.87 | 45.58 | 96.97 | 33.06 | 57.34 | 130.45 |
| 17 | 80 (1) | 60 (1) | 8.5 (0) | 46 | 36.05 | 2.64 | 31.04 | 74.26 | 17.88 | 38.55 | 83.57 |
Table 4.
Mathematical models and coefficients of the studied responses using coded values for the Box–Behnken design applied to the bath-type ultrasound-assisted extraction of exhausted olive pomace (EOP).
Table 4.
Mathematical models and coefficients of the studied responses using coded values for the Box–Behnken design applied to the bath-type ultrasound-assisted extraction of exhausted olive pomace (EOP).
| Dependent Variable | Equation
No. | Model 1 | CV (%) | R2 | Adjusted R2 | F-Value | p-Value | Lack of Fit (p-Value) |
|---|
| Extraction yield (%) | (1) | 48.79 − 7.66∙A + 1.31∙t − 0.67∙B − 14.50∙A∙B − 3.62∙A − 9.51∙t + 5.94∙B | 5.44 | 0.955 | 0.901 | 21.49 | <0.0001 | 0.6938 |
| PC (g GAE/L) | (2) | 3.79 − 0.50∙A + 0.19∙t + 2.59∙B − 0.42∙A∙B + 0.18∙t∙B − 0.55∙A − 0.40∙t + 0.17∙B | 4.25 | 0.997 | 0.994 | 307.30 | <0.0001 | 0.357 |
| TPC (mg GAE/g EOP) | (3) | 44.67 − 6.37∙A + 1.88∙t − 3.71∙B − 10.57∙A | 3.72 | 0.972 | 0.962 | 96.64 | <0.0001 | 0.498 |
| TFC (mg RE/g EOP) | (4) | 96.27 − 6.99∙A + 4.16∙t + 3.66∙A∙t − 18.72 A − 6.63∙t | 2.94 | 0.971 | 0.954 | 59.64 | <0.0001 | 0.423 |
| DPPH (mg TE/g EOP) | (5) | 31.60 − 6.67∙A − 0.69∙t − 18.99∙B − 3.25∙A∙B − 5.35∙A − 1.45∙t + 5.41∙B | 2.02 | 0.998 | 0.997 | 807.84 | <0.0001 | 0.787 |
| FRAP (mg TE/g EOP) | (6) | 57.81 − 7.82∙A + 4.13∙t − 0.67∙B + 1.46∙A∙t − 1.82∙A∙B −3.03∙t∙B − 15.93∙A | 2.47 | 0.993 | 0.986 | 140.97 | <0.0001 | 0.193 |
| ABTS (mg TE/g EOP) | (7) | 132.06 − 22.74∙A − 1.42∙t − 11.11∙B − 8.40∙A∙t − 37.34∙A − 9.86∙t + 7.34∙B | 2.72 | 0.990 | 0.981 | 113.26 | <0.0001 | 0.2584 |
Table 5.
Predicted and experimental values obtained by bath- (B) and probe (P)-ultrasound-assisted extraction (UAE) for exhausted olive pomace (EOP) under the optimal conditions, which simultaneously maximized the seven responses. Data represent the average value and standard deviation (n = 5).
Table 5.
Predicted and experimental values obtained by bath- (B) and probe (P)-ultrasound-assisted extraction (UAE) for exhausted olive pomace (EOP) under the optimal conditions, which simultaneously maximized the seven responses. Data represent the average value and standard deviation (n = 5).
| Response Variable | B-UAE | P-UAE |
|---|
| Predicted Values | Experimental Values | Error | Predicted Values | Experimental Values | Error |
|---|
| Extraction Yield (%) | 49.98 | 47.32 ± 0.79 b | 5.62 | 59.76 | 56.79 ± 3.58 a | 5.23 |
| Phenolic concentration (g GAE/L) | 3.96 | 3.62 ± 0.05 b | 9.39 | 4.19 | 3.91 ± 0.27 a | 7.16 |
| TPC (mg GAE/g EOP) | 46.20 | 42.05 ± 0.56 b | 9.87 | 48.83 | 45.41 ± 3.16 a | 7.53 |
| TFC (mg RE/g EOP) | 96.85 | 91.59 ± 1.14 b | 5.74 | 111.32 | 100.81 ± 7.02 a | 10.43 |
| DPPH (mg TE/g EOP) | 32.54 | 31.44 ± 0.54 b | 3.50 | 34.69 | 35.61 ± 0.53 a | 2.36 |
| FRAP (mg TE/g EOP) | 59.87 | 61.08 ± 2.23 b | 1.98 | 75.66 | 68.08 ± 4.32 a | 11.13 |
| ABTS (mg TE/g EOP) | 137.7 | 135.0 ± 1.39 a | 2.00 | 147.13 | 140.67 ± 3.61 a | 4.59 |
Table 6.
