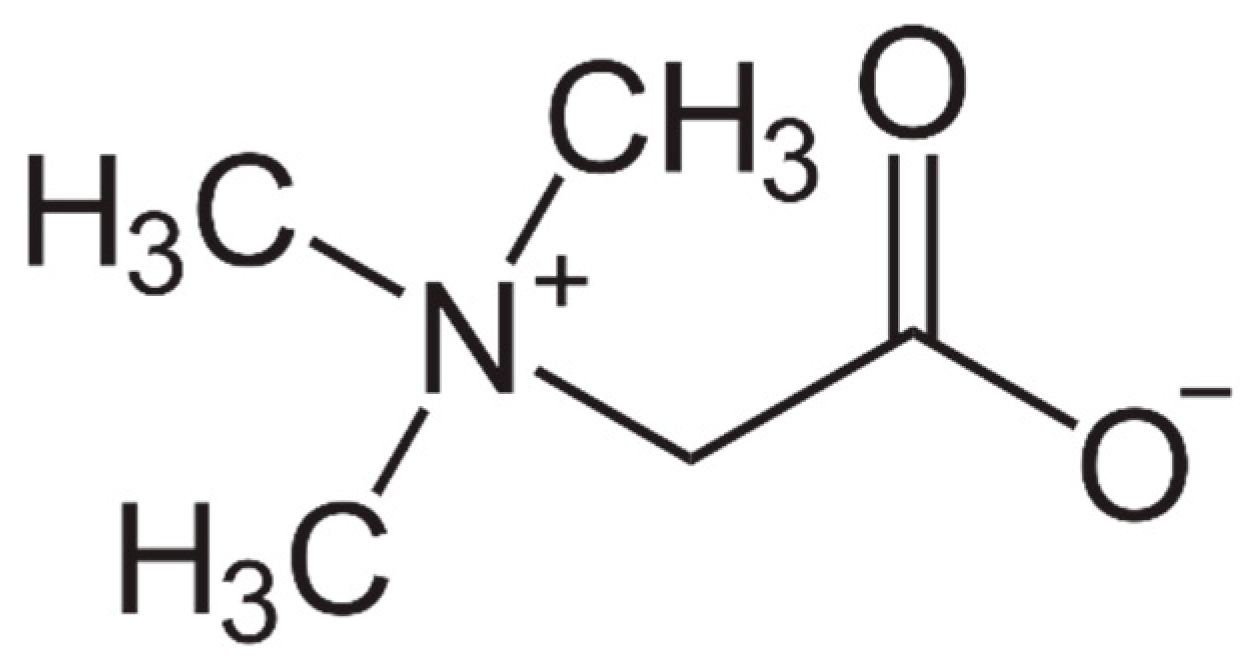
Beneficial Effects of Betaine: A Comprehensive Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Dietary Betaine Uptake
3. Important Roles of Betaine
4. Disease Prevention by Betaine Administration
4.1. ALD
4.1.1. Stages of ALD
4.1.2. Betaine Protects against the Development of Alcohol-Induced Hepatic Steatosis
4.1.3. Betaine Prevents Other Indices of Early Alcohol-Induced Liver Damage
4.1.4. Betaine Prevents Oxidative Stress and Inflammation in ALD
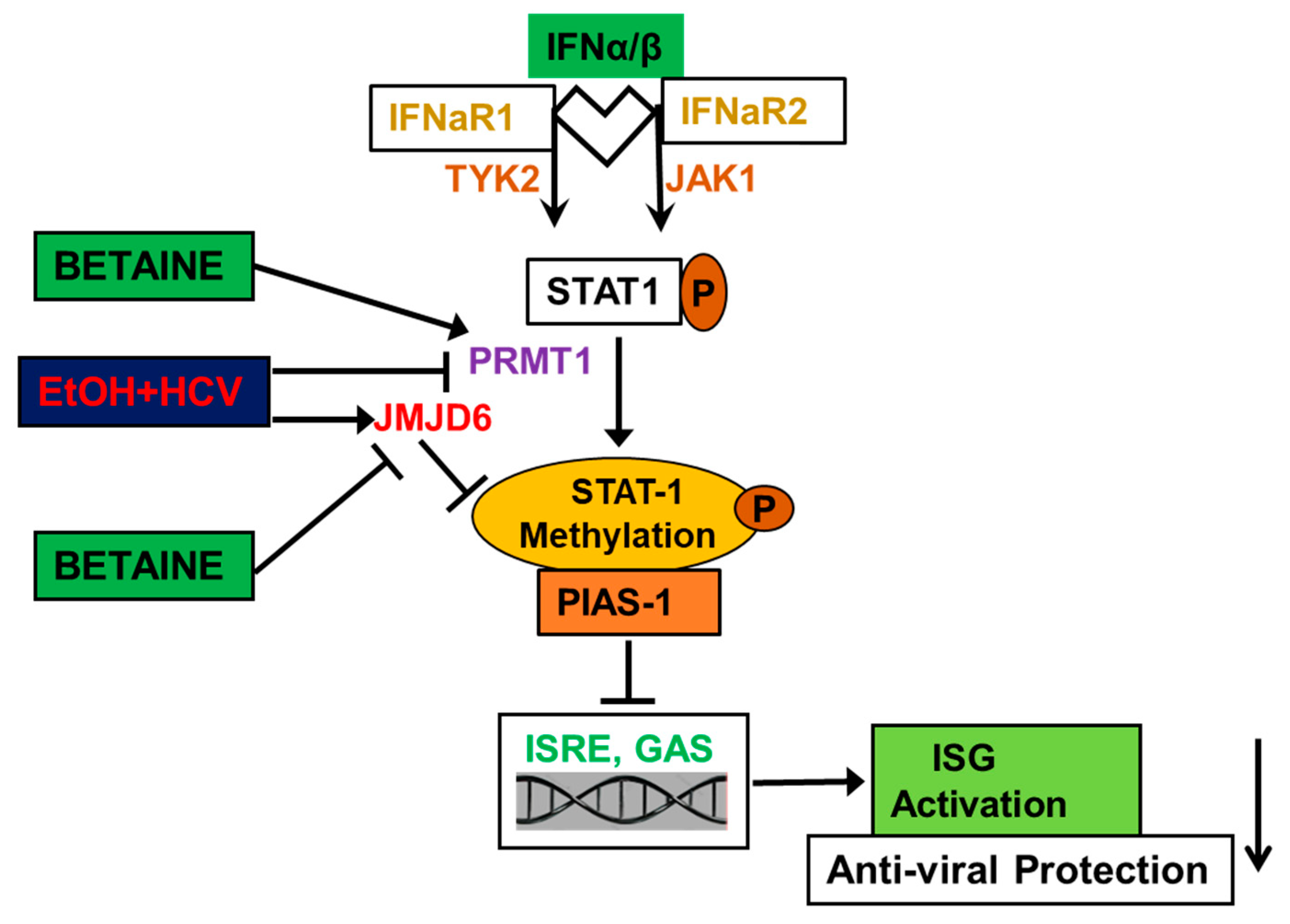
4.1.5. Betaine Protects against the Detrimental Effects of HCV and Ethanol on Innate Immunity
4.1.6. Betaine Protects against Fulminant Liver Failure and Toxin-Induced Liver Damage
4.2. MAFLD
4.3. Alterations in Gut–Liver and Adipose–Liver Axes in Promoting Hepatic Damage
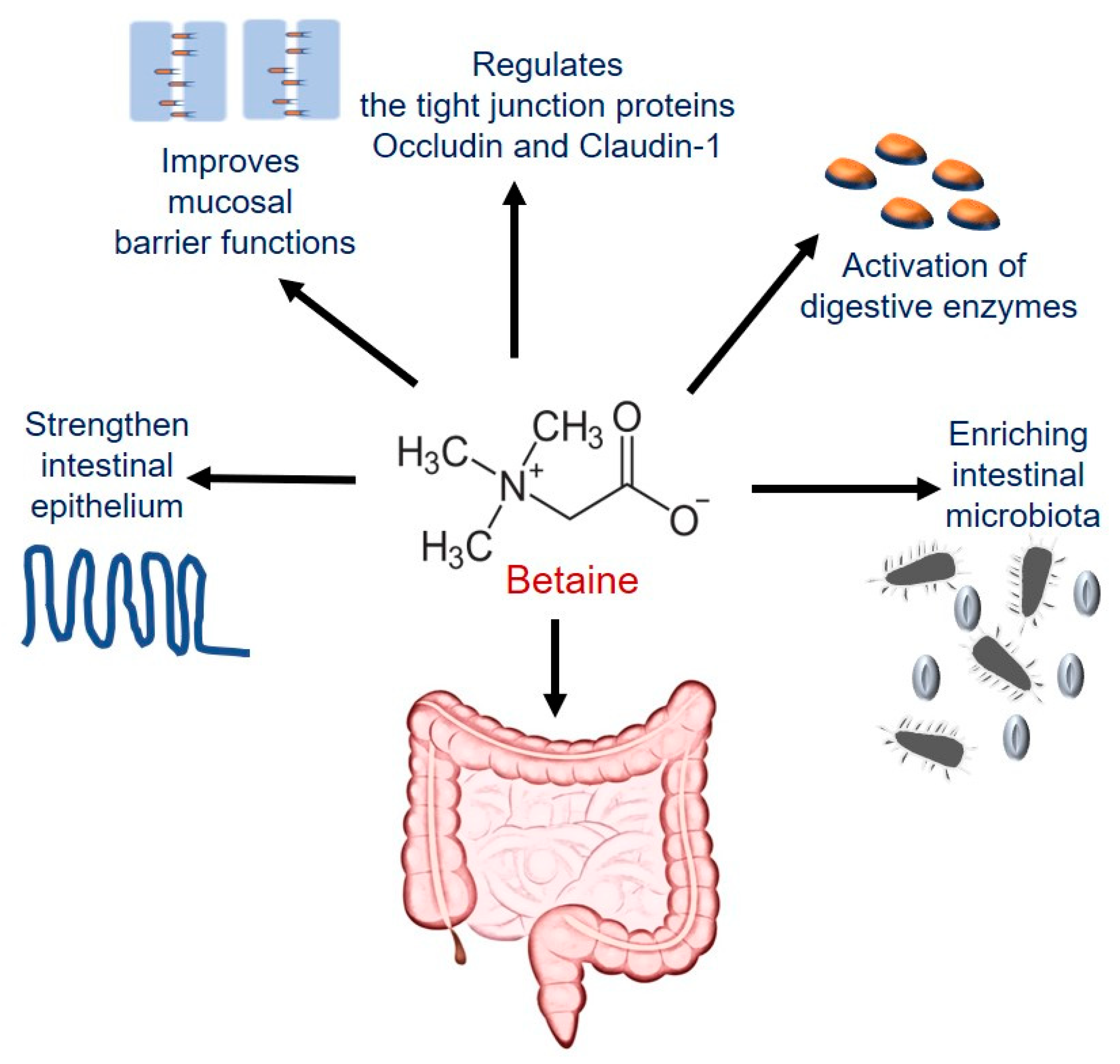
4.3.1. Betaine Maintains Intestinal Epithelial Barrier Integrity
4.3.2. Betaine Maintains Adipose Function
4.4. Protective Effects of Betaine on Other Tissues
4.5. Anti-Cancer Effect of Betaine
5. Other Beneficial Effects of Betaine
5.1. Effects of Betaine on General Well-Being
5.2. Effects of Maternal Betaine Supplementation on Offspring
6. Safety Studies with Betaine
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Li, S.; Li, H.-B. Dietary Natural Products for Prevention and Treatment of Liver Cancer. Nutrients 2016, 8, 156. [Google Scholar] [CrossRef]
- Domitrović, R.; Potočnjak, I. A comprehensive overview of hepatoprotective natural compounds: Mechanism of action and clinical perspectives. Arch. Toxicol. 2016, 90, 39–79. [Google Scholar] [CrossRef]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.A.S. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef]
- Willingham, B.D.; Ragland, T.J.; Ormsbee, M.J. Betaine Supplementation May Improve Heat Tolerance: Potential Mechanisms in Humans. Nutrients 2020, 12, 2939. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Chen, N.; Shi, M.; Xian, M.; Song, Y.; Liu, J. The metabolism and biotechnological application of betaine in microorganism. Appl. Microbiol. Biotechnol. 2016, 100, 3865–3876. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Ratamess, N.A.; Kang, J.; Rashti, S.L.; Faigenbaum, A.D. Effect of betaine supplementation on power performance and fatigue. J. Int. Soc. Sports Nutr. 2009, 6, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, M.; Weik, C.; Holneicher, C.; Häussinger, D. Betaine as an osmolyte in rat liver: Metabolism and cell-to-cell interactions. Hepatology 1998, 27, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Cholewa, J.M.; Guimarães-Ferreira, L.; Zanchi, N.E. Effects of betaine on performance and body composition: A review of recent findings and potential mechanisms. Amino Acids 2014, 46, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, S.V.; Tell, G.S.; Vollset, S.E.; Nygård, O.; Bleie, Ø.; Ueland, P.M. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J. Nutr. 2008, 138, 914–920. [Google Scholar] [CrossRef]
- Horio, M.; Ito, A.; Matsuoka, Y.; Moriyama, T.; Orita, Y.; Takenaka, M.; Imai, E. Apoptosis induced by hypertonicity in Madin Darley canine kidney cells: Protective effect of betaine. Nephrol. Dial. Transplant. 2001, 16, 483–490. [Google Scholar] [CrossRef]
- Courtenay, E.S.; Capp, M.W.; Anderson, A.C.F.; Record, M.T., Jr. Vapor Pressure Osmometry Studies of Osmolyte−Protein Interactions: Implications for the Action of Osmoprotectants in Vivo and for the Interpretation of “Osmotic Stress” Experiments in Vitro. Biochemistry 2000, 39, 4455–4471. [Google Scholar] [CrossRef]
- Hundahl, C.; Fago, A.; Malte, H.; Weber, R.E. Allosteric Effect of Water in Fish and Human Hemoglobins. J. Biol. Chem. 2003, 278, 42769–42773. [Google Scholar] [CrossRef]
- Zhang, F.; Warskulat, U.; Wettstein, M.; Häussinger, D. Identification of betaine as an osmolyte in rat liver macrophages (Kupffer cells). Gastroenterology 1996, 110, 1543–1552. [Google Scholar] [CrossRef]
- Moeckel, G.W.; Shadman, R.; Fogel, J.M.; Sadrzadeh, S.M.H. Organic osmolytes betaine, sorbitol and inositol are potent inhibitors of erythrocyte membrane ATPases. Life Sci. 2002, 71, 2413–2424. [Google Scholar] [CrossRef]
- Ortiz-Costa, S.; Sorenson, M.; Sola-Penna, M. Counteracting effects of urea and methylamines in function and structure of skeletal muscle myosin. Arch. Biochem. Biophys. 2002, 408, 272–278. [Google Scholar] [CrossRef]
- Kettunen, H.; Peuranen, S.; Tiihonen, K. Betaine aids in the osmoregulation of duodenal epithelium of broiler chicks, and affects the movement of water across the small intestinal epithelium in vitro. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 129, 595–603. [Google Scholar] [CrossRef]
- Kempson, S.A.; Vovor-Dassu, K.; Day, C. Betaine Transport in Kidney and Liver: Use of Betaine in Liver Injury. Cell. Physiol. Biochem. 2013, 32, 32–40. [Google Scholar] [CrossRef]
