Treating the Metabolic Syndrome by Fecal Transplantation—Current Status
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Gut Microbiota and Dysbiosis
1.2. Gut Microbiota and Intestinal Dysmotility
1.3. Microbial Signaling, Gastrointestinal Motility, and Metabolic Disease
2. Gastrointestinal Microbiome
2.1. Gut Microbiota and the Gut–Brain Axis
2.2. Metabolic Disorders and Gut Dysbiosis
2.3. MAFLD, the Metabolic Syndrome, and the Relation to the Intestinal Microbiome
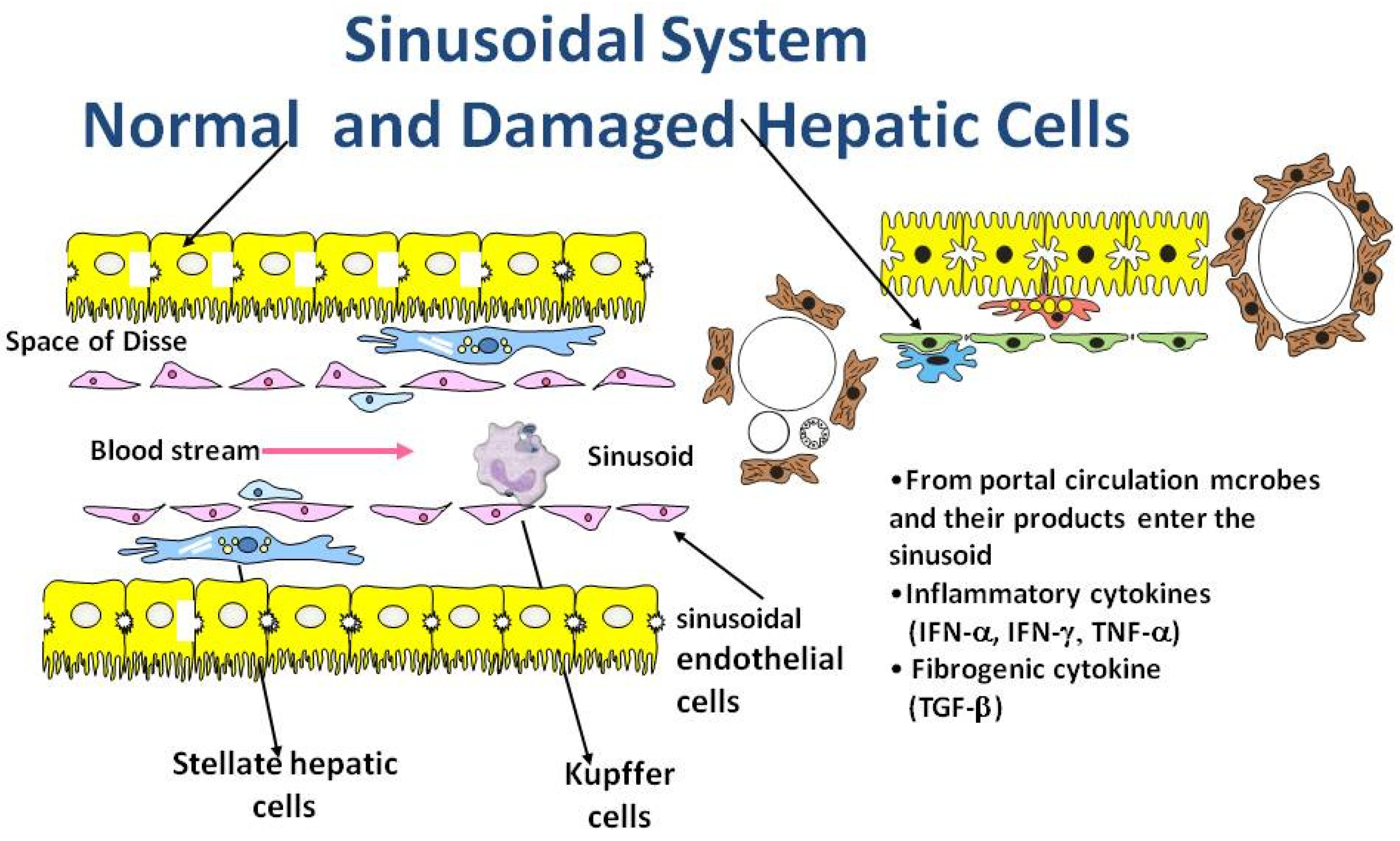
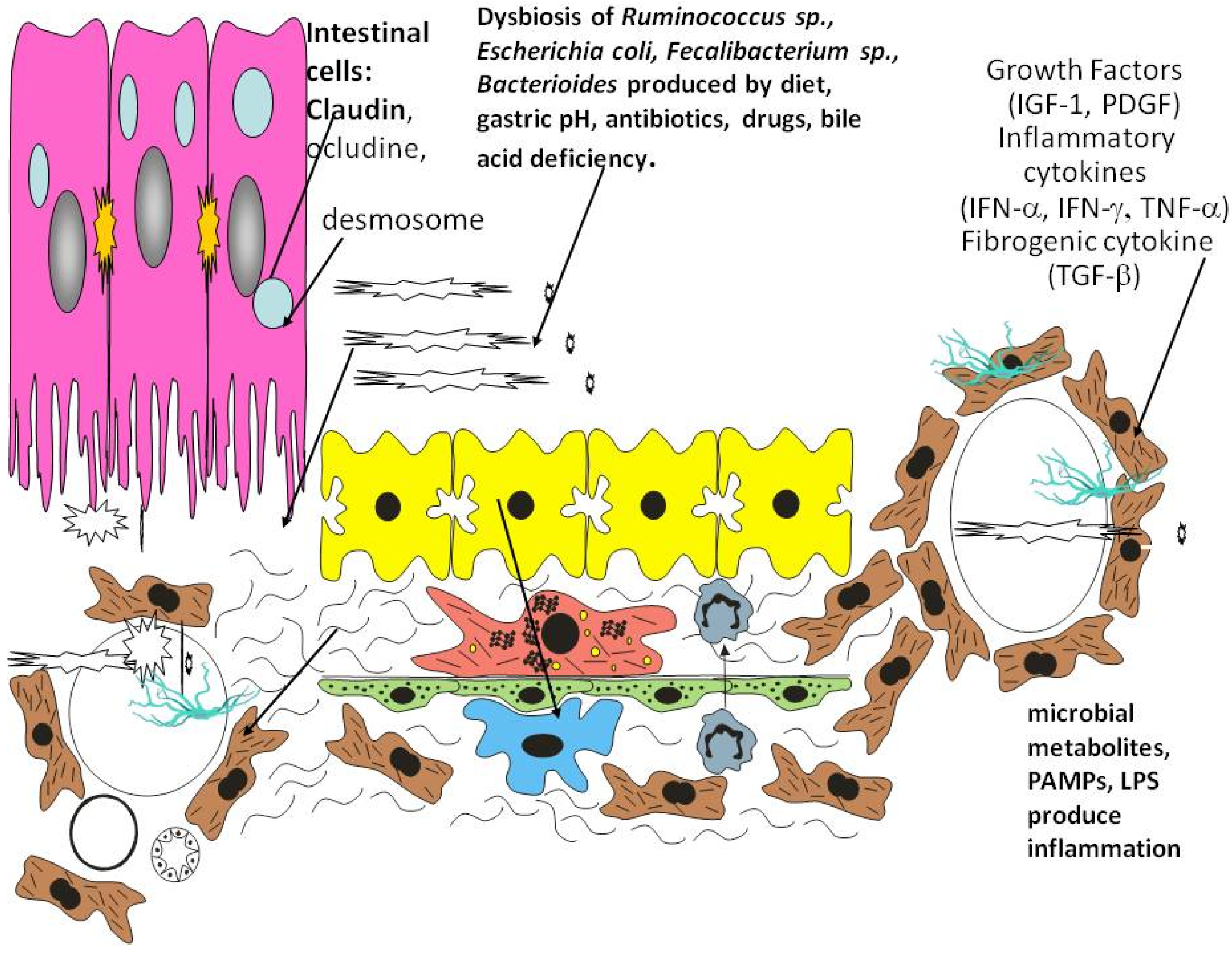
3. Role of Inflammation
4. FMT as Treatment for the Metabolic Syndrome
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALD | Alcoholic liver disease |
| AHR | Aryl hydrogen receptor |
| BMI | Body mass index |
| DAFLD | Dysbiosis-associated fatty liver disease; |
| DIO | Diet-induced obesity |
| FFAR 2 and 3 | Free fatty acid receptor 2 and 3 |
| FMT | Fecal microbial transplantation |
| FMT- TRIM | Fecal microbiota transplantation for the improvement of metabolism in obesity |
| GI | Gastrointestinal tract |
| GLP-1 | Glucagon-like peptide-1 = gluco-regulatory incretin like hormone |
| GLUT-2 | Glucose transporter 2 |
| GPR 41 and 43 | G protein coupled receptor 41and 43 |
| HbAc1 | Hemoglobin Ac1 |
| HDL | High density lipoprotein |
| HOMA-IR | Homeostatic model assessment-insulin resistance |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| IFNα,γ | Interferon alpha, gamma |
| IM | Intestinal microbiome |
| L cells | Enteroendocrine L cells. |
| LDL | Low density lipoprotein |
| LPS | Lipopolysaccharide |
| MAFLD | Metabolic associated fatty liver disease |
| MS | Metabolic syndrome |
| NAFLD | Nonalcoholic fatty liver disease |
| NFκB | Nuclear factor kappa B |
| OTUs | Operational taxonomic units |
| PAMP | Pathogen associated molecular pattern |
| SCFA | Short chain fatty acids |
| SIBO | Small intestine bacterial overgrowth |
| TLR | Toll-like receptor |
| TMAO | Trimethyl-amine-N-oxide |
| TGFβ | Transforming growth factor beta |
| TNFα | Tumor necrosis factor alpha |
References
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Bakken, J.S.; Borody, T.; Brandt, L.J.; Brill, J.V.; Demarco, D.C.; Franzos, M.A.; Kelly, C.; Khoruts, A.; Louie, T.; Martinelli, L.P.; et al. Treating clostridium difficile infection with fecal microbiota transplantation. Clin. Gastroenterol. Hepatol. 2011, 9, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Brody, H. The gut microbiome. Nature 2020, 577, S5. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Ghoshal, U. Small intestinal bacterial overgrowth and other intestinal disorders. Gastroenterol. Clin. N. Am. 2017, 46, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Gut microbiota: Changes in gut microbes and host metabolism: Squaring the circle? Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 563–564. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Riordan, S.M.; McIver, C.J.; Wakefield, D.; Duncombe, V.M.; Thomas, M.C.; Bolin, T.D. Small intestinal mucosal immunity and morphometry in luminal overgrowth of indigenous gut flora. Am. J. Gastroenterol. 2001, 96, 494–500. [Google Scholar] [CrossRef]
- Peralta, S.; Cottone, C.; Doveri, T.; Almasio, P.L.; Craxi, A. Small intestine bacterial overgrowth and irritable bowel syndrome-related symptoms: Experience with rifaximin. World J. Gastroenterol. 2009, 15, 2628–2631. [Google Scholar] [CrossRef]
- Erdogan, A.; Rao, S.S.C.; Gulley, D.; Jacobs, C.; Lee, Y.Y.; Badger, C. Small intestinal bacterial overgrowth: Duodenal aspiration vs. glucose breath test. Neurogastroenterol. Motil. 2009, 27, 481–489. [Google Scholar] [CrossRef]
- Ziegler, T.R.; Cole, C.R. Small bowel bacterial overgrowth in adults: A potential contributor to intestinal failure. Curr. Gastroenterol. Rep. 2007, 9, 463–467. [Google Scholar] [CrossRef]
- Luissint, A.-C.; Parkos, C.A.; Nusrat, A. Inflammation and the intestinal barrier: Leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 2016, 151, 616–632. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Köhler, H.; McCormick, B.A.; Walker, W.A. Bacterial-enterocyte crosstalk: Cellular mechanisms in health and disease. J. Pediatric Gastroenterol. Nutr. 2003, 36, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Shindo, K.; Machida, M.; Koide, K.; Fukumura, M.; Yamazaki, R. Deconjugation ability of bacteria isolated from the jejunal fluid of patients with progressive systemic sclerosis and its gastric pH. Hepato Gastroenterol. 1998, 45, 1643–1650. [Google Scholar]
- Gorbach, S.L. Probiotics and gastrointestinal health. Am. J. Gastroenterol. 2000, 95, S2–S4. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Shukla, R.; Ghoshal, U. Small intestinal bacterial overgrowth and irritable bowel syndrome: A bridge between functional organic dichotomy. Gut Liver 2017, 11, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Malinen, E.; Rinttilä, T.; Kajander, K.; Mättö, J.; Kassinen, A.; Krogius, L.; Saarela, M.; Korpela, R.; Palva, A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am. J. Gastroenterol. 2005, 100, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Ghoshal, U.; Dhole, T.N.; Ghoshal, U.C. Fecal microbiota in patients with irritable bowel syndrome compared with healthy controls using real-time polymerase chain reaction: An evidence of dysbiosis. Dig. Dis. Sci. 2015, 60, 2953–2962. [Google Scholar] [CrossRef]