Factorial experimental design in terms of actual and coded factors applied to the probe-type ultrasound-assisted extraction of exhausted olive pomace (EOP) and experimental values of the response variables: yield (%), phenolic concentration (PC) (g gallic acid equivalents/L), total phenolic content (TPC) (mg gallic acid equivalents/g EOP), total flavonoid content (TFC) (mg rutin equivalents/g EOP), and antioxidant activity (DPPH, FRAP, and ABTS) (mg Trolox equivalents/g EOP).
Table 6.
Factorial experimental design in terms of actual and coded factors applied to the probe-type ultrasound-assisted extraction of exhausted olive pomace (EOP) and experimental values of the response variables: yield (%), phenolic concentration (PC) (g gallic acid equivalents/L), total phenolic content (TPC) (mg gallic acid equivalents/g EOP), total flavonoid content (TFC) (mg rutin equivalents/g EOP), and antioxidant activity (DPPH, FRAP, and ABTS) (mg Trolox equivalents/g EOP).
| Run | C 1 | t 1 | T 2 | Yield | PC | TPC | TFC | DPPH | FRAP | ABTS |
|---|
| 1 | 30 (−1) | 12 (1) | 32 | 47.09 | 3.36 | 39.06 | 89.37 | 32.55 | 59.82 | 101.89 |
| 2 | 50 (0) | 7 (0) | 34 | 44.73 | 2.98 | 34.62 | 87.22 | 32.55 | 58.79 | 115.35 |
| 3 | 70 (1) | 12 (1) | 50 | 60.74 | 4.32 | 50.27 | 113.63 | 35.10 | 76.73 | 144.78 |
| 4 | 70 (1) | 2 (−1) | 28 | 45.75 | 3.30 | 38.38 | 88.73 | 32.87 | 58.58 | 102.39 |
| 5 | 50 (0) | 7 (0) | 34 | 48.71 | 3.31 | 38.43 | 91.48 | 33.25 | 61.45 | 115.85 |
| 6 | 50 (0) | 7 (0) | 33 | 46.44 | 3.20 | 37.21 | 85.31 | 32.62 | 59.08 | 112.33 |
| 7 | 30 (−1) | 2 (−1) | 25 | 43.39 | 2.94 | 34.24 | 81.31 | 32.93 | 53.84 | 100.51 |
| 8 | 50 (0) | 7 (0) | 33 | 47.57 | 3.25 | 37.81 | 87.52 | 33.14 | 59.97 | 119.37 |
| 9 | 50 (0) | 7 (0) | 33 | 49.94 | 3.57 | 41.46 | 94.03 | 31.53 | 62.34 | 120.25 |
Table 7.
Mathematical models and coefficients of the studied responses using coded values for the factorial design applied to the probe-type ultrasound-assisted extraction of exhausted olive pomace (EOP).
Table 7.
Mathematical models and coefficients of the studied responses using coded values for the factorial design applied to the probe-type ultrasound-assisted extraction of exhausted olive pomace (EOP).
| Dependent Variable | Equation | Model 1 | CV (%) | R2 | Adjusted R2 | F-Value | p-Value | Lack of Fit (p-Value) |
|---|
| Extraction Yield (%) | (8) | +48.26 + 4.00∙C + 4.67∙t + 2.83∙C∙t | 4.46 | 0.888 | 0.820 | 13.18 | 0.0082 | 0.262 |
| PC (g GAE/L) | (9) | +3.36 + 0.33∙C + 0.36∙t + 0.15∙C∙t | 7.50 | 0.714 | 0.6190 | 7.50 | 0.0233 | 0.2207 |
| TPC (mg GAE/g EOP) | (10) | +39.05 + 3.84∙C + 4.18∙t + 1.77∙C∙t | 7.50 | 0.714 | 0.6190 | 7.50 | 0.0233 | 0.2207 |
| TFC (mg RE/g EOP) | (11) | +90.96 + 7.92∙C + 8.24∙t + 4.21∙C∙t | 4.63 | 0.870 | 0.792 | 11.16 | 0.0112 | 0.156 |
| DPPH (mg TE/g EOP) | (12) | +32.95 + 0.63∙C + 0.46∙t + 0.65∙C∙t | 2.38 | 0.573 | 0.316 | 2.23 | 0.2022 | 0.179 |
| FRAP (mg TE/g EOP) | (13) | +61.18 + 5.41∙C + 6.03∙t + 3.04∙C∙t | 3.06 | 0.945 | 0.912 | 28.49 | 0.0014 | 0.135 |
| ABTS (mg TE/g EOP) | (14) | +182.92 + 17.89∙C + 17.48∙t + 16.38∙C∙t | 3.52 | 0.945 | 0.912 | 28.70 | 0.0014 | 0.121 |
Table 8.
Phenolic compounds characterized by high-performance chromatography coupled to mass spectrometry in water–acetone extracts obtained by bath- and probe-type ultrasound-assisted extraction under the best obtained conditions.
Table 8.