- Dalloul, R.A.; Lillehoj, H.S. Poultry coccidiosis: Recent advancements in control measures and vaccine development. Expert Rev. Vaccines 2006, 5, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Amerah, A.M.; Ravindran, V. Effect of coccidia challenge and natural betaine supplementation on performance, nutrient utilization, and intestinal lesion scores of broiler chickens fed suboptimal level of dietary methionine. Poult. Sci. 2015, 94, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.E.; Chalmers, R.A.; Randall, E.W.; Iles, R.A. Betaine metabolism in human neonates and developing rats. Clin. Chim. Acta 1988, 178, 241–249. [Google Scholar] [CrossRef]
- Thomes, P.G.; Osna, N.A.; Bligh, S.M.; Tuma, D.J.; Kharbanda, K.K. Role of defective methylation reactions in ethanol-induced dysregulation of intestinal barrier integrity. Biochem. Pharmacol. 2015, 96, 30–38. [Google Scholar] [CrossRef]
- Kharbanda, K.K.; Mailliard, M.E.; Baldwin, C.R.; Beckenhauer, H.C.; Sorrell, M.F.; Tuma, D.J. Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J. Hepatol. 2007, 46, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Ismaeel, A. Effects of Betaine Supplementation on Muscle Strength and Power: A Systematic Review. J. Strength Cond. Res. 2017, 31, 2338–2346. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Osna, N.A.; Kharbanda, K.K. Treatment options for alcoholic and non-alcoholic fatty liver disease: A review. World J. Gastroenterol. 2017, 23, 6549–6570. [Google Scholar] [CrossRef] [PubMed]
- Bakir, M.B.; Salama, M.A.; Refaat, R.; Ali, M.A.; Khalifa, E.A.; Kamel, M.A. Evaluating the therapeutic potential of one-carbon donors in nonalcoholic fatty liver disease. Eur. J. Pharmacol. 2019, 847, 72–82. [Google Scholar] [CrossRef]
- Kathirvel, E.; Morgan, K.; Nandgiri, G.; Sandoval, B.C.; Caudill, M.A.; Bottiglieri, T.; French, S.W.; Morgan, T.R. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: A potential mechanism for hepatoprotection by betaine. Am. J. Physiol. Liver Physiol. 2010, 299, G1068–G1077. [Google Scholar] [CrossRef]
- Veskovic, M.; Mladenovic, D.; Milenkovic, M.; Tosic, J.; Borozan, S.; Gopcevic, K.; Labudovic-Borovic, M.; Dragutinovic, V.; Vucevic, D.; Jorgacevic, B.; et al. Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur. J. Pharmacol. 2019, 848, 39–48. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Baj, J.; Garruti, G.; Celano, G.; De Angelis, M.; Wang, H.H.; Di Palo, D.M.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk. J. Clin. Med. 2020, 9, 2648. [Google Scholar] [CrossRef]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Lefkowitch, J.H. Morphology of Alcoholic Liver Disease. Clin. Liver Dis. 2005, 9, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Maurantonio, M.; Marrazzo, A.; Rinaldi, L.; Adinolfi, L.E. Nonalcoholic fatty liver disease: Evolving paradigms. World J. Gastroenterol. 2017, 23, 6571–6592. [Google Scholar] [CrossRef] [PubMed]
- Osna, N.A.; Donohue, T.M., Jr.; Kharbanda, K.K. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol. Res. 2017, 38, 147–161. [Google Scholar] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global status report on alcohol and health 2018. WHO Alcohol. Drugs Addict. Behav. 2018, 8, e010454. [Google Scholar]
- Shah, N.J.; Royer, A.; John, S. Alcoholic Hepatitis; Statpearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Rehm, J.; Shield, K.D. Global Burden of Alcohol Use Disorders and Alcohol Liver Disease. Biomedicines 2019, 7, 99. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Im, G.Y.; Cameron, A.M.; Lucey, M.R. Liver transplantation for alcoholic hepatitis. J. Hepatol. 2019, 70, 328–334. [Google Scholar] [CrossRef]
- Diehl, A.M.; Day, C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2017, 377, 2063–2072. [Google Scholar] [CrossRef]
- Leite, N.C.; Salles, G.F.; Araujo, A.L.E.; Villela-Nogueira, C.A.; Cardoso, C.R.L. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009, 29, 113–119. [Google Scholar] [CrossRef]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Campbell–Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef]
- Abdelmalek, M.F.; Sanderson, S.O.; Angulo, P.; Soldevila-Pico, C.; Liu, C.; Peter, J.; Keach, J.; Cave, M.; Chen, T.; McClain, C.J.; et al. Betaine for nonalcoholic fatty liver disease: Results of a randomized placebo-controlled trial. Hepatology 2009, 50, 1818–1826. [Google Scholar] [CrossRef]
- Best, C.H.; Huntsman, M.E. The effects of the components of lecithine upon deposition of fat in the liver. J. Physiol. 1932, 75, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wu, N.; Chen, X.; Wang, W.; Chu, Y.; Liu, H.; Li, W.; Chen, D.; Li, X.; Xu, B. Pathogenesis of and major animal models used for nonalcoholic fatty liver disease. J. Int. Med. Res. 2019, 47, 1453–1466. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, N.; Shor, J.; Szabo, G. Alcoholic Hepatitis: A Review. Alcohol Alcohol. 2019, 54, 408–416. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Status Report on Alcohol and Health. P. Xiv. 2014 ed. Available online: http://www.who.int/substance_abuse/publications/global_alcohol_report/msb_gsr_2014_1.pdf?ua=1.2017 (accessed on 18 January 2017).
