Evaluation of Sown Cover Crops and Spontaneous Weed Flora as a Potential Reservoir of Black-Foot Pathogens in Organic Viticulture
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Plots and Vineyards
2.2. Sampling and Fungal Isolation
2.3. DNA Isolation, Sequencing and Phylogenetic Analyses
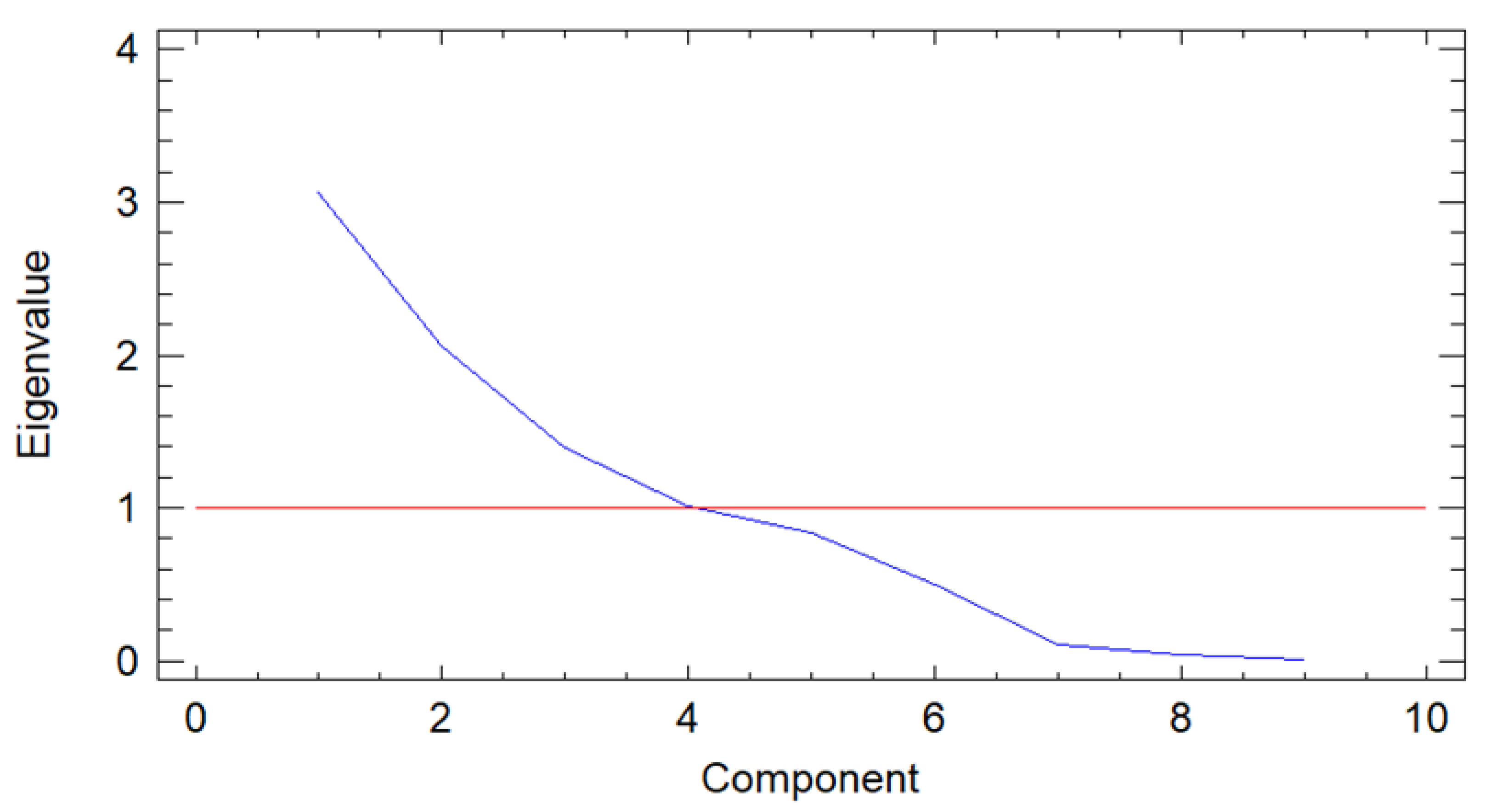
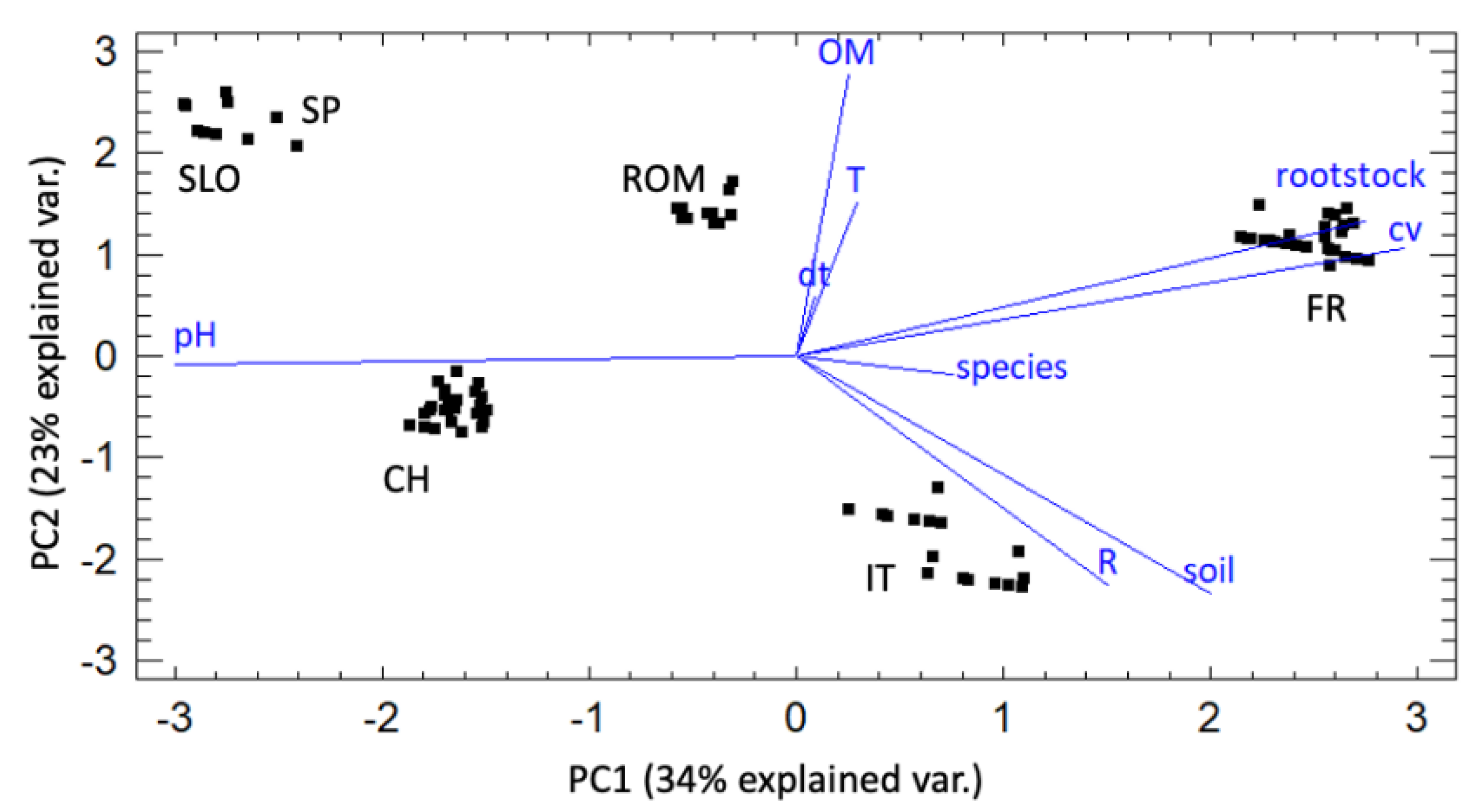
2.4. Principal Component Analysis
3. Results
3.1. Cylindrocarpon-Like Asexual Morphs Detection and Identification
3.2. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Agustí-Brisach, C.; Armengol, J. Black-foot disease of grapevine: An update on taxonomy, epidemiology and management strategies. Phytopathol. Mediterr. 2013, 52, 245–261. [Google Scholar]
- Carlucci, A.; Francesco, L.; Mostert, L.; Halleen, F.; Raimondo, M.L. Occurrence fungi causing black-foot on young grapevines and nursery rootstock plants in Italy. Phytopathol. Mediterr. 2017, 56, 10–39. [Google Scholar]
- Gramaje, D.; Urbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef]
- Aigoun-Mouhous, W.; Elena, G.; Cabral, A.; León, M.; Sabaou, N.; Armengol, J.; Chaouia, C.; Mahamedi, A.E.; Berraf-Tebbal, A. Characterization and pathogenicity of Cylindrocarpon-like asexual morphs associated with black-foot disease in Algerian grapevine nurseries, with the description of Pleiocarpon algeriense sp. nov. Eur. J. Plant Pathol. 2019, 154, 887–901. [Google Scholar] [CrossRef]
- Berlanas, C.; Andrés-Sodupe, M.; López-Manzanares, B.; Maldonado-González, M.M.; Gramaje, D. Effect of white mustard cover crop residue, soil chemical fumigation and Trichoderma spp. root treatment on black-foot disease control in grapevine. Pest. Manag. Sci. 2018, 74, 2864–2873. [Google Scholar] [CrossRef] [PubMed]
- Vukicevich, E.; Lowery, T.; Bowen, P.; Úrbez-Torres, J.R.; Hart, M. Cover crops to increase soil microbial diversity and decline in perennial agriculture. A review. Agron. Sustain. Dev. 2016, 36, 48. [Google Scholar] [CrossRef]