Phenolic compounds characterized by high-performance chromatography coupled to mass spectrometry in water–acetone extracts obtained by bath- and probe-type ultrasound-assisted extraction under the best obtained conditions.
| RT (min) | [M-H]− | MS/MS | Compound |
|---|
| Hydroxytyrosol derivatives |
| 1.1 | 153 | 123 | Hydroxytyrosol 1 |
| 1.2 | 315 | 153, 135, 123 | Hydroxytyrosol hexoside |
| 1.9 | 299 | 179, 161, 119, 101 | Tyrosol hexoside |
| 6.4 | 195 | 153, 151, 59 | Hydroxytyrosol acetate |
| 9.3 | 483 | 347, 123 | Oleacein derivative (+hexose + H2) |
| 9.9 | 623 | 461, 315 | Verbascoside |
| 10.3 | 701 | 539, 437, 377, 307, 275 | Oleuropein hexoside isomer 1 |
| 10.6 | 335 | 317, 199, 153 | Hydroxy oleacein isomer 1 |
| 10.7 | 623 | 461 | Isoverbascoside |
| 10.9 | 335 | 317, 199, 153, 111 | Hydroxy oleacein isomer 2 |
| 10.6 | 701 | 539, 377, 307, 275 | Oleuropein hexoside isomer 2 |
| 11.4 | 539 | 403, 223 | Oleouropein isomer 1 |
| 11.6 | 539 | 403, 377, 307, 275, 223 | Oleuropein 1 |
| 11.8 | 701 | 377, 307, 275 | Oleuropein hexoside isomer 3 |
| 12.4 | 539 | 377, 307, 275, 223 | Oleouropein isomer 2 |
| 12.7 | 539 | 403, 377, 307, 275, 223 | Oleouropein isomer 3 |
| 13.1 | 319 | 183, 181, 153, 111 | 3,4-DHPEA-EDA 2 or oleacein |
| 13.5 | 523 | 361, 291, 259, 223 | Ligustroside |
| 18.7 | 361 | 329, 291, 225, 193, 181 | Hydroxytyrosol linked to desoxy elenolic acid |
| Non-hydroxytyrosol derivatives |
| 6.0 | 137 | Not fragmented | Hydroxybenzoic acid |
| 9.7 | 463 | 347, 301 | Quercetin hexoside |
| 10.0 | 447 | 285 | Luteolin 7-O-glucoside 1 |
| 10.2 | 593 | 285 | Luteolin O-deoxyhexosyl-hexoside |
| 10.5 | 593 | 447, 285 | Luteolin O-deoxyhexoside O-hexoside |
| 11.7 | 551 | 507, 389, 341, 281, 251, 179 | Caffeoyl-6′-secologanoside |
| 13.2 | 535 | 491, 389, 345, 265, 163 | p-Coumaroyl-6′-secologanoside |
| 14.2 | 285 | 175, 151 | Luteolin |
Table 9.
Chemical composition of the exhausted olive pomace (EOP) before and after bath (B)- and probe (P)-ultrasound assisted extraction (UAE). Data (%, dry weight basis) represent the average value and standard deviation (n = 3).
Table 9.
Chemical composition of the exhausted olive pomace (EOP) before and after bath (B)- and probe (P)-ultrasound assisted extraction (UAE). Data (%, dry weight basis) represent the average value and standard deviation (n = 3).
| Component | Raw EOP 2 | B-UAE | P-UAE |
|---|
| Extractives | 42 ± 2 | 15.5 ± 0.4 | 20 ± 1 |
| Aqueous extractives | 38 ± 2 | 4.9 ± 0.2 | 8.0 ± 1 |
| Ethanol extractives | 3.8 ± 0.2 | 10.8 ± 0.4 | 12 ± 1 |
| Cellulose | 9.7 ± 0.8 | 11.5 ± 0.7 | 15.1 ± 0.8 |
| Hemicellulose | 10.9 ± 0.5 | 15.8 ± 0.8 | 20.1 ± 0.9 |
| Xylan 1 | 9.8 ± 0.5 | 11.3 ± 0.8 | 15.8 ± 0.9 |
| Galactan 1 | 0.3 ± 0.3 | 2.84 ± 0.09 | 2.6 ± 0.6 |
| Arabinan 1 | 1.82 ± 0.03 | 2.27 ± 0.02 | 2.76 ± 0.01 |
| Mannan 1 | 0.42 ± 0.02 | 1.3 ± 0.2 | 1.41 ± 0.03 |
| Acetyl groups | 1.5 ± 0.2 | 1.4 ± 0.2 | 1.84 ± 0.05 |
| Lignin | 21.8 ± 0.9 | 30.1 ± 0.3 | 29.79 ± 0.07 |
| Acid insoluble lignin | 20.3 ± 0.7 | 29.6 ± 0.3 | 28.5 ± 0.7 |
| Acid soluble lignin | 1.5 ± 0.5 | 0.55 ± 0.06 | 1.27 ± 0.07 |
| Ash | 6.4 ± 0.2 | 1.67 ± 0.06 | 1.6 ± 0.2 |