- Sudhinaraset, M.; Wigglesworth, C.; Takeuchi, D.T. Social and cultural contexts of alcohol use: Influences in a social-ecological framework. Alcohol Res. 2016, 38, 35–45. [Google Scholar] [PubMed]
- Rahman, M.A.; Patters, B.J.; Kodidela, S.; Kumar, S. Extracellular Vesicles: Intercellular Mediators in Alcohol-Induced Pathologies. J. Neuroimmune Pharmacol. 2019, 15, 409–421. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Prim. 2018, 4, 16. [Google Scholar] [CrossRef]
- Sayette, M.A. The effects of alcohol on emotion in social drinkers. Behav. Res. Ther. 2017, 88, 76–89. [Google Scholar] [CrossRef]
- Schulze, R.J.; Ding, W.-X. Lipid droplet dynamics in alcoholic fatty liver disease. Liver Res. 2019, 3, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J. The Risks Associated With Alcohol Use and Alcoholism. Alcohol Res. Health 2011, 34, 135–143. [Google Scholar] [PubMed]
- NHS. Alcohol-related liver disease. NHS 2018, 74, 280. [Google Scholar]
- Oshino, N.; Oshino, R.; Chance, B. The characteristics of the ‘peroxidatic’ reaction of catalase in ethanol oxidation. Biochem. J. 1973, 131, 555–563. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol Metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef]
- Zakhari, S. Overview: How Is Alcohol Metabolized by the Body? Alcohol Res. Health 2006, 29, 245–254. [Google Scholar]
- Kharbanda, K.K. Role of transmethylation reactions in alcoholic liver disease. World J. Gastroenterol. 2007, 13, 4947–4954. [Google Scholar] [CrossRef]
- Kharbanda, K.K.; Todero, S.L.; King, A.L.; Osna, N.A.; McVicker, B.L.; Tuma, D.J.; Wisecarver, J.L.; Bailey, S.M. Betaine Treatment Attenuates Chronic Ethanol-Induced Hepatic Steatosis and Alterations to the Mitochondrial Respiratory Chain Proteome. Int. J. Hepatol. 2011, 2012, 1–10. [Google Scholar] [CrossRef]
- Listenberger, L.; Townsend, E.; Rickertsen, C.; Hains, A.; Brown, E.; Inwards, E.G.; Stoeckman, A.K.; Matis, M.P.; Sampathkumar, R.S.; Osna, N.A.; et al. Decreasing Phosphatidylcholine on the Surface of the Lipid Droplet Correlates with Altered Protein Binding and Steatosis. Cells 2018, 7, 230. [Google Scholar] [CrossRef]
- Fernando, H.; Wiktorowicz, J.E.; Soman, K.V.; Kaphalia, B.S.; Khan, M.F.; Ansari, G.A.S. Liver proteomics in progressive alcoholic steatosis. Toxicol. Appl. Pharmacol. 2013, 266, 470–480. [Google Scholar] [CrossRef]
- Donohue, T.M., Jr. Alcohol-induced steatosis in liver cells. World J. Gastroenterol. 2007, 13, 4974–4978. [Google Scholar] [PubMed]
- Lieber, C.S.; Rubin, E. Alcoholic Fatty Liver. N. Engl. J. Med. 1969, 280, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Bergheim, I.; McClain, C.J.; Arteel, G.E. Treatment of Alcoholic Liver Disease. Dig. Dis. 2005, 23, 275–284. [Google Scholar] [CrossRef] [PubMed]
- McClain, C.J.; Barve, S.; Deaciuc, I.; Kugelmas, M.; Hill, D. Cytokines in Alcoholic Liver Disease. Semin. Liver Dis. 1999, 19, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Celli, R.; Zhang, X. Pathology of Alcoholic Liver Disease. J. Clin. Transl. Hepatol. 2014, 2, 103–109. [Google Scholar] [CrossRef]
- Patel, R.; Mueller, M. Alcoholic Liver Disease; Statpearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Van Der Heide, D.; Weiskirchen, R.; Bansal, R. Therapeutic Targeting of Hepatic Macrophages for the Treatment of Liver Diseases. Front. Immunol. 2019, 10, 2852. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; John, S. Hepatic Cirrhosis; Statpearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Li, D.; Friedman, S. Liver fibrogenesis and the role of hepatic stellate cells: New insights and prospects for therapy. J. Gastroenterol. Hepatol. 1999, 14, 618–633. [Google Scholar] [CrossRef]
- Ankoma-Sey, V.; Friedman, S.L. Hepatic stellate cells. Liver Growth Repair. 1998, 14, 512–537. [Google Scholar] [CrossRef]
- Teli, M.; Day, C.; James, O.; Burt, A.; Bennett, M. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995, 346, 987–990. [Google Scholar] [CrossRef]
- Nieto, N.; Greenwel, P.; Friedman, S.L.; Zhang, F.; Dannenberg, A.J.; Cederbaum, A.I. Ethanol and Arachidonic Acid Increase α2(I) Collagen Expression in Rat Hepatic Stellate Cells Overexpressing Cytochrome P450 2E1. J. Biol. Chem. 2000, 275, 20136–20145. [Google Scholar] [CrossRef]
- Nieto, N.; Friedman, S.L.; Greenwel, P.; Cederbaum, A.I. CYP2E1-mediated oxidative stress induces collagen type I expression in rat hepatic stellate cells. Hepatology 1999, 30, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.; Nieto, N. Hepatic stellate cells and alcoholic liver disease. Rev. Esp. Enferm. Dig. 2006, 98, 674–684. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chan, Y.-T.; Wang, N.; Tan, H.Y.; Li, S.; Feng, Y. Targeting Hepatic Stellate Cells for the Treatment of Liver Fibrosis by Natural Products: Is It the Dawning of a New Era? Front. Pharmacol. 2020, 11, 548. [Google Scholar] [CrossRef]
- Purohit, V.; Abdelmalek, M.F.; Barve, S.; Benevenga, N.J.; Halsted, C.H.; Kaplowitz, N.; Kharbanda, K.K.; Liu, Q.-Y.; Lu, S.C.; McClain, C.J.; et al. Role of S-adenosylmethionine, folate, and betaine in the treatment of alcoholic liver disease: Summary of a symposium. Am. J. Clin. Nutr. 2007, 86, 14–24. [Google Scholar] [CrossRef]
- Halsted, C.H.; Villanueva, J.A.; Devlin, A.M. Folate deficiency, methionine metabolism, and alcoholic liver disease. Alcohol 2002, 27, 169–172. [Google Scholar] [CrossRef]
- Kharbanda, K.K. Alcoholic Liver Disease and Methionine Metabolism. Semin. Liver Dis. 2009, 29, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, K.K. Methionine metabolic pathway in alcoholic liver injury. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Kaplowitz, N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 2003, 124, 1488–1499. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, Z.; Chen, T.; Hill, D.; Kang, J.; Barve, S.; McClain, C. S-adenosylmethionine (SAMe) protects against acute alcohol induced hepatotoxicity in mice. J. Nutr. Biochem. 2003, 14, 591–597. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Liang, B.; Su, Y.; Sun, S.; Xia, S.; Shao, J.; Zhang, Z.; Hong, M.; Zhang, F.; et al. Methionine metabolism in chronic liver diseases: An update on molecular mechanism and therapeutic implication. Signal Transduct. Target. Ther. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Barak, A.J.; Beckenhauer, H.C.; Kharbanda, K.K.; Tuma, D.J. Chronic ethanol consumption increases homocysteine accumulation in hepatocytes. Alcohol 2001, 25, 77–81. [Google Scholar] [CrossRef]
- Trimble, K.C.; Molloy, A.; Scot, J.M.; Weir, D.G. The effect of ethanol on one-carbon metabolism: Increased methionine catabolism and lipotrope methyl-group wastage. Hepatology 1993, 18, 984–989. [Google Scholar] [CrossRef]
- Barak, A.J.; Beckenhauer, H.C.; Mailliard, M.E.; Kharbanda, K.K.; Tuma, D.J. Betaine Lowers Elevated S-Adenosylhomocysteine Levels in Hepatocytes from Ethanol-Fed Rats. J. Nutr. 2003, 133, 2845–2848. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.; Banfield, K. S-adenosylmethionine-dependent methyltransferases. In Homocysteine in Health and Disease; Carmel, R., Jacobsen, D.W., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 63–78. [Google Scholar]
- Kharbanda, K.K.; Todero, S.L.; Ward, B.W.; Cannella, J.J., 3rd; Tuma, D.J. Betaine administration corrects ethanol-induced defective VLDL secretion. Mol. Cell. Biochem. 2009, 327, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, K.K.; Rogers, D.D., 2nd; Mailliard, M.E.; Siford, G.L.; Barak, A.J.; Beckenhauer, H.C.; Sorrell, M.F.; Tuma, D.J. Role of elevated s-adenosylhomocysteine in rat hepatocyte apoptosis: Protection by betaine. Biochem. Pharmacol. 2005, 70, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, K.K.; Mailliard, M.E.; Baldwin, C.R.; Sorrell, M.F.; Tuma, D.J. Accumulation of proteins bearing atypical isoaspartyl residues in livers of alcohol-fed rats is prevented by betaine administration: Effects on protein-l-isoaspartyl methyltransferase activity. J. Hepatol. 2007, 46, 1119–1125. [Google Scholar] [CrossRef]
- Kharbanda, K.K.; Todero, S.L.; Moats, J.C.; Harris, R.M.; Osna, N.A.; Thomes, P.G.; Tuma, D.J. Alcohol Consumption Decreases Rat Hepatic Creatine Biosynthesis Via Altered Guanidinoacetate Methyltransferase Activity. Alcohol. Clin. Exp. Res. 2013, 38, 641–648. [Google Scholar] [CrossRef]