- Hanning, N.; Edwinson, A.L.; Ceuleers, H.; Peters, S.A.; De Man, J.G.; Hassett, L.C.; De Winter, B.Y.; Grover, M. Intestinal barrier dysfunction in irritable bowel syndrome: A systematic review. Therp. Adv. Gastroenterol. 2021, 14, 1–3. [Google Scholar]
- Posserud, I.; Stotzer, P.-O.; Björnsson, E.S.; Abrahamsson, H.; Simren, M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 2007, 56, 802–808. [Google Scholar] [CrossRef]
- Bala, L.; Ghoshal, U.C.; Ghoshal, U.; Tripathi, P.; Misra, A.; Gowda, G.A.; Khetrapal, C.L. Malabsorption syndrome with and without small intestinal bacterial overgrowth: A study on upper-gut aspirate using 1H NMR spectroscopy. Magn. Reson. Med. 2006, 56, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Srivastava, D. Irritable bowel syndrome and small intestinal bacterial overgrowth: Meaningful association or unnecessary hype. World J. Gastroenterol. 2014, 20, 2482–2491. [Google Scholar] [CrossRef]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-X.; Wang, C.; Zhang, Z.M.; Jaeger, C.D.; Krager, S.L.; Bottum, K.M.; Liu, J.; Liao, D.-F.; Tischkau, S.A. Aryl hydrocarbon receptor deficiency protects mice from diet-induced adiposity and metabolic disorders through increased energy expenditure. Int. J. Obes. 2015, 39, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Bock, K.W. Aryl hydrocarbon receptor (AHR) functions in NAD (+) metabolism, myelopoiesis and obesity. Biochem. Pharmacol. 2019, 163, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Castaño, Á.; Boeing, S.; Bon-Frauches, A.C.; Fung, C.; Fallesen, T.; de Agüero, M.G.; Yilmaz, B.; Lopes, R.A.; Horswell, S.; et al. Neuronal programming by microbiota regulates intestinal physiology. Nature 2020, 578, 284–289. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Fava, S. Glucagon-like peptide 1 and the cardiovascular system. Curr. Diabetes Rev. 2014, 10, 302–310. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Malnick, S.; Abdul-Hai, A.; Abdullah, A.; Gharaba, Y.; Brand, A.; Basevitz, A.; Alin, P.; Binder, Y.; Raz, O.; Melzer, E.; et al. Bloody diarrhoea in a hunger striker: Starvation colitis. Lancet 2015, 385, 1696. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Microbiota-brain-gut axis and neuro-degenerative diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Garcovich, M.; Zocco, M.A.; Roccarina, D.; Ponziani, F.R.; Gasbarrini, A. Prevention and treatment of hepatic encephalopathy: Focusing on gut microbiota. World J. Gastroenterol. 2012, 18, 6693–6700. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Collins, S.M.; Bercik, P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes 2014, 5, 419–429. [Google Scholar] [CrossRef]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef]
