Nutrient Deficiency Promotes the Entry of Helicobacter pylori Cells into Candida Yeast Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
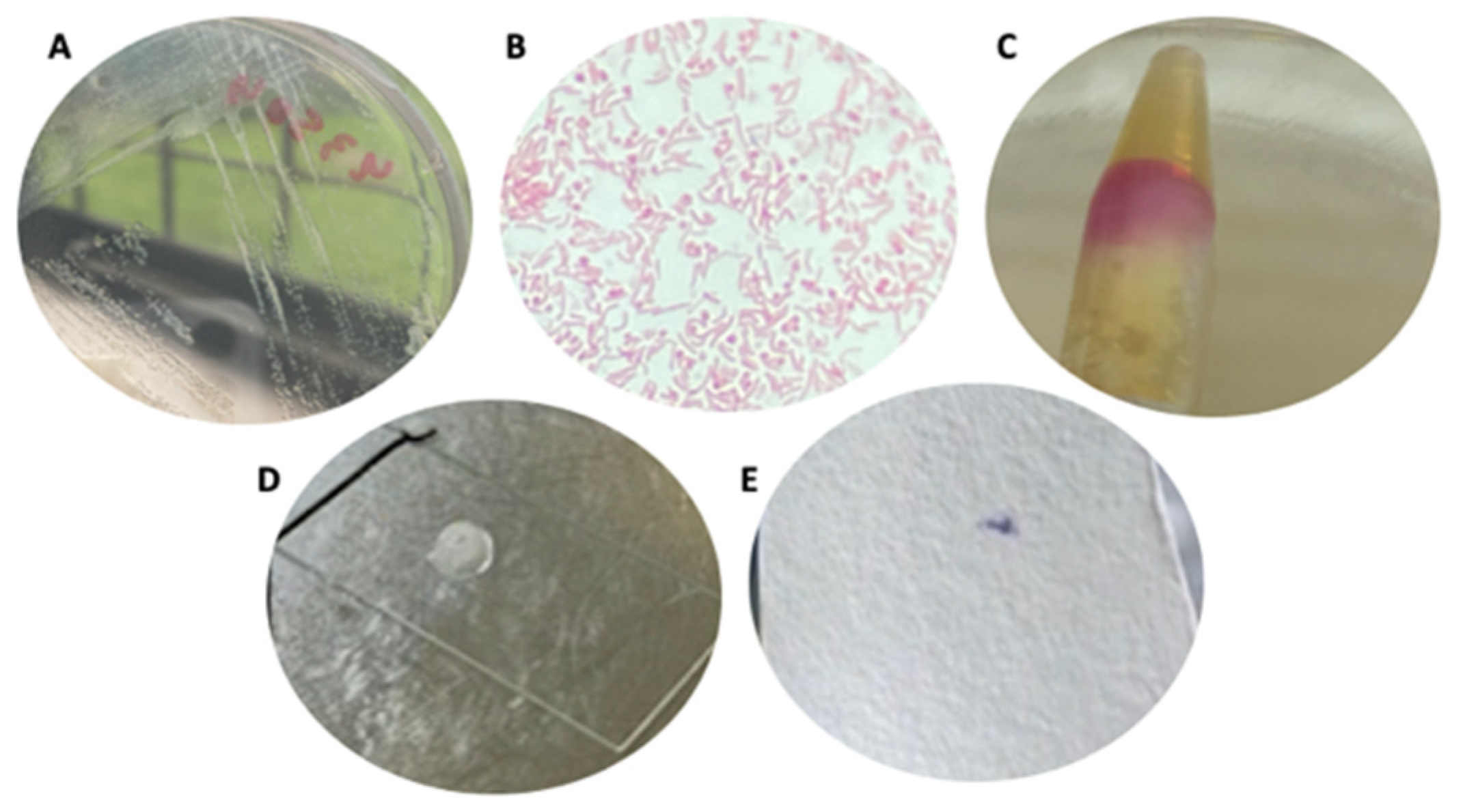
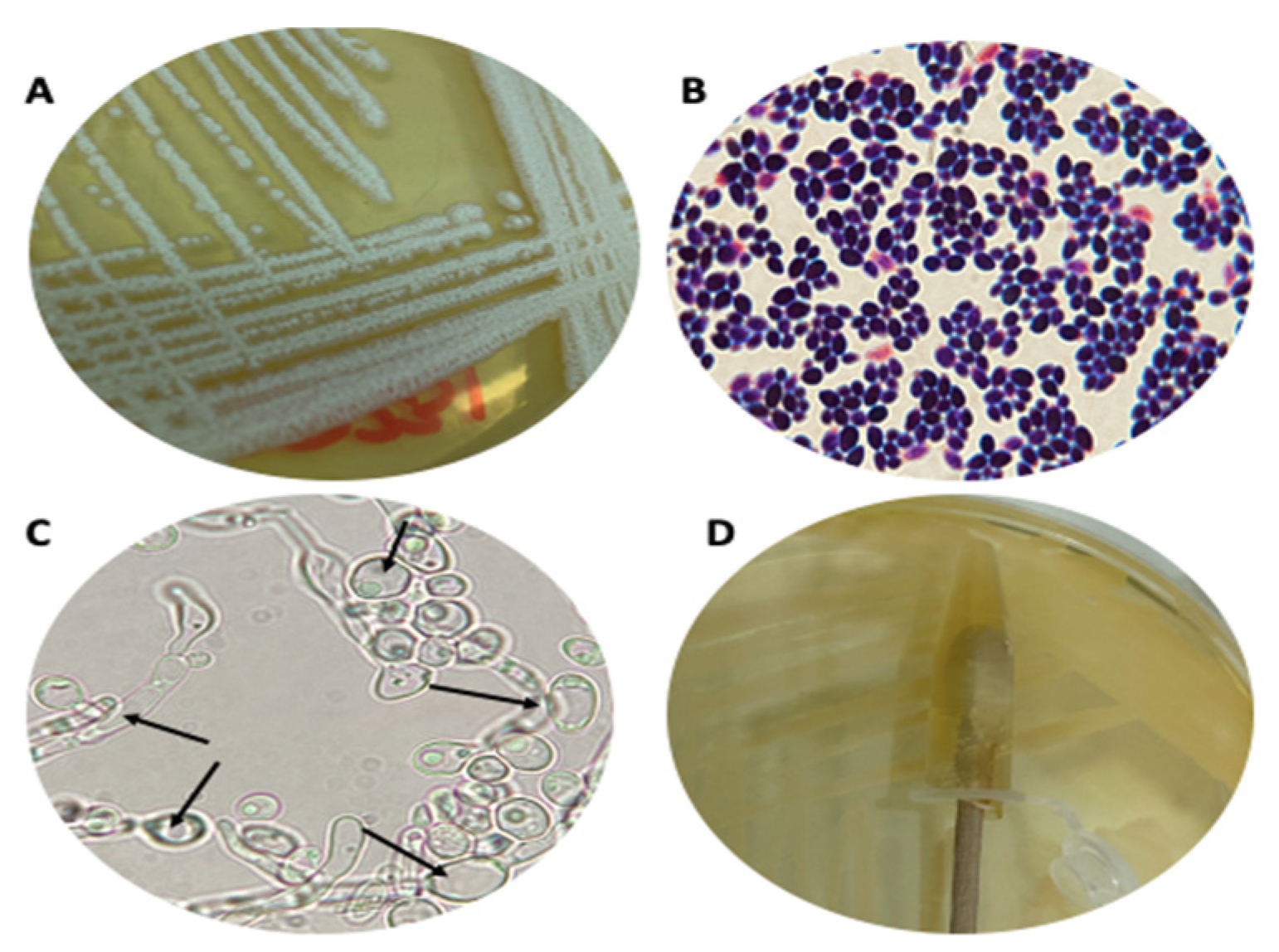
2.1. Bacterial Strains and Growth Conditions