- You, M.; Arteel, G.E. Effect of ethanol on lipid metabolism. J. Hepatol. 2019, 70, 237–248. [Google Scholar] [CrossRef]
- Rasineni, K.; Casey, C.A. Molecular mechanism of alcoholic fatty liver. Indian J. Pharmacol. 2012, 44, 299–303. [Google Scholar] [CrossRef]
- Jump, D.B. Fatty acid regulation of hepatic lipid metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 115–120. [Google Scholar] [CrossRef]
- Watkins, S.M.; Zhu, X.; Zeisel, S.H. Phosphatidylethanolamine-N-methyltransferase Activity and Dietary Choline Regulate Liver-Plasma Lipid Flux and Essential Fatty Acid Metabolism in Mice. J. Nutr. 2003, 133, 3386–3391. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chung, S.; Shelness, G.S.; Parks, J.S. Hepatic ABCA1 and VLDL triglyceride production. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2012, 1821, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Nishimaki-Mogami, T.; Yao, Z.; Fujimori, K. Inhibition of phosphatidylcholine synthesis via the phosphatidylethanolamine methylation pathway impairs incorporation of bulk lipids into VLDL in cultured rat hepatocytes. J. Lipid Res. 2002, 43, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Noga, A.A.; Zhao, Y.; Vance, D.E. An Unexpected Requirement for PhosphatidylethanolamineN-Methyltransferase in the Secretion of Very Low Density Lipoproteins. J. Biol. Chem. 2002, 277, 42358–42365. [Google Scholar] [CrossRef]
- Moslehi, A.; Hamidi-Zad, Z. Role of SREBPs in Liver Diseases: A Mini-review. J. Clin. Transl. Hepatol. 2018, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Rao, S.; Reddy, J.K. Peroxisome Proliferator-Activated Receptors, Fatty Acid Oxidation, Steatohepatitis and Hepatocarcinogenesis. Curr. Mol. Med. 2003, 3, 561–572. [Google Scholar] [CrossRef]
- You, M.; Crabb, D.W. Recent Advances in Alcoholic Liver Disease II. Minireview: Molecular mechanisms of alcoholic fatty liver. Am. J. Physiol. Liver Physiol. 2004, 287, G1–G6. [Google Scholar] [CrossRef]
- You, M.; Matsumoto, M.; Pacold, C.M.; Cho, W.K.; Crabb, D.W. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology 2004, 127, 1798–1808. [Google Scholar] [CrossRef]
- Meng, F.-G.; Zhang, X.-N.; Liu, S.-X.; Wang, Y.-R.; Zeng, T. Roles of peroxisome proliferator-activated receptor α in the pathogenesis of ethanol-induced liver disease. Chem. Interact. 2020, 327, 109176. [Google Scholar] [CrossRef]
- Nanji, A.A.; Dannenberg, A.J.; Jokelainen, K.; Bass, N.M. Alcoholic liver injury in the rat is associated with reduced expression of peroxisome proliferator-alpha (pparalpha)-regulated genes and is ameliorated by pparalpha activation. J. Pharmacol. Exp. Ther. 2004, 310, 417–424. [Google Scholar] [CrossRef]
- Liangpunsakul, S.; Wou, S.-E.; Zeng, Y.; Ross, R.A.; Jayaram, H.N.; Crabb, D.W. Effect of ethanol on hydrogen peroxide-induced AMPK phosphorylation. Am. J. Physiol. Liver Physiol. 2008, 295, G1173–G1181. [Google Scholar] [CrossRef]
- Liangpunsakul, S.; Sozio, M.S.; Shin, E.; Zhao, Z.; Xu, Y.; Ross, R.A.; Zeng, Y.; Crabb, D.W. Inhibitory effect of ethanol on AMPK phosphorylation is mediated in part through elevated ceramide levels. Am. J. Physiol. Liver Physiol. 2010, 298, G1004–G1012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, T.; Su, Z.; Wang, X.; Wang, Y.; Ni, Y.; Zuo, Y.; Gu, H. Reduced methylation of PP2Ac promotes ethanol–induced lipid accumulation through FOXO1 phosphorylation in vitro and in vivo. Toxicol. Lett. 2020, 331, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Cahill, A.; Cunningham, C.C.; Adachi, M.; Ishii, H.; Bailey, S.M.; Fromenty, B.; Davies, A. Effects of alcohol and oxidative stress on liver pathology: The role of the mitochondrion. Alcohol. Clin. Exp. Res. 2002, 26, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Hoek, J.B.; Cahill, A.; Pastorino, J.G. Alcohol and mitochondria: A dysfunctional relationship. Gastroenterology 2002, 122, 2049–2063. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, S.; Ward, R.; Peters, T. Effect of chronic ethanol feeding on the hepatic secretion of very-low-density lipoproteins. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1988, 960, 61–66. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Keshaw, H.; Xu, L.Y.; Lam, K.S.; Cooper, G.J. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J. Clin. Investig. 2003, 112, 91–100. [Google Scholar] [CrossRef]
- Kharbanda, K.K.; Ronis, M.J.J.; Shearn, C.T.; Petersen, D.R.; Zakhari, S.; Warner, D.R.; Feldstein, A.E.; McClain, C.J.; Kirpich, I.A. Role of nutrition in alcoholic liver disease: Summary of the symposium at the esbra 2017 congress. Biomolecules 2018, 8, 16. [Google Scholar] [CrossRef]
- Yang, W.; Huang, L.; Gao, J.; Wen, S.; Tai, Y.; Chen, M.; Huang, Z.; Liu, R.; Tang, C.; Li, J. Betaine attenuates chronic alcohol-induced fatty liver by broadly regulating hepatic lipid metabolism. Mol. Med. Rep. 2017, 16, 5225–5234. [Google Scholar] [CrossRef]
- Bingül, I.; Başaran-Küçükgergin, C.; Aydın, A.; Çoban, J.; Doğan-Ekici, I.; Doğru-Abbasoğlu, S.; Uysal, M. Betaine treatment decreased oxidative stress, inflammation, and stellate cell activation in rats with alcoholic liver fibrosis. Environ. Toxicol. Pharmacol. 2016, 45, 170–178. [Google Scholar] [CrossRef]
- Mato, J.M.; Martínez-Chantar, M.L.; Lu, S.C. S-adenosylmethionine metabolism and liver disease. Ann. Hepatol. 2013, 12, 183–189. [Google Scholar] [CrossRef]
- Ji, C.; Shinohara, M.; Vance, D.; Than, T.A.; Ookhtens, M.; Chan, C.; Kaplowitz, N. Effect of Transgenic Extrahepatic Expression of Betaine-Homocysteine Methyltransferase on Alcohol or Homocysteine-Induced Fatty Liver. Alcohol. Clin. Exp. Res. 2008, 32, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Barak, A.J.; Beckenhauer, H.C.; Tuma, D.J. Betaine effects on hepatic methionine metabolism elicited by short-term ethanol feeding. Alcohol 1996, 13, 483–486. [Google Scholar] [CrossRef]
- Barak, A.J.; Beckenhauer, H.C.; Badakhsh, S.; Tuma, D.J. The effect of betaine in reversing alcoholic steatosis. Alcohol. Clin. Exp. Res. 1997, 21, 1100–1102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, T.; Song, Z. Involvement and mechanism of DGAT2 upregulation in the pathogenesis of alcoholic fatty liver disease. J. Lipid Res. 2010, 51, 3158–3165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, T.; Song, Z. Extracellular signal-regulated kinases 1/2 suppression aggravates transforming growth factor-beta1 hepatotoxicity: A potential mechanism for liver injury in methionine-choline deficient-diet-fed mice. Exp. Biol. Med. 2010, 235, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zhou, Z.; Deaciuc, I.; Chen, T.; McClain, C.J. Inhibition of adiponectin production by homocysteine: A potential mechanism for alcoholic liver disease. Hepatology 2007, 47, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Wang, X.; Wang, Y.; Feng, J. Betaine affects muscle lipid metabolism via regulating the fatty acid uptake and oxidation in finishing pig. J. Anim. Sci. Biotechnol. 2017, 8, 72. [Google Scholar] [CrossRef]
- Yang, W.; Gao, J.; Tai, Y.; Chen, M.; Huang, L.; Wen, S.; Huang, Z.; Liu, R.; Li, J.; Tang, C. Betaine Attenuates Alcohol-Induced Pancreatic Steatosis. Pancreas 2016, 45, 836–845. [Google Scholar] [CrossRef]
- Osna, N.A.; White, R.L.; Donohue, T.M., Jr.; Beard, M.R.; Tuma, D.J.; Kharbanda, K.K. Impaired methylation as a novel mechanism for proteasome suppression in liver cells. Biochem. Biophys. Res. Commun. 2010, 391, 1291–1296. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J. Oxidative stress: The paradox of aerobic life. Biochem. Soc. Symp. 1995, 61, 1–31. [Google Scholar] [CrossRef]
- Freitas, I.; Boncompagni, E.; Tarantola, E.; Gruppi, C.; Bertone, V.; Ferrigno, A.; Milanesi, G.; Vaccarone, R.; Tira, M.E.; Vairetti, M. In SituEvaluation of Oxidative Stress in Rat Fatty Liver Induced by a Methionine- and Choline-Deficient Diet. Oxidative Med. Cell. Longev. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ceni, E.; Mello, T.; Galli, A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J. Gastroenterol. 2014, 20, 17756–17772. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. The Discovery of the Microsomal Ethanol Oxidizing System and Its Physiologic and Pathologic Role. Drug Metab. Rev. 2004, 36, 511–529. [Google Scholar] [CrossRef]
- Cichoz-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Kim, S.J.; Kwon, D.Y.; Ahn, C.W.; Kim, Y.S.; Choi, D.W. Alleviation of alcoholic liver injury by betaine involves an enhancement of antioxidant defense via regulation of sulfur amino acid metabolism. Food Chem. Toxicol. 2013, 62, 292–298. [Google Scholar] [CrossRef]