- Singh, R.; Zogg, H.; Wei, L.; Bartlett, A.; Ghoshal, U.C.; Rajender, S.; Ro, S. Gut microbial dysbiosis in the pathogenesis of gastrointestinal dysmotility and metabolic disorders. J. Neurogastroenterol. Motil. 2021, 27, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Choung, R.S.; Locke III, G.R.; Schleck, C.D.; Zinsmeister, A.R.; Melton, L.J., III; Talley, N.J. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am. J. Gastroenterol. 2012, 107, 82–88. [Google Scholar] [CrossRef]
- Halland, M.; Bharucha, A.E. Relationship between control of glycemia and gastric emptying disturbances in diabetes mellitus. Clin. Gastroenterol. Hepatol. 2016, 14, 929–936. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Sorini, C.; Cosorich, I.; Lo Conte, M.; De Giorgi, L.; Facciotti, F.; Lucianò, R.; Rocchi, M.; Ferrarese, R.; Sanvito, F.; Canducci, F.; et al. Loss of gut barrier integrity triggers activation of islet-reactive T cells and autoimmune diabetes. Proc. Natl. Acad. Sci. USA 2019, 116, 15140–15149. [Google Scholar] [CrossRef]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017, 26, 611–619. [Google Scholar] [CrossRef]
- Barbara, G.; Feinle-Bisset, C.; Ghoshal, U.C.; Quigley, E.M.; Santos, J.; Vanner, S.; Vergnolle, N.; Zoetendal, E.G. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology 2016, 150, 1305–1318. [Google Scholar] [CrossRef]
- Bouter, K.E.; van Raalte, D.H.; Groen, A.K.; Nieuwdorp, M. Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction. Gastroenterology 2017, 152, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Balercia, G.; Barrea, L.; Cignarelli, A.; Giorgino, F.; Holst, J.J.; Laudisio, D.; Orio, F.; Tirabassi, G.; Colao, A. Gut: A key player in the pathogenesis of type 2 diabetes? Crit. Rev. Food Sci. Nutr. 2018, 58, 1294–1309. [Google Scholar] [CrossRef]
- Johnson, A.M.F.; Olefsky, J.M. The origins and drivers of insulin resistance. Cell 2013, 152, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria’, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Le Roy, T.; Llopis, M.; Lepage, P.; Bruneau, A.; Rabot, S.; Bevilacqua, C.; Martin, P.; Philippe, C.; Walker, F.; Bado, A. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 2013, 62, 1787–1794. [Google Scholar] [CrossRef]
- Caesar, R.; Reigstad, C.S.; Bäckhed, H.K.; Reinhardt, C.; Ketonen, M.; Lundén, G.Ö.; Cani, P.D.; Bäckhed, F. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut 2012, 61, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Membrez, M.; Blancher, F.; Jaquet, M.; Bibiloni, R.; Cani, P.D.; Burcelin, R.G.; Corthesy, I.; Macé, K.; Chou, C.J. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008, 22, 2416–2426. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, B.; He, X.; Nie, Y.; Wu, K.; Fan, D. Microbiota transplantation: Concept, methodology and strategy for its modernization. Protein Cell 2018, 9, 462–473. [Google Scholar] [CrossRef]