- Winter, S.; Bauer, T.; Strauss, P.; Kratschmer, S.; Paredes, D.; Popescu, D.; Landa, B.; Guzmán, G.; Gómez, J.A.; Guernion, M.; et al. Effects of vegetation management intensity on biodiversity and ecosystem services in vineyards: A meta-analysis. J. Appl. Ecol. 2018, 55, 2484–2495. [Google Scholar] [CrossRef]
- Finney, D.M.; Buyer, J.S.; Kayne, J.P. Living cover crops have immediate impacts on soil microbial community structure and function. J. Soil Water Conserv. 2017, 72. [Google Scholar] [CrossRef]
- Diti, I.; Legler, S.E.; Caffi, T.; Rossi, V.; Canali, G.; Bosso, A.; Cancila, E.; Anelli, S.; Trioli, G.; Kleshcheva, E.; et al. A new integrated approach for management of soil threats in the vineyard ecosystem. Catena 2020, 195, 104788. [Google Scholar] [CrossRef]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Marín, D.; Armengol, J.; Carbonell-Bejerano, P.; Escalona, J.M.; Gramaje, D.; Hernández-Montes, E.; Intrigliolo, D.S.; Martínez-Zapater, J.M.; Medrano, H.; Miras-Ávalos, J.M.; et al. Challenges of viticulture adaptation to global change: Tackling the issue from the roots. Aust. J. Grape Wine Res. 2021, 27, 8–25. [Google Scholar] [CrossRef]
- Walker, G.E.; Stirling, G.R. Plant-parasitic nematodes in Australian viticulture: Key pests, current management practices and opportunities for future improvements. Australas. Plant. Pathol. 2008, 37, 268–278. [Google Scholar] [CrossRef]
- Baginsky, C.; Contreras, A.; Covarrubias, J.I.; Seguel, O.; Aballay, E. Control of plant-parasitic nematodes using cover crops in table grape cultivation in Chile. Cienc. Investig. Agrar. 2013, 40, 547–557. [Google Scholar] [CrossRef][Green Version]
- Kruger, D.H.M.; Fourie, J.C.; Malan, A.P. Cover crops with biofumigation properties for the suppression of plant-parasitic nematodes: A review. S. Afr. J. Enol. Vitic. 2013, 34, 287–295. [Google Scholar] [CrossRef]
- Barbour, J.E.; Ridgway, H.J.; Jones, E.E. Influence of mustard biofumigation on growth, conidial germination and propagule recovery of Ilyonectria macrodidyma-complex species. Phytopathol. Mediterr. 2014, 53, 582–583. [Google Scholar]
- Withelaw-Weckert, M.; Rahman, M.; Capello, J.; Bartrop, K. Preliminary findings on the grapevine yield response to Brassica biofumigation soil. Phytopathol. Mediterr. 2014, 53, 587. [Google Scholar]