- Medici, V.; Schroeder, D.I.; Woods, R.; LaSalle, J.M.; Geng, Y.; Shibata, N.M.; Peerson, J.; Hodzic, E.; Dayal, S.; Tsukamoto, H.; et al. Methylation and gene expression responses to ethanol feeding and betaine supplementation in the cystathionine beta synthase-deficient mouse. Alcohol. Clin. Exp. Res. 2014, 38, 1540–1549. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Caudill, M.; Malysheva, O.; Bardag-Gorce, F.; Oliva, J.; French, B.; Gorce, E.; Morgan, K.; Kathirvel, E.; et al. Betaine feeding prevents the blood alcohol cycle in rats fed alcohol continuously for 1month using the rat intragastric tube feeding model. Exp. Mol. Pathol. 2011, 91, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.-Z.; Wang, L.-W.; Zhang, W.; Gong, Z.-J. Betaine inhibits Toll-like receptor 4 expression in rats with ethanol-induced liver injury. World J. Gastroenterol. 2010, 16, 897–903. [Google Scholar]
- Jangra, A.; Sriram, C.S.; Pandey, S.; Choubey, P.; Rajput, P.; Saroha, B.; Bezbaruah, B.K.; Lahkar, M. Epigenetic Modifications, Alcoholic Brain and Potential Drug Targets. Ann. Neurosci. 2016, 23, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Bardag-Gorce, F.; Li, J.; French, B.A.; Nguyen, S.K.; Lu, S.C.; French, S.W. Betaine prevents Mallory-Denk body formation in drug-primed mice by epigenetic mechanisms. Exp. Mol. Pathol. 2009, 86, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Belouzard, S.; Danneels, A.; Fénéant, L.; Séron, K.; Rouillé, Y.; Dubuisson, J. Entry and Release of Hepatitis C Virus in Polarized Human Hepatocytes. J. Virol. 2017, 91, e00478-17. [Google Scholar] [CrossRef]
- Ganesan, M.; Poluektova, L.Y.; Tuma, D.J.; Kharbanda, K.K.; Osna, N.A. Acetaldehyde Disrupts Interferon Alpha Signaling in Hepatitis C Virus-Infected Liver Cells by Up-Regulating USP18. Alcohol. Clin. Exp. Res. 2016, 40, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, M.; Tikhanovich, I.; Vangimalla, S.S.; Dagur, R.S.; Wang, W.; Poluektova, L.I.; Sun, Y.; Mercer, D.F.; Tuma, D.; Weinman, S.A.; et al. Demethylase JMJD6 as a New Regulator of Interferon Signaling: Effects of HCV and Ethanol Metabolism. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 101–112. [Google Scholar] [CrossRef]
- Ganesan, M.; Hindman, J.; Tillman, B.; Jaramillo, L.; Poluektova, L.I.; French, B.A.; Kharbanda, K.K.; French, S.W.; Osna, N.A. FAT10 suppression stabilizes oxidized proteins in liver cells: Effects of HCV and ethanol. Exp. Mol. Pathol. 2015, 99, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, M.; Zhang, J.; Bronich, T.; Poluektova, L.I.; Donohue, T.M., Jr.; Tuma, D.J.; Kharbanda, K.K.; Osna, N.A. Acetaldehyde accelerates HCV-induced impairment of innate immunity by suppressing methylation reactions in liver cells. Am. J. Physiol. Liver Physiol. 2015, 309, G566–G577. [Google Scholar] [CrossRef]
- Gotthardt, D.; Riediger, C.; Weiss, K.H.; Encke, J.; Schemmer, P.; Schmidt, J.; Sauer, P. Fulminant hepatic failure: Etiology and indications for liver transplantation. Nephrol. Dial. Transplant. 2007, 22 (Suppl. S8), viii5–viii8. [Google Scholar] [CrossRef]
- Rasineni, K.; Lee, S.M.L.; McVicker, B.L.; Osna, N.A.; Casey, C.A.; Kharbanda, K.K. Susceptibility of Asialoglycoprotein Receptor-Deficient Mice to Lps/Galactosamine Liver Injury and Protection by Betaine Administration. Biology 2020, 10, 19. [Google Scholar] [CrossRef]
- Junnila, M.; Barak, A.J.; Beckenhauer, H.C.; Rahko, T. Betaine reduces hepatic lipidosis induced by carbon tetrachloride in Sprague-Dawley rats. Vet. Hum. Toxicol. 1998, 40, 263–266. [Google Scholar]
- Junnila, M.; Rahko, T.; Sukura, A.; Lindberg, L.-A. Reduction of Carbon Tetrachloride-Induced Hepatotoxic Effects by Oral Administration of Betaine in Male Han-Wistar Rats: A Morphometric Histological Study. Vet. Pathol. 2000, 37, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-T.; Chen, C.-Y.; Pan, Y.-H.; Wang, S.-H.; Mersmann, H.J.; Ding, S.-T. Alleviation of Carbon-Tetrachloride-Induced Liver Injury and Fibrosis by Betaine Supplementation in Chickens. Evid. Based Complement. Altern. Med. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Maurice, J.; Manousou, P. Non-alcoholic fatty liver disease. Clin. Med. 2018, 18, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.P.; Eudy, B.J.; Deminice, R. One-Carbon Metabolism in Fatty Liver Disease and Fibrosis: One-Carbon to Rule Them All. J. Nutr. 2020, 150, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.K.C.; Zhang, X.; Yu, J. Animal models of non-alcoholic fatty liver disease: Current perspectives and recent advances. J. Pathol. 2017, 241, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.-M.; Linfoot, P.; Dare, D.; Aghajanian, K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am. J. Clin. Nutr. 2003, 77, 43–50. [Google Scholar] [CrossRef]
- Kwanten, W.J.; Martinet, W.; Michielsen, P.P.; Francque, S.M. Role of autophagy in the pathophysiology of nonalcoholic fatty liver disease: A controversial issue. World J. Gastroenterol. 2014, 20, 7325–7338. [Google Scholar] [CrossRef] [PubMed]
- Paglialunga, S.; Dehn, C.A. Clinical assessment of hepatic de novo lipogenesis in non-alcoholic fatty liver disease. Lipids Health Dis. 2016, 15, 159. [Google Scholar] [CrossRef]
- Lu, S.C.; Alvarez, L.; Huang, Z.-Z.; Chen, L.; An, W.; Corrales, F.J.; Avila, M.A.; Kanel, G.; Mato, J.M. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc. Natl. Acad. Sci. USA 2001, 98, 5560–5565. [Google Scholar] [CrossRef]
- Radziejewska, A.; Muzsik, A.; Milagro, F.I.; Martínez, J.A.; Chmurzynska, A. One-Carbon Metabolism and Nonalcoholic Fatty Liver Disease: The Crosstalk between Nutrients, Microbiota, and Genetics. Lifestyle Genom. 2019, 13, 53–63. [Google Scholar] [CrossRef]
- Deminice, R.; Da Silva, R.P.; Lamarre, S.G.; Kelly, K.B.; Jacobs, R.L.; Brosnan, M.E.; Brosnan, J.T. Betaine supplementation prevents fatty liver induced by a high-fat diet: Effects on one-carbon metabolism. Amino Acids 2015, 47, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Tan, Y.; Wei, J.; Chang, Y.; Jin, T.; Zhu, H. Betaine supplement alleviates hepatic triglyceride accumulation of apolipoprotein E deficient mice via reducing methylation of peroxisomal proliferator-activated receptor alpha promoter. Lipids Health Dis. 2013, 12, 34. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Xu, M.; Jiang, L.; Zhou, M.; Liu, W.; Chen, Z.; Wang, Y.; Zou, Q.; Wang, L. Betaine prevented high-fat diet-induced nafld by regulating the fgf10/ampk signaling pathway in ApoE−/− mice. Eur. J. Nutr. 2020, 60, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Deaciuc, I.; Zhou, Z.; Song, M.; Chen, T.; Hill, D.; McClain, C.J. Involvement of AMP-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis. Am. J. Physiol. Liver Physiol. 2007, 293, G894–G902. [Google Scholar] [CrossRef]
- Vesković, M.; Labudović-Borović, M.; MladenoviĆ, D.; Jadžić, J.; Jorgačević, B.; Vukićević, D.; Vučević, D.; Radosavljević, T. Effect of Betaine Supplementation on Liver Tissue and Ultrastructural Changes in Methionine–Choline-Deficient Diet-Induced NAFLD. Microsc. Microanal. 2020, 26, 1–10. [Google Scholar] [CrossRef]
- Abu Ahmad, N.; Raizman, M.; Weizmann, N.; Wasek, B.; Arning, E.; Bottiglieri, T.; Tirosh, O.; Troen, A.M. Betaine attenuates pathology by stimulating lipid oxidation in liver and regulating phospholipid metabolism in brain of methionine-choline–deficient rats. FASEB J. 2019, 33, 9334–9349. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Jung, Y.S.; Kim, S.J.; Park, H.K.; Park, J.H.; Kim, Y.C. Impaired Sulfur-Amino Acid Metabolism and Oxidative Stress in Nonalcoholic Fatty Liver Are Alleviated by Betaine Supplementation in Rats. J. Nutr. 2008, 139, 63–68. [Google Scholar] [CrossRef]
- Sookoian, S.; Puri, P.; Castaño, G.O.; Scian, R.; Mirshahi, F.; Sanyal, A.J.; Pirola, C.J. Nonalcoholic steatohepatitis is associated with a state of betaine-insufficiency. Liver Int. 2017, 37, 611–619. [Google Scholar] [CrossRef]
- Mukherjee, S.; Tamara, B.; Kharbanda, K.; Barak, A.J.; Sorrell, M.F.; Tuma, D.J. Impact of betaine on hepatic fibrosis and homocysteine in nonalcoholic steatohepatitis—A prospective, cohort study. Open Transl. Med. J. 2011, 3, 1–4. [Google Scholar] [CrossRef]