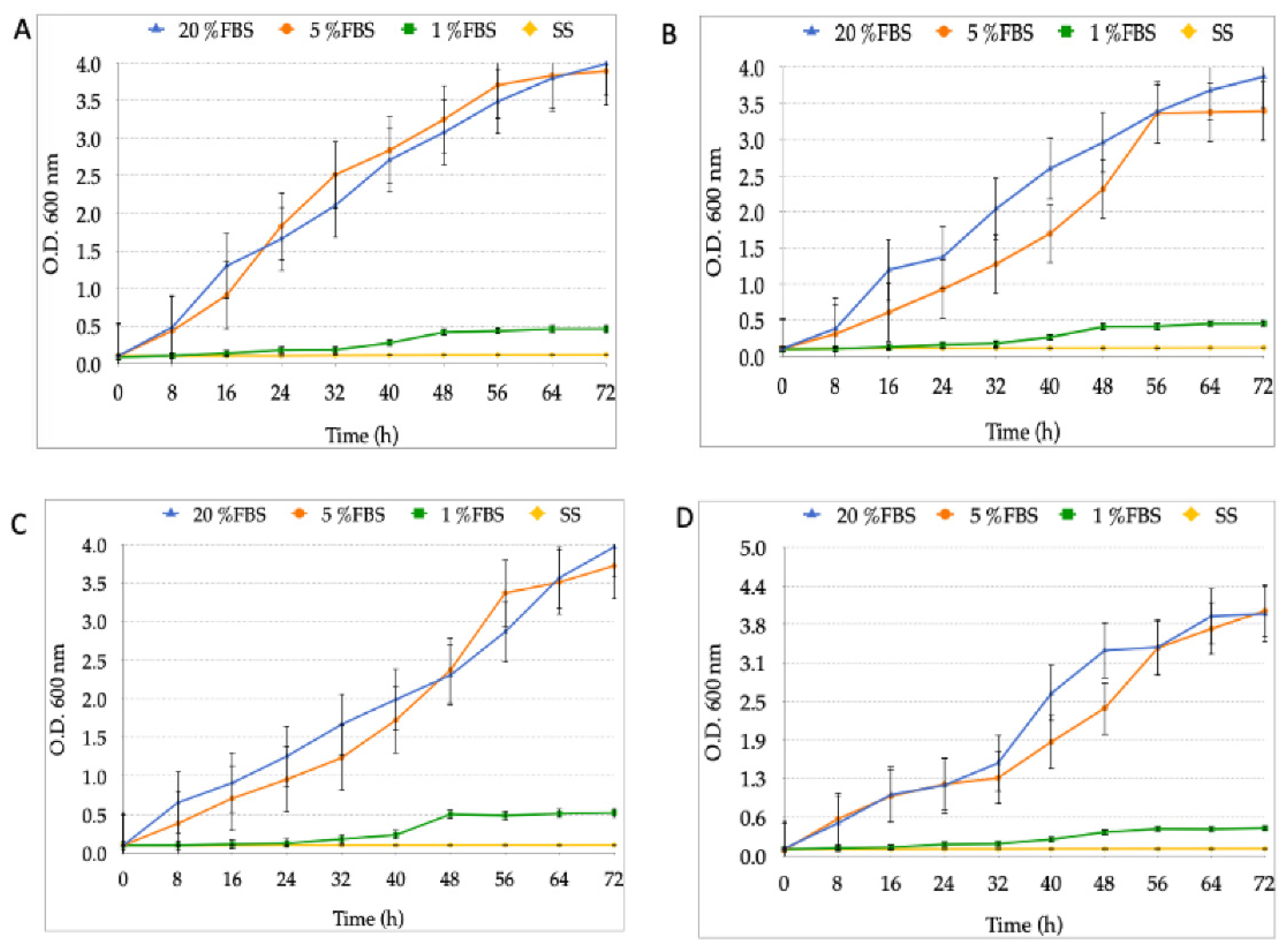
2.2. Growth Curves at Different Concentrations of FBS for H. pylori and Candida Strains
2.3. Co-Cultures of H. pylori