- Mantovani, A.; Dalbeni, A. NAFLD, MAFLD and DAFLD. Dig. Liver Dis. 2020, 52, 1519–1520. [Google Scholar] [CrossRef]
- Neuman, M.; Hilzenrat, N.; Cohen, L.; Winkler, R.E.; Nanau, R. Multiple factors involved in nonalcoholic hepatitis pathogenesis. Int. J. Hepatol. 2012, 2012, 1–2. [Google Scholar] [CrossRef]
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef]
- Schäfer, C.; Parlesak, A.; Schütt, C.; Bode, J.C.; Bode, C. Concentrations of lipopolysaccharide-binding protein, bactericidal/permeability-increasing protein, soluble CD14 and plasma lipids in relation to endotoxaemia in patients with alcoholic liver disease. Alcohol Alcohol. 2002, 37, 81–86. [Google Scholar] [CrossRef]
- Vera-Barajas, A.; Abenavoli, L.; Scarpellini, E.; Méndez-Sánchez, N.; Valencia-Rodríguez, A.; Ponciano-Rodríguez, G.; Wang, D.Q.-H. The mechanism of dysbiosis in alcoholic liver disease leading to liver cancer. Hepatoma Res. 2020, 6, 5. [Google Scholar] [CrossRef]
- Usami, M.; Miyoshi, M.; Yamashita, H. Gut microbiota and host metabolism in liver cirrhosis. World J. Gastroenterol. 2015, 21, 11597–11608. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Seitz, H.K.; Neuman, M.G. The history of alcoholic liver disease: From an unrecognized disease to one of the most frequent diseases in hepatology. J. Clin. Med. 2021, 10, 858. [Google Scholar] [CrossRef]
- Nanau, R.M.; Neuman, M.G. Metabolome and inflammasome in inflammatory bowel disease. Transl. Res. 2012, 160, 1–28. [Google Scholar] [CrossRef]
- Oz, H.S.; Chen, T.S.; Neuman, M. Nutrition intervention: A strategy against systemic inflammatory syndrome. J. Parenter. Enter. Nutr. 2009, 33, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G. Immune dysfunction in inflammatory bowel disease. Transl. Res. 2007, 149, 173–186. [Google Scholar] [CrossRef]
- Eiseman, B.; Silen, W.; Bascom, G.S.; Kauvar, A.J. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958, 44, 854–859. [Google Scholar] [PubMed]
- Persky, S.E.; Brandt, L.J. Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am. J. Gastroenterol. 2000, 95, 3283–3285. [Google Scholar] [PubMed]
- Ng, S.C.; Kamm, M.A.; Yeoh, Y.K.; Chan, P.K.S.; Zuo, T.; Tang, W.; Sood, A.; Andoh, A.; Ohmiya, N.; Zhou, Y.; et al. Scientific frontiers in faecal microbiota transplantation: Joint document of Asia-Pacific Association of Gastroenterology (APAGE) and Asia-Pacific Society for Digestive Endoscopy (APSDE). Gut 2020, 69, 83–91. [Google Scholar] [CrossRef]