- Vukicevich, E.; Lowery, D.T.; Bennet, J.A.; Hart, M. Influence of ground cover vegetation, soil physicochemical properties, and irrigation practices on soil fungi in semi-arid vineyards. Front. Ecol. Evol. 2019, 7, 118. [Google Scholar] [CrossRef]
- Richards, A.; Estaki, M.; Úrbez-Torres, J.R.; Bowen, P.; Lowery, T.; Hart, M. Cover crop diversity as a tool to mitigate vine decline and reduce pathogens in vineyard soils. Diversity 2020, 12, 128. [Google Scholar] [CrossRef]
- Agustí-Brisach, C.; Gramaje, D.; León, M.; García-Jiménez, J.; Armengol, J. Evaluation of vineyard weeds as potential hosts of black-foot and petri disease pathogens. Plant Dis. 2011, 95, 803–810. [Google Scholar] [CrossRef]
- Dhingra, O.D.; Sinclair, J.B. Basic Plant Pathology Methods, 2nd ed.; CRC Press: Boca Ratón, FL, USA, 1995; 448p. [Google Scholar]
- Cabral, A.; Rego, C.; Nascimento, T.; Oliveira, H.; Groenewald, J.Z.; Crous, P.W. Multi-gene analysis and morphology reveal novel Ilyonectria species associated with black-foot disease of grapevines. Fungal Biol. 2012, 116, 62–80. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z.; Risède, J.M.; Simoneau, P.; Hywel-Jones, N.L. Calonectria species and their Cylindrocladium anamorphs: Species with sphaeropedunculate vesicles. Stud. Mycol. 2004, 50, 415–430. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Cabral, A.; Groenewald, J.Z.; Rego, C.; Oliveira, H.; Crous, P.W. Cylindrocarpon root rot: Multi-gene analysis reveals novel species within Ilyonectria radicicola species complex. Mycol. Prog. 2012, 11, 655–688. [Google Scholar] [CrossRef]
- Halleen, F.; Fourie, P.H.; Crous, P.W. A review of black-foot disease of grapevine. Phytopathol. Mediterr. 2006, 45, S55–S67. [Google Scholar]
- Berlanas, C.; López-Manzanares, B.; Gramaje, D. Estimation of viable propagules of black-foot disease pathogens in grapevine cultivated soils and their relation to production systems and soil properties. Plant Soil. 2017, 417, 467–479. [Google Scholar] [CrossRef]