- Abdelmalek, M.F.; Angulo, P.; Jorgensen, R.A.; Sylvestre, P.B.; Lindor, K.D. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: Results of a pilot study. Am. J. Gastroenterol. 2001, 96, 2711–2717. [Google Scholar] [CrossRef]
- Miglio, F.; Rovati, L.; Santoro, A.; Setnikar, I. Efficacy and Safety of Oral Betaine Glucuronate in Non-alcoholic Steatohepatitis. A double-blind, randomized, parallel-group, placebo-controlled prospective clinical study. Arzneimittelforschung 2000, 50, 722–727. [Google Scholar] [CrossRef][Green Version]
- Mukherjee, S. Role of betaine in liver disease-worth revisiting or has the die been cast? World J. Gastroenterol. 2020, 26, 5745–5748. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nat. Cell Biol. 2012, 482, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Csak, T.; Ganz, M.; Pespisa, J.; Kodys, K.; Dolganiuc, A.; Szabo, G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology 2011, 54, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Schnabl, B. Role of innate immunity and the microbiota in liver fibrosis: Crosstalk between the liver and gut. J. Physiol. 2012, 590, 447–458. [Google Scholar] [CrossRef]
- Campo, L.; Eiseler, S.; Apfel, T.; Pyrsopoulos, N. Fatty Liver Disease and Gut Microbiota: A Comprehensive Update. J. Clin. Transl. Hepatol. 2018, 7, 1–5. [Google Scholar] [CrossRef]
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Flier, J.S. Adipose Tissue as an Endocrine Organ. Trends Endocrinol. Metab. 2000, 11, 327–332. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Dou, X.-B.; Zhou, Z.-X.; Song, Z.-Y. Adipose tissue-liver axis in alcoholic liver disease. World J. Gastrointest. Pathophysiol. 2016, 7, 17–26. [Google Scholar] [CrossRef]
- Li, Y.; Ding, W.-X. Adipose tissue autophagy and homeostasis in alcohol-induced liver injury. Liver Res. 2017, 1, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Pestana, D.; Teixeira, D.; Meireles, M.; Marques, C.; Norberto, S.; Sá, C.; Fernandes, V.C.; Correia-Sá, L.; Faria, A.; Guardão, L.; et al. Adipose tissue dysfunction as a central mechanism leading to dysmetabolic obesity triggered by chronic exposure to p,p’-DDE. Sci. Rep. 2017, 7, 2738. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shi, X.; Zhong, W.; Zhao, Y.; Tang, Y.; Sun, W.; Yin, X.; Bogdanov, B.; Kim, S.; McClain, C.; et al. Chronic Alcohol Exposure Disturbs Lipid Homeostasis at the Adipose Tissue-Liver Axis in Mice: Analysis of Triacylglycerols Using High-Resolution Mass Spectrometry in Combination with In Vivo Metabolite Deuterium Labeling. PLoS ONE 2013, 8, e55382. [Google Scholar] [CrossRef]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef]
- Fabbrini, E.; Mohammed, B.S.; Magkos, F.; Korenblat, K.M.; Patterson, B.W.; Klein, S. Alterations in Adipose Tissue and Hepatic Lipid Kinetics in Obese Men and Women With Nonalcoholic Fatty Liver Disease. Gastroenterology 2008, 134, 424–431. [Google Scholar] [CrossRef]
- Kang, L.; Chen, X.; Sebastian, B.M.; Pratt, B.T.; Bederman, I.R.; Alexander, J.C.; Previs, S.F.; Nagy, L.E. Chronic ethanol and triglyceride turnover in white adipose tissue in rats: Inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J. Biol. Chem. 2007, 282, 28465–28473. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Y.; Xiao, J.; Liu, L.; Chen, S.; Mohammadi, M.; McClain, C.J.; Li, X.; Feng, W. FGF21 mediates alcohol-induced adipose tissue lipolysis by activation of systemic release of catecholamine in mice. J. Lipid Res. 2015, 56, 1481–1491. [Google Scholar] [CrossRef]
- Zhong, W.; Zhao, Y.; Tang, Y.; Wei, X.; Shi, X.; Sun, W.; Sun, X.; Yin, X.; Sun, X.; Kim, S.; et al. Chronic Alcohol Exposure Stimulates Adipose Tissue Lipolysis in Mice: Role of Reverse Triglyceride Transport in the Pathogenesis of Alcoholic Steatosis. Am. J. Pathol. 2012, 180, 998–1007. [Google Scholar] [CrossRef]
- Parker, R. The role of adipose tissue in fatty liver diseases. Liver Res. 2018, 2, 35–42. [Google Scholar] [CrossRef]
- Parker, R.; Kim, S.-J.; Gao, B. Alcohol, adipose tissue and liver disease: Mechanistic links and clinical considerations. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.L.; Lang, C.H. Alcohol, Adipose Tissue and Lipid Dysregulation. Biomolecules 2017, 7, 16. [Google Scholar] [CrossRef]
- Tan, X.; Sun, X.; Li, Q.; Zhao, Y.; Zhong, W.; Sun, X.; Jia, W.; McClain, C.J.; Zhou, Z. Leptin Deficiency Contributes to the Pathogenesis of Alcoholic Fatty Liver Disease in Mice. Am. J. Pathol. 2012, 181, 1279–1286. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- He, Z.; Li, M.; Zheng, D.; Chen, Q.; Liu, W.; Feng, L. Adipose tissue hypoxia and low-grade inflammation: A possible mechanism for ethanol-related glucose intolerance? Br. J. Nutr. 2015, 113, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, B.R.; McKenzie, C.M.; Hempel, K.W.; Lang, A.L.; Arteel, G.E.; Beier, J.I. Adipose tissue-liver crosstalk during pathologic changes caused by vinyl chloride metabolites in mice. Toxicol. Appl. Pharmacol. 2020, 399, 115068. [Google Scholar] [CrossRef] [PubMed]
- Duwaerts, C.C.; Amin, A.M.; Siao, K.; Her, C.; Fitch, M.; Beysen, C.; Turner, S.M.; Goodsell, A.; Baron, J.L.; Grenert, J.P.; et al. Specific Macronutrients Exert Unique Influences on the Adipose-Liver Axis to Promote Hepatic Steatosis in Mice. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Xia, Y.; Chen, J.; Qian, Y.; Li, S.; Zhang, X.; Song, Z. Rectification of impaired adipose tissue methylation status and lipolytic response contributes to hepatoprotective effect of betaine in a mouse model of alcoholic liver disease. Br. J. Pharmacol. 2014, 171, 4073–4086. [Google Scholar] [CrossRef]
- Wang, Z.; Dou, X.; Yao, T.; Song, Z. Homocysteine inhibits adipogenesis in 3T3-L1 preadipocytes. Exp. Biol. Med. 2011, 236, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Bistritz, L.; Meddings, J.B. Alterations in intestinal permeability. Gut 2006, 55, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Y.; Jiao, F.; Shi, C.; Pei, M.; Wang, L.; Gong, Z. Betaine inhibits Toll-like receptor 4 responses and restores intestinal microbiota in acute liver failure mice. Sci. Rep. 2020, 10, 21850. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; He, C.; Bu, J.; Luo, Y.; Yang, S.; Ye, C.; Yu, S.; He, B.; Yin, Y.; Yang, X. Betaine attenuates LPS-induced downregulation of Occludin and Claudin-1 and restores intestinal barrier function. BMC Vet. Res. 2020, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, H.; Tiihonen, K.; Peuranen, S.; Saarinen, M.; Remus, J. Dietary betaine accumulates in the liver and intestinal tissue and stabilizes the intestinal epithelial structure in healthy and coccidia-infected broiler chicks. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 130, 759–769. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Fang, S.; Yang, X.; Feng, J. Betaine Improves Intestinal Functions by Enhancing Digestive Enzymes, Ameliorating Intestinal Morphology, and Enriching Intestinal Microbiota in High-salt stressed Rats. Nutrients 2018, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, T.; Pini, M.; Zhou, Z.; Fantuzzi, G.; Song, Z. Betaine improved adipose tissue function in mice fed a high-fat diet: A mechanism for hepatoprotective effect of betaine in nonalcoholic fatty liver disease. Am. J. Physiol. Liver Physiol. 2010, 298, G634–G642. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, J.; Chen, J.; Wu, W.; Wang, X.; Wang, Y. The beneficial effects of betaine on dysfunctional adipose tissue and N6-methyladenosine mRNA methylation requires the AMP-activated protein kinase α1 subunit. J. Nutr. Biochem. 2015, 26, 1678–1684. [Google Scholar] [CrossRef]
- Du, J.; Shen, L.; Tan, Z.; Zhang, P.; Zhao, X.; Xu, Y.; Gan, M.; Yang, Q.; Ma, J.; Jiang, A.; et al. Betaine Supplementation Enhances Lipid Metabolism and Improves Insulin Resistance in Mice Fed a High-Fat Diet. Nutrients 2018, 10, 131. [Google Scholar] [CrossRef]
- Olli, K.; Lahtinen, S.; Rautonen, N.; Tiihonen, K. Betaine reduces the expression of inflammatory adipokines caused by hypoxia in human adipocytes. Br. J. Nutr. 2013, 109, 43–49. [Google Scholar] [CrossRef]
- Ganesan, B.; Anandan, R. Protective effect of betaine on changes in the levels of lysosomal enzyme activities in heart tissue in isoprenaline-induced myocardial infarction in Wistar rats. Cell Stress Chaperones 2009, 14, 661–667. [Google Scholar] [CrossRef]