Strains with Candida Strains
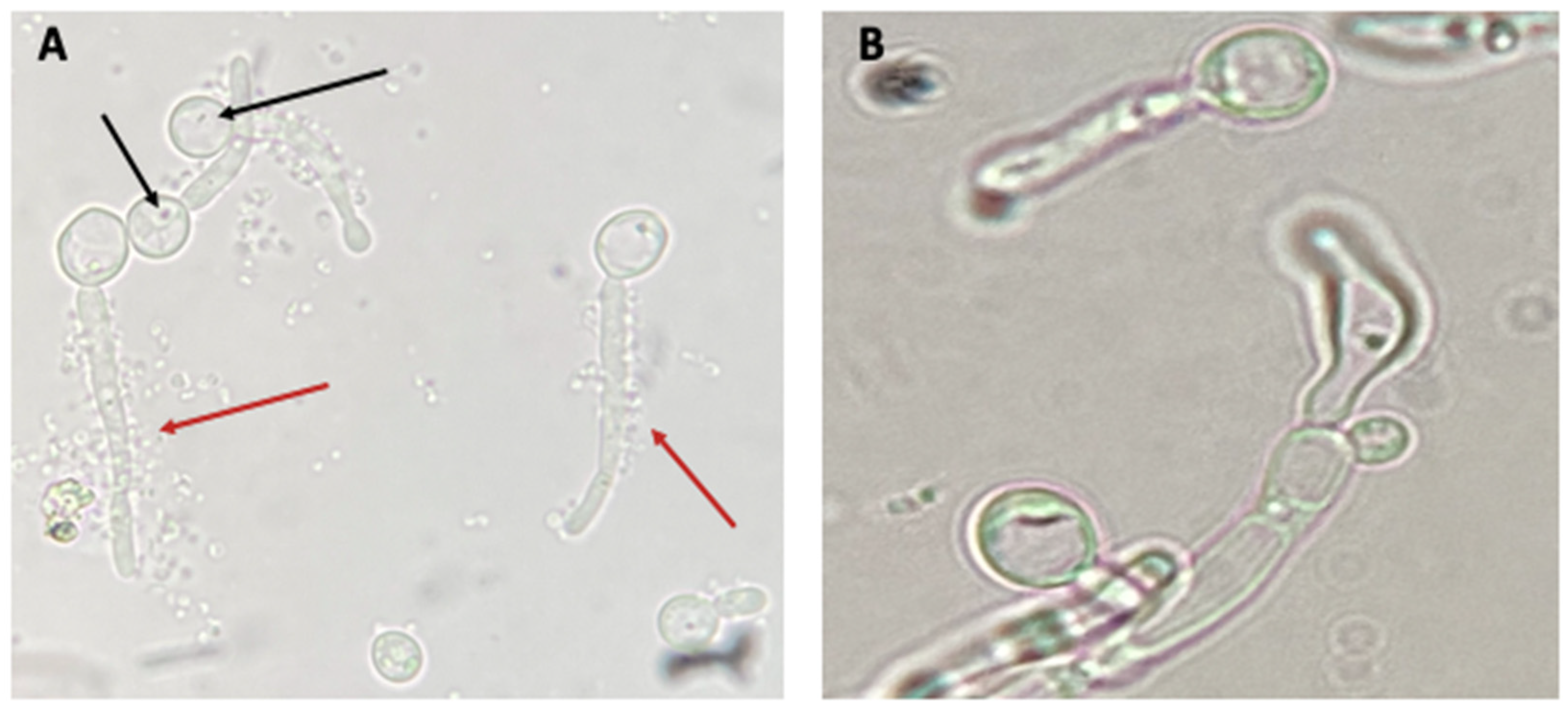
2.4. Search for Bacteria-Like Bodies (BLBs) and Culture of Yeast Cells Containing BLBs