- Cabral, A.; Rego, C.; Crous, P.W.; Oliveira, H. Virulence and cross-infection potential of Ilyonectria spp. to grapevine. Phytopathol. Mediterr. 2012, 51, 340–354. [Google Scholar] [CrossRef]
- Berlanas, C.; Ojeda, S.; López-Manzanares, B.; André-Sodupe, M.; Bujanda, R.; Martínez-Diz, M.P.; Díaz-Losada, E.; Gramaje, D. Occurrence and diversity of black-foot disease fungi in symptomless grapevine nursery stock in Spain. Plant Dis. 2020, 104, 94–104. [Google Scholar] [CrossRef]
- Lombard, L.; Van Der Merwe, A.; Groenewald, J.Z.; Crous, P.W. Lineages in Nectriaceae: Re-evaluating the generic status of Ilyonectria and allied genera. Phytopathol. Mediterr. 2014, 53, 515–532. [Google Scholar]
- Mangla, S.; Callaway, R.M. Exotic invasive plant accumulates native soil pathogens which inhibit native plants. J. Ecol. 2008, 96, 58–67. [Google Scholar] [CrossRef]
- Agustí-Brisach, C.; Mostert, L.; Armengol, J. Detection and quantification of Ilyonectria spp. associated with black-foot disease of grapevine in nursery soils using multiplex nested PCR and quantitative PCR. Plant Path. 2014, 63, 316–322. [Google Scholar] [CrossRef]
- Berlanas, C.; Berbegal, C.; Elena, G.; Laidani, M.; Cibriain, J.F.; Sagües, A.; Gramaje, D. The fungal and bacterial rhizosphere microbiome associated with grapevine rootstock genotypes in mature and young vineyards. Front. Microbiol. 2019, 10, 1142. [Google Scholar] [CrossRef] [PubMed]
- Nerva, L.; Moffa, L.; Giudice, G.; Giorgianni, A.; Tomasi, D.; Chitarra, W. Microscale analysis of soil characteristics and microbiomes reveals potential impacts on plants and fruit: Vineyard as a model case study. Plant Soil 2021, 462, 525–541. [Google Scholar] [CrossRef]
- Agustí-Brisach, C.; Armengol, J. Effects of temperature, pH and water potential on mycelial growth, sporulation and chlamydospore production in culture of Cylindrocarpon spp. associated with black foot of grapevines. Phytopathol. Mediterr. 2012, 51, 37–50. [Google Scholar]
| Country | Location | Cultivar | Rootstock | Age (Years) | Soil Texture | Soil pH | Organic Matter | Rainfall Jan–Oct (mm) | Mean Temperature Jan–Oct (°C) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | ||||||||