- Quraishi, M.N.; Widlak, M.; Bhala, N.; Moore, D.; Price, M.; Sharma, N.; Iqbal, T.H. Systematic review with meta-analysis: The efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment. Pharmacol. Ther. 2017, 46, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, C.P.; Neut, C.; Desreumaux, P.; Colombel, J.F. Dysbiosis as a prerequisite for IBD. Gut 2004, 53, 1057. [Google Scholar] [PubMed]
- Walker, A.W.; Lawley, T.D. Therapeutic modulation of intestinal dysbiosis. Pharmacol. Res. 2013, 69, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of fecal microbiota transplantation on obesity and metabolic syndrome—A systematic review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef]
- Smits, L.P.; Kootte, R.S.; Levin, E.; Prodan, A.; Fuentes, S.; Zoetendal, E.G.; Wang, Z.; Levison, B.S.; Cleophas, M.C.P.; Kemper, E.M.; et al. Effect of vegan fecal microbiota transplantation on carnitine- and choline-derived trimethylamine-N-oxide production and vascular inflammation in patients with metabolic syndrome. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Zipursky, J.S.; Sidorsky, T.I.; Freedman, C.A.; Sidorsky, M.N.; Kirkland, K.B. Patient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin. Infect. Dis. 2012, 55, 1652–1658. [Google Scholar] [CrossRef]
- Youngster, I.; Mahabamunuge, J.; Systrom, H.K.; Sauk, J.; Khalili, H.; Levin, J.; Kaplan, J.L.; Hohmann, E.L. Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016, 14, 1–4. [Google Scholar] [CrossRef]
- Bhutiani, N.; Schucht, J.E.; Miller, K.R.; McClave, S.A. Technical aspects of Fecal Microbial Transplantation (FMT). Curr. Gastroenterol. Rep. 2018, 20, 30. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Kassam, Z.; Mullish, B.H.; Chiang, A.; Carrellas, M.; Hurtado, J.; Marchesi, J.R.; McDonald, J.A.K.; Pechlivanis, A.; Barker, G.F.; et al. Effects of fecal microbiota transplantation with oral capsules in obese patients. Clin. Gastroenterol. Hepatol. 2020, 18, 855–863. [Google Scholar] [CrossRef]
- Yu, E.W.; Gao, L.; Stastka, P.; Cheney, M.C.; Mahabamunuge, J.; Torres Soto, M.; Ford, C.B.; Bryant, J.A.; Henn, M.R.; Hohmann, E.L. Fecal microbiota transplantation for the improvement of metabolism in obesity: The fmt-trim double-blind placebo-controlled pilot trial. PLoS Med. 2020, 17, 1–19. [Google Scholar] [CrossRef]
- Koutnikova, H.; Genser, B.; Monteiro-Sepulveda, M.; Faurie, J.M.; Rizkalla, S.; Schrezenmeir, J.; Clément, K. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Gilijamse, P.W.; Hartstra, A.V.; Levin, E.; Wortelboer, K.; Serlie, M.J.; Ackermans, M.T.; Herrema, H.; Nederveen, A.J.; Imangaliyev, S.; Aalvink, S.; et al. Treatment with Anaerobutyricum soehngenii: A pilot study of safety and dose-response effects on glucose metabolism in human subjects with metabolic syndrome. NPJ Biofilms Microbiomes 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.J.; de Aguiar, V.T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Ghiassi, S.; Morton, J.M. Safety and efficacy of bariatric and metabolic surgery. Curr. Obes. Rep. 2020, 9, 159–164. [Google Scholar] [CrossRef]