2.5. Identification of BLBs within Yeast Cells Using Fluorescent In Situ Hybridization (FISH) Technique
2.6. Detection of H. pylori 16S rRNA
2.7. Cell Viability Assay
2.8. Statistical Analysis
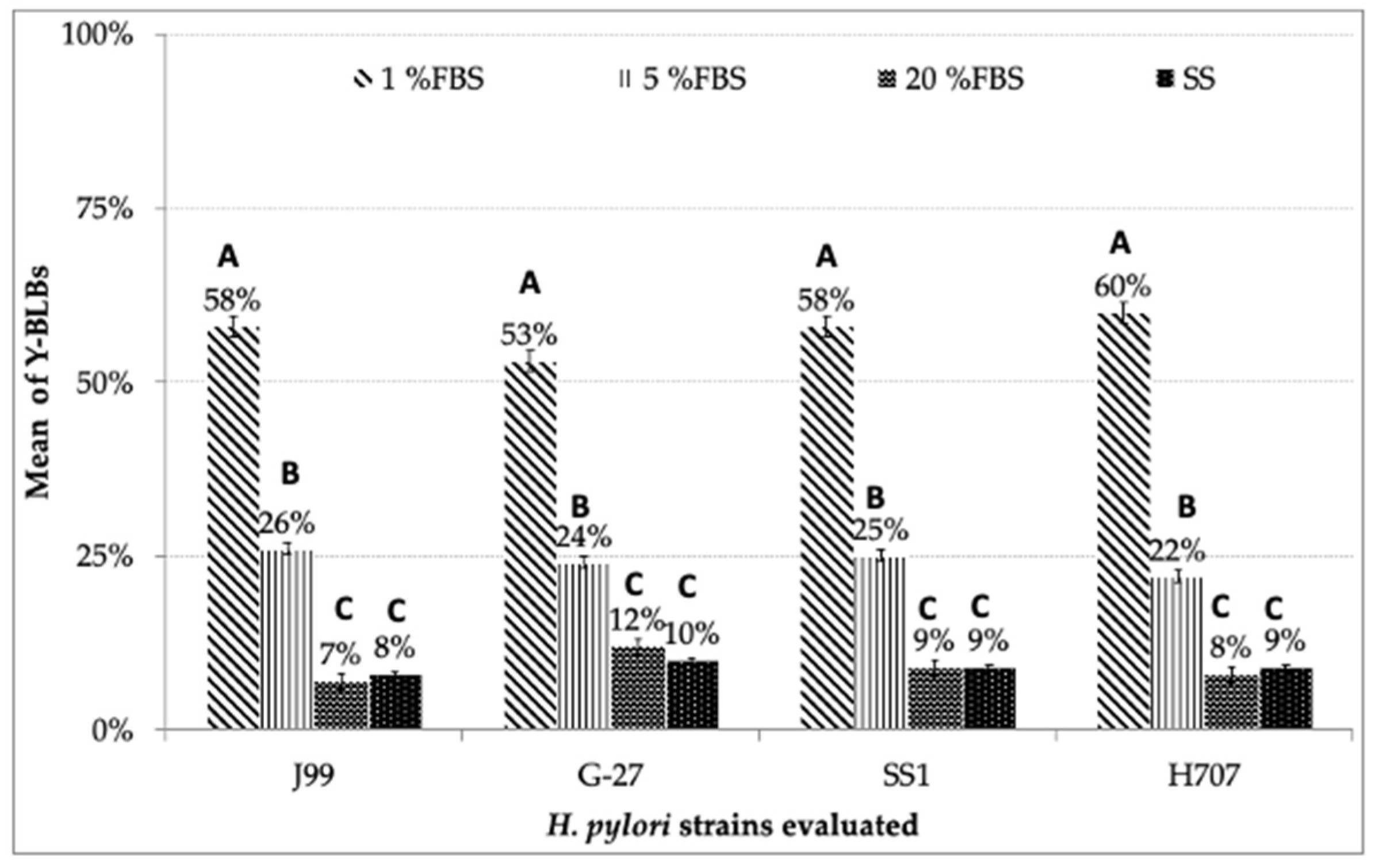
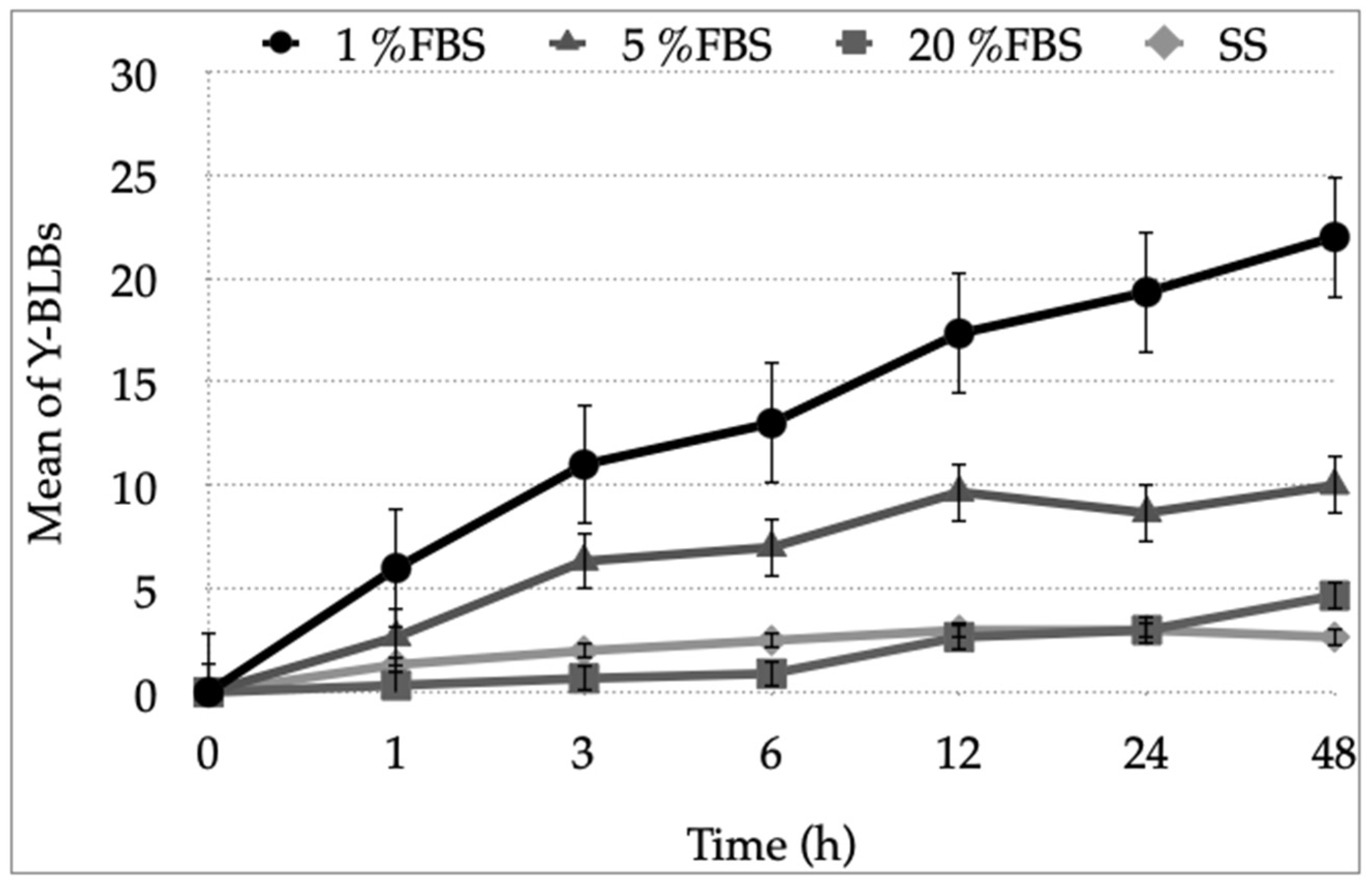