| France | Premeaux-Prissey (Burgundy) | Pinot Noir | Teleki 5C | 20 | silty-clay | 5.8 | 3.0% | -- | 1060 | -- | 10.1 |
| Italy | Res Uvea farm in Castell’Arquato (Piacenza) | Croatina | Kober 5BB | 20 | silty-clay-loam | 6.9 | 1.3% | 1422 | 921 | 10.0 | 9.8 |
| Romania | Murfatlar vineyard, (Dobrodgea region) | Feteasca neagra | SO4 | 18 | loam | 7.9 | 2.3% | 354 | 400 | 9.7 | 9.5 |
| Slovenia | Hruševica, Vinakras (Primorska region) | Refošk | SO4 | 2 | loam | 5.3 | 2.8% | 1352 | 1358 | 10.0 | 9.6 |
| Spain | Villar del Arzobispo (Valencia province) | Cabernet Sauvignon | 110 Richter | 10 | clay-loam | 8.3 | 2.6% | 223 | 444 | 14.1 | 14.1 |
| Switzerland | Nyon | Chasselas | Rootstock 3309 | 23 | loam | 7.8 | 2.4% | 1075 | 1025 | 7.5 | 8.2 |
| France | Romania (Summer) | Spain (Autumn) | |
|---|---|---|---|
| Not evaluated | 1. Lolium perenne 2. Lolium perenne 3. Onobrychis sp. 4. Onobrychis sp. 5. Sinapis sp. 6. Sinapis sp. 7. Tagetes erecta L. 8. Tagetes erecta 9. Trifolium repens DT (P1/1; P2/1) 10. Trifolium repens DT (P4/1) 11. Vicia faba L. 12. Vicia faba | 1. Cyperus rotundus L. 2. Diplotaxis erucoides (L.) DC. 3. Salsola kali L. DT (P1/1) | |
| Italy (Summer) | Slovenia (Summer) | Switzerland (Summer) | Switzerland (Autumn) |
| 1. Armoracia rusticana G. Gaertn., B. Mey. & Scherb. 2. Armoracia rusticana DT (P2/1) a 3. Armoracia rusticana 4. Lolium perenne L. 5. Lolium perenne 6. Lolium perenne 7. Onobrychis viciifolia Scop 8. Onobrychis viciifolia 9. Onobrychis viciifolia 10. Sinapis sp. 11. Sinapis sp. 12. Sinapis sp. 13. Trifolium repens L. 14. Trifolium repens 15. Trifolium repens DT (P1/1) 16. Trifolium repens 17. Trifolium repens 18. Vicia sativa L. 19. Vicia sativa 20. Vicia sativa | 1. Phacelia sp. DT (P2/1) 2. Sinapis alba L. 3. Trifolium incarnatum L. 4. Vicia pannonica Crantz | 1. Bromus tectorum L. 2. Geranium columbinum L. DT (P1/1; P2/1) 3. Hordeum murinum L. 4.Lolium perenne 5. Plantago lanceolata L. DT (P1/2; P4/1) 6. Trifolium repens DT (P1/1) | 1. Bromus tectorum 2. Hordeum murinum DT (P1/2) 3. Lolium perenne DT (P4/1; P5/1) 4. Lolium perenne 5. Medicago maculata Willd. DT (P3/1; P5/2) 6. Plantago lanceolata DT (P2/1; P4/1; P5/3) 7. Plantago lanceolata DT (P1/2) 8. Sanguisorba minor Scop. 9. Trifolium repens DT (P1/5; P2/1) 10. Trifolium repens DT (P3/1) 11. Veronica persica Poir. |