- Liou, A.P.; Paziuk, M.; Luevano, J.-M.J.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013, 5, 178ra41. [Google Scholar] [CrossRef]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef]
- De Groot, P.F.; Belzer, C.; Aydin, Ö.; Levin, E.; Levels, J.H.; Aalvink, S.; Boot, F.; Holleman, F.; van Raalte, D.H.; Scheithauer, T.P.; et al. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS ONE 2017, 12, e0188475. [Google Scholar] [CrossRef]
- Rinott, E.; Youngster, I.; Yaskolka, M.A.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Fava, F.; Scholz, M.U.; et al. Effects of diet-modulated autologous fecal microbiota transplantation on weight regain. Gastroenterology 2021, 160, 158–173. [Google Scholar] [CrossRef]
- Taur, Y.; Coyte, K.; Schluter, J.; Robilotti, E.; Figueroa, C.; Gjonbalaj, M.; Littmann, E.R.; Ling, L.; Miller, L.; Gyaltshen, Y.; et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci. Transl. Med. 2018, 10, eaap9489. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.M. Keystone taxa indispensable for microbiome recovery. Nat. Microbiol. 2020, 5, 1067–1068. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Clément, K.; Nieuwdorp, M. Fecal microbiota transplantation: A future therapeutic option for obesity/diabetes? Curr. Diabetes Rep. 2019, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Bojanova, D.P.; Bordenstein, S.R. Fecal transplants: What is being transferred? PLoS Biol. 2016, 14, e1002503. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malnick, S.D.H.; Fisher, D.; Somin, M.; Neuman, M.G. Treating the Metabolic Syndrome by Fecal Transplantation—Current Status. Biology 2021, 10, 447. https://doi.org/10.3390/biology10050447
Malnick SDH, Fisher D, Somin M, Neuman MG. Treating the Metabolic Syndrome by Fecal Transplantation—Current Status. Biology. 2021; 10(5):447. https://doi.org/10.3390/biology10050447
Chicago/Turabian StyleMalnick, Stephen D. H., David Fisher, Marina Somin, and Manuela G. Neuman. 2021. "Treating the Metabolic Syndrome by Fecal Transplantation—Current Status" Biology 10, no. 5: 447. https://doi.org/10.3390/biology10050447
APA StyleMalnick, S. D. H., Fisher, D., Somin, M., & Neuman, M. G. (2021). Treating the Metabolic Syndrome by Fecal Transplantation—Current Status. Biology, 10(5), 447. https://doi.org/10.3390/biology10050447