- Hagar, H.; Al Malki, W. Betaine supplementation protects against renal injury induced by cadmium intoxication in rats: Role of oxidative stress and caspase-3. Environ. Toxicol. Pharmacol. 2014, 37, 803–811. [Google Scholar] [CrossRef]
- Pourmehdi, A.; Sakhaei, Z.; Alirezaei, M.; Dezfoulian, O. Betaine effects against asthma-induced oxidative stress in the liver and kidney of mice. Mol. Biol. Rep. 2020, 47, 1–7. [Google Scholar] [CrossRef]
- Borsook, M.E.; Billig, H.K.; Golseth, J.G. Betaine and glycocyamine in the treatment of disability resulting from acute anterior poliomyelitis. Ann. West. Med. Surg. 1952, 6, 423–427. [Google Scholar] [PubMed]
- Lee, E.C.; Maresh, C.M.; Kraemer, W.J.; Yamamoto, L.M.; Hatfield, D.L.; Bailey, B.L.; Armstrong, L.E.; Volek, J.S.; McDermott, B.P.; Craig, S.A. Ergogenic effects of betaine supplementation on strength and power performance. J. Int. Soc. Sports Nutr. 2010, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.K.; Sternbach, S.; Fleming, S.; Alkhayer, K.; Shelestak, J.; Popescu, D.; Weaver, A.; Clements, R.; Wasek, B.; Bottiglieri, T.; et al. Betaine restores epigenetic control and supports neuronal mitochondria in the cuprizone mouse model of multiple sclerosis. Epigenetics 2020, 15, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nguyen, N.; Colditz, G.A. Links between Alcohol Consumption and Breast Cancer: A Look at the Evidence. Women’s Health 2015, 11, 65–77. [Google Scholar] [CrossRef]
- McDonald, J.A.; Goyal, A.; Terry, M.B. Alcohol Intake and Breast Cancer Risk: Weighing the Overall Evidence. Curr. Breast Cancer Rep. 2013, 5, 208–221. [Google Scholar] [CrossRef]
- Hong, Z.; Lin, M.; Zhang, Y.; He, Z.; Zheng, L. Role of betaine in inhibiting the induction of RNA Pol III gene transcription and cell growth caused by alcohol. Chem. Interact. 2020, 325, 109129. [Google Scholar] [CrossRef]
- Brown, A.L.; Conrad, K.; Allende, D.S.; Gromovsky, A.D.; Zhang, R.; Neumann, C.K.; Owens, A.P.; Tranter, M.; Helsley, R.N. Dietary Choline Supplementation Attenuates High-Fat-Diet–Induced Hepatocellular Carcinoma in Mice. J. Nutr. 2019, 150, 775–783. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Liu, Y.; Wang, X.; Guan, K.; Zhu, H.-L. Higher serum concentrations of betaine rather than choline is associated with better profiles of DXA-derived body fat and fat distribution in Chinese adults. Int. J. Obes. 2014, 39, 465–471. [Google Scholar] [CrossRef]
- Al-Musharaf, S.; Aljuraiban, G.S.; Hussain, S.D.; Alnaami, A.M.; Saravanan, P.; Al-Daghri, N. Low Serum Vitamin B12 Levels Are Associated with Adverse Lipid Profiles in Apparently Healthy Young Saudi Women. Nutrients 2020, 12, 2395. [Google Scholar] [CrossRef]
- Adaikalakoteswari, A.; Finer, S.; Voyias, P.D.; McCarthy, C.M.; Vatish, M.; Moore, J.; Smart-Halajko, M.; Bawazeer, N.; Al-Daghri, N.M.; McTernan, P.G.; et al. Vitamin B12 insufficiency induces cholesterol biosynthesis by limiting s-adenosylmethionine and modulating the methylation of SREBF1 and LDLR genes. Clin. Epigenet. 2015, 7, 1–14. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, K.; Pu, Y.; Chang, L.; Wang, S.; Tan, Y.; Wang, X.; Zhang, J.; Ohnishi, T.; Yoshikawa, T.; et al. Betaine supplementation is associated with the resilience in mice after chronic social defeat stress: A role of brain–gut–microbiota axis. J. Affect. Disord. 2020, 272, 66–76. [Google Scholar] [CrossRef]
- Murata, Y.; Ikegame, T.; Koike, S.; Saito, T.; Ikeda, M.; Sasaki, T.; Iwata, N.; Kasai, K.; Bundo, M.; Iwamoto, K. Global DNA hypomethylation and its correlation to the betaine level in peripheral blood of patients with schizophrenia. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2020, 99, 109855. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, S.; Rezaei, H.; Razavi, S.M. Anti-nociceptive and antioxidant activity of betaine on formalin- and writhing tests induced pain in mice. Behav. Brain Res. 2020, 390, 112699. [Google Scholar] [CrossRef]
- Idriss, A.A.; Hu, Y.; Sun, Q.; Hou, Z.; Yang, S.; Omer, N.A.; Abobaker, H.; Zhao, R. Fetal betaine exposure modulates hypothalamic expression of cholesterol metabolic genes in offspring cockerels with modification of promoter DNA methylation. Poult. Sci. 2020, 99, 2533–2542. [Google Scholar] [CrossRef]
- Ala, F.S.; Hassanabadi, A.; Golian, A. Effects of dietary supplemental methionine source and betaine replacement on the growth performance and activity of mitochondrial respiratory chain enzymes in normal and heat-stressed broiler chickens. J. Anim. Physiol. Anim. Nutr. 2018, 103, 87–99. [Google Scholar] [CrossRef]
- Zhang, L.; Ying, S.; An, W.; Lian, H.; Zhou, G.; Han, Z. Effects of dietary betaine supplementation subjected to heat stress on milk performances and physiology indices in dairy cow. Genet. Mol. Res. 2014, 13, 7577–7586. [Google Scholar] [CrossRef]
- Leng, Z.; Fu, Q.; Yang, X.; Ding, L.; Wen, C.; Zhou, Y. Increased fatty acid beta-oxidation as a possible mechanism for fat-reducing effect of betaine in broilers. Anim. Sci. J. 2016, 87, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhao, S.; Dai, S.; Liu, D.; Bokhari, S.G. Effects of dietary betaine on growth performance, fat deposition and serum lipids in broilers subjected to chronic heat stress. Anim. Sci. J. 2015, 86, 897–903. [Google Scholar] [CrossRef]
- Zulkifli, I.; Mysahra, S.A.; Jin, L.Z. Dietary Supplementation of Betaine (Betafin®) and Response to High Temperature Stress in Male Broiler Chickens. Asian Australas. J. Anim. Sci. 2004, 17, 244–249. [Google Scholar] [CrossRef]
- Digiacomo, K.; Simpson, S.; Leury, B.J.; Dunshea, F.R. Dietary Betaine Impacts the Physiological Responses to Moderate Heat Conditions in a Dose Dependent Manner in Sheep. Animals 2016, 6, 51. [Google Scholar] [CrossRef]
- Van Lee, L.; Tint, M.T.; Aris, I.M.; Quah, P.L.; Fortier, M.V.; Lee, Y.S.; Yap, F.K.; Saw, S.M.; Godfrey, K.M.; Gluckman, P.D.; et al. Prospective associations of maternal betaine status with offspring weight and body composition at birth: The Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort study. Am. J. Clin. Nutr. 2016, 104, 1327–1333. [Google Scholar] [CrossRef]
- Joselit, Y.; Nanobashvili, K.; Jack-Roberts, C.; Greenwald, E.; Malysheva, O.V.; Caudill, M.A.; Saxena, A.; Jiang, X. Maternal betaine supplementation affects fetal growth and lipid metabolism of high-fat fed mice in a temporal-specific manner. Nutr. Diabetes 2018, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Brougham, B.-J.; Weaver, A.C.; Swinbourne, A.M.; Baida, B.E.L.; Kelly, J.M.; Walker, S.K.; Kleemann, D.O.; Van Wettere, W.H. Maternal Supplementation with Dietary Betaine during Gestation to Improve Twin Lamb Survival. Animals 2020, 10, 1749. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yang, S.; Sun, B.; Feng, Y.; Zhao, R. Maternal betaine protects rat offspring from glucocorticoid-induced activation of lipolytic genes in adipose tissue through modification of DNA methylation. Eur. J. Nutr. 2019, 59, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, Y.; Ding, Z.; Lv, L.; Sui, Y.; Sun, Q.; Abobaker, H.; Cai, D.; Zhao, R. Maternal betaine supplementation decreases hepatic cholesterol deposition in chicken offspring with epigenetic modulation of SREBP2 and CYP7A1 genes. Poult. Sci. 2020, 99, 3111–3120. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, W.; Yang, S.; Qi, F.; Zhao, R. Transgenerational Inheritance of Betaine-Induced Epigenetic Alterations in Estrogen-Responsive IGF-2/IGFBP2 Genes in Rat Hippocampus. Mol. Nutr. Food Res. 2020, 64, e1900823. [Google Scholar] [CrossRef]
- Kharbanda, K.K.; Rogers, D.D., 2nd; Mailliard, M.E.; Siford, G.L.; Barak, A.J.; Beckenhauer, H.C.; Sorrell, M.F.; Tuma, D.J. A comparison of the effects of betaine and s-adenosylmethionine on ethanol-induced changes in methionine metabolism and steatosis in rat hepatocytes. J. Nutr. 2005, 135, 519–524. [Google Scholar] [CrossRef]
- Barak, A.J.; Beckenhauer, H.C.; Junnila, M.; Tuma, D.J. Dietary Betaine Promotes Generation of Hepatic S-Adenosylmethionine and Protects the Liver from Ethanol-Induced Fatty Infiltration. Alcohol. Clin. Exp. Res. 1993, 17, 552–555. [Google Scholar] [CrossRef]
- DHHS/FDA, Dhhs/fda. Electronic Orange Book-Approved Drug Products with Therapeutic Equivalence Evaluations. Cancer Epidemiol. Biomark. Prev. 2006, 15. Available online: https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm (accessed on 15 November 2006).