| France (Autumn) | Romania (Summer) | Spain (Summer) | |
|---|---|---|---|
| 1. Arenaria serpyllifolia L. 2. Avena strigosa Schreb. 3. Brassica carinata L. DT (P4/1) a 4. Brassica carinata 5. Geranium sp. 6. Lathyrus sativus L. 7. Lens culinaris Medik. 8. Linum usitatissimum L. 9.Pisum sativum L. 10. Pisum sativum 11. Pisum sativum IR (P1/1) 12. Raphanus sativus L. longipinnatus Bailey 13. Raphanus sativus longipinnatus DT (P2/1; P4/1) 14. Secale cereale L. 15. Trifolium alexandrinum DT (P4/2) 16. Trifolium alexandrinum DT (P1/1; P4/3) 17.Trifolium alexandrinum DT (P1/1; P4/1) 18. Trifolium subterraneum L. DT (P3/1; P5/1) 19. Vicia faba 20. Vicia faba 21. Vicia villosa DT (P5/1) 22. Vicia. villosa DT (P2/1; P4/1) 23. Vicia. villosa | 1. Lolium perenne 2. Lolium perenne 3. Onobrychis sp. 4. Onobrychis sp. 5. Sinapis sp. 6. Sinapis sp. 7. Tagetes erecta 8. Tagetes erecta | 1. Anacyclus clavatus (Desf.) Pers. 2. Avena sterilis L. 3. Cichorium intybus L. DT (P5/1) 4. Conyza sumatrensis (Retz.) E. Walker 5. Plantago albicans L. 6. Sonchus oleraceus L. DT (P3/1) 7. Xantium orientale L. subsp. italicum (Moretti) Greuter | |
| Italy (Summer) | Slovenia (Autumn) | Switzerland (Summer) | Switzerland (Autumn) |
| 1. Armoracia rusticana 2. Armoracia rusticana 3. Armoracia rusticana 4. Lolium perenne 5. Lolium perenne 6. Onobrychis viciifolia 7. Sinapis sp 8. Sinapis sp 9. Trifolium repens DT (P4/1) 10. Trifolium repens 11. Trifolium repens 12. Vicia sativa 13. Vicia sativa 14. Vicia sativa | 1. Raphanus sativus L. 2. Raphanus sp. 3. Rorippa sylvestris (L.) Bess. DT (P3/7; P5/1) 4. Sinapis alba DT (P5/1) | 1. Lolium perenne 2. Lolium perenne DT (P3/1; P4/1; P5/1) 3. Lolium perenne 4. Lolium perenne 5. Plantago lanceolata 6. Plantago lanceolata 7. Plantago lanceolata 8. Plantago lanceolata 9. Prunella