- Schwab, U.; Törrönen, A.; Meririnne, E.; Saarinen, M.; Alfthan, G.; Aro, A.; Uusitupa, M. Orally Administered Betaine Has an Acute and Dose-Dependent Effect on Serum Betaine and Plasma Homocysteine Concentrations in Healthy Humans. J. Nutr. 2006, 136, 34–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA); Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; et al. Safety of betaine as a novel food pursuant to regulation (ec) no 258/97. EFSA J. 2017, 15, e05057. [Google Scholar]
- Rotzsch, W.; Lorenz, I.; Strack, E. On the toxicity of carnitine and some related substances. Acta Boil. Med. Ger. 1959, 3, 28–36. [Google Scholar]
- Dechezlepretre, S.; Portet, R.; Cheymol, J. Comparative toxicity of trimethylamine (TMA), of its oxide trimethylaminoxide (TMAO), and of their combination. Med. Pharmacol. Exp. Int. J. Exp. Med. 1967, 16, 529–535. [Google Scholar] [PubMed]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of alkyl betaines as used in cosmetics. Int. J. Toxicol. 2018, 37, 28S–46S. [Google Scholar] [CrossRef]

| Therapeutic Effects of Betaine Administration | Experimental Model | Authors |
|---|---|---|
| Prevents hepatic fat accumulation in ALD | Male Wistar rats; C57BL/6 mice; Balb/c mice | [23,27,83,115,121,157,158,160] |
| Preserves/restores hepatic SAM: SAH ratios by regenerating SAM and lowering SAH and homocysteine levels in ALD | Male Wistar rats; hepatocytes; male C57BL/6 mice | [23,60,61,81,82,83,84,86,88,91,92,117,119,121,234,235] |
| Restores activities of various liver methyltransferases (PEMT, ICMT, PIMT, PRMT) to increase phosphatidylcholine levels, preventing apoptosis and accumulation of damaged proteins, and restoring proteasome activity | Male Wistar rats; hepatocytes | [23,90,91,92] |
| Suppresses the synthesis of DGAT2, a rate-limiting enzyme in triglyceride synthesis, by alleviating ERK1/2 inhibition in ALD | Male C57BL/6 mice | [121] |
| Upregulates antioxidant defense system and improves oxyradical scavenging activity in ALD | Male Wistar rats | [133] |
| Prevents/attenuates ER stress in ALD | Male C57BL/6 mice | [83] |
| Exerts hepatoprotection by preserving mitochondrial function in ALD | Male Wistar rats | [61] |
| Restores the serum adiponectin levels in ALD | Mice | [123] |
| Prevents elevations of CD14, TNFα, COX2, GADD45β, LITAF, JAK3, TLR2, TLR4, IL1β, and PDCD4 and NOS2 mRNA levels in alcoholic liver injury | Male Wistar rats | [115,133] |
| Prevents serum ALT and AST activity elevations in models of ALD and MAFLD | Male Wistar rats | [27,115,121] |
| Reduces liver oxidant stress, inflammation, and apoptosis in MAFLD | Male C57BL/6 mice | [28] |
| Remethylates homocysteine, protecting from oxidant stress and restoring phosphatidylcholine generation in MAFLD | C57BL/6 mice | [161] |
| Stimulates β-oxidation in livers of MCD diet-induced MAFLD | Male Sprague-Dawley rats | [162] |
| Alleviates steatosis and increases autophagosomes numbers in mouse livers with MAFLD | Male C57BL/6 mice; rats | [120,161] |
| Enhances the conversion of existing WAT to brown adipose tissue through stimulating mitochondrial biogenesis in MAFLD | Mice | [203] |
| Alleviates ROS-induced mitochondrial respiratory chain dysfunction in MAFLD | Male Sprague-Dawley rats | [163]. |
| Attenuates different grades of steatosis, inflammation, and fibrosis in MAFLD patients | Human trials | [45,165,166,167] |
| Prevents adipose tissue dysfunction in ALD | Male C57BL/6 mice | [194] |
| Reduces the inflammatory adipokines, IL6, TNFα, and leptin in human adipocytes | Human visceral adipocytes | [204] |
| Inhibits lipid peroxidation, hepatic inflammation, and expression of transforming growth factor-β1 in liver fibrosis | Male chicks | [148] |
| Suppresses alcoholic liver fibrosis | Rats | [116] |
| Prevents the formation of Mallory–Denk bodies through epigenetic means by attenuating the decrease of MAT1A, SAHH, BHMT, and AMD1 expression | C3H male mice | [138] |
| Reverses the inhibitory effects of acetaldehyde on IFN signaling and decreases de-methylation of STAT1 by JMJD6 | HCV-infected Huh7.5 CYP2E1 (+) cells and human hepatocytes | [141,143] |
| Enhances expression of PPARα and elevates fatty acid catabolism | Male C57BL/6 and ApoE−/− mice | [158]. |
| Inhibits lipogenic activity in liver by activation of AMPK | ApoE−/− mice; Male C57BL/6 mice | [159,160] |
| Regulates colonic fluid balance | Rats | [21,200] |
| Improves intestinal barrier function and maintains the gut microbiota | Porcine epithelial cells; Caco-2 cells; rat small intestinal cell line IEC-18 | [22,197,198] |
| Activates GI digestive enzymes and ameliorates intestinal morphology and microbiota dysbiosis | Male Sprague Dawley rats | [200] |
| Attenuates alcoholic-induced pancreatic steatosis | Male Wistar rats | [125] |
| Associated with resilience to anhedonia and prevention of stress-related psychiatric disorders | Male C57BL/6 mice | [218] |
| Treats asthma-induced oxidative stress, thus improving airway function of lung tissue | BALB/C mice | [207] |
| Protects against cadmium nephrotoxicity | Male Wistar rats | [206] |
| Protects against isoprenaline-induced myocardial dysfunction | Male Wistar rats | [205] |
| Anti-nociceptive and sedative role via interactions with opioidergic and GABA receptors | Male albino mice | [220] |
| Normalizes fetal growth and reduces adiposity of progeny from obese mice | C57BL/6J mice | [229] |
| Anti-cancer effect in alcohol-associated breast cancer cell growth and development | Breast adenocarcinoma cell line (MCF-7) | [213] |
| Reduces rectal temperature in broiler chickens | Chickens | [226,227] |
| Improves post-natal lamb survival | Lambs | [230] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arumugam, M.K.; Paal, M.C.; Donohue, T.M., Jr.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial Effects of Betaine: A Comprehensive Review. Biology 2021, 10, 456. https://doi.org/10.3390/biology10060456
Arumugam MK, Paal MC, Donohue TM Jr., Ganesan M, Osna NA, Kharbanda KK. Beneficial Effects of Betaine: A Comprehensive Review. Biology. 2021; 10(6):456. https://doi.org/10.3390/biology10060456
Chicago/Turabian StyleArumugam, Madan Kumar, Matthew C. Paal, Terrence M. Donohue, Jr., Murali Ganesan, Natalia A. Osna, and Kusum K. Kharbanda. 2021. "Beneficial Effects of Betaine: A Comprehensive Review" Biology 10, no. 6: 456. https://doi.org/10.3390/biology10060456
APA StyleArumugam, M. K., Paal, M. C., Donohue, T. M., Jr., Ganesan, M., Osna, N. A., & Kharbanda, K. K. (2021). Beneficial Effects of Betaine: A Comprehensive Review. Biology, 10(6), 456. https://doi.org/10.3390/biology10060456