vulgaris L. DT (P1/1; P4/1; P5/1) 10. Trifolium repens 11. Trifolium repens DT (P1/1) 12. Trifolium repens 13. Trifolium repens | 1. Lolium perenne 2. Lolium perenne DT (P1/1; P5/1) 3. Lolium perenne 4. Lolium perenne 5. Plantago lanceolata DT (P4/1; P5/2) 6. Plantago lanceolata 7. Plantago lanceolata DT (P1/1; P3/1; P4/2) 8. Plantago lanceolata DA (P4/1) and DT (P1/1) 9. Taraxacum officinale Weber et Wiggers DT (P2/1; P3/1; P4/1; P5/1) and IR (P3/1) 10. Taraxacum officinale DT (P1/2; P2/3; P4/1) 11. Trifolium repens DT (P2/1) 12. Trifolium repens |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León, M.; Berbegal, M.; Abad-Campos, P.; Ramón-Albalat, A.; Caffi, T.; Rossi, V.; Hasanaliyeva, G.; Noceto, P.A.; Wipf, D.; Širca, S.; et al. Evaluation of Sown Cover Crops and Spontaneous Weed Flora as a Potential Reservoir of Black-Foot Pathogens in Organic Viticulture. Biology 2021, 10, 498. https://doi.org/10.3390/biology10060498
León M, Berbegal M, Abad-Campos P, Ramón-Albalat A, Caffi T, Rossi V, Hasanaliyeva G, Noceto PA, Wipf D, Širca S, et al. Evaluation of Sown Cover Crops and Spontaneous Weed Flora as a Potential Reservoir of Black-Foot Pathogens in Organic Viticulture. Biology. 2021; 10(6):498. https://doi.org/10.3390/biology10060498
Chicago/Turabian StyleLeón, Maela, Mónica Berbegal, Paloma Abad-Campos, Antonio Ramón-Albalat, Tito Caffi, Vittorio Rossi, Gultakin Hasanaliyeva, Pierre Antoine Noceto, Daniel Wipf, Saša Širca, and et al. 2021. "Evaluation of Sown Cover Crops and Spontaneous Weed Flora as a Potential Reservoir of Black-Foot Pathogens in Organic Viticulture" Biology 10, no. 6: 498. https://doi.org/10.3390/biology10060498
APA StyleLeón, M., Berbegal, M., Abad-Campos, P., Ramón-Albalat, A., Caffi, T., Rossi, V., Hasanaliyeva, G., Noceto, P. A., Wipf, D., Širca, S., Razinger, J., Fragnière, A.-L., Kehrli, P., Ranca, A., Petrescu, A., & Armengol, J. (2021). Evaluation of Sown Cover Crops and Spontaneous Weed Flora as a Potential Reservoir of Black-Foot Pathogens in Organic Viticulture. Biology, 10(6), 498. https://doi.org/10.3390/biology10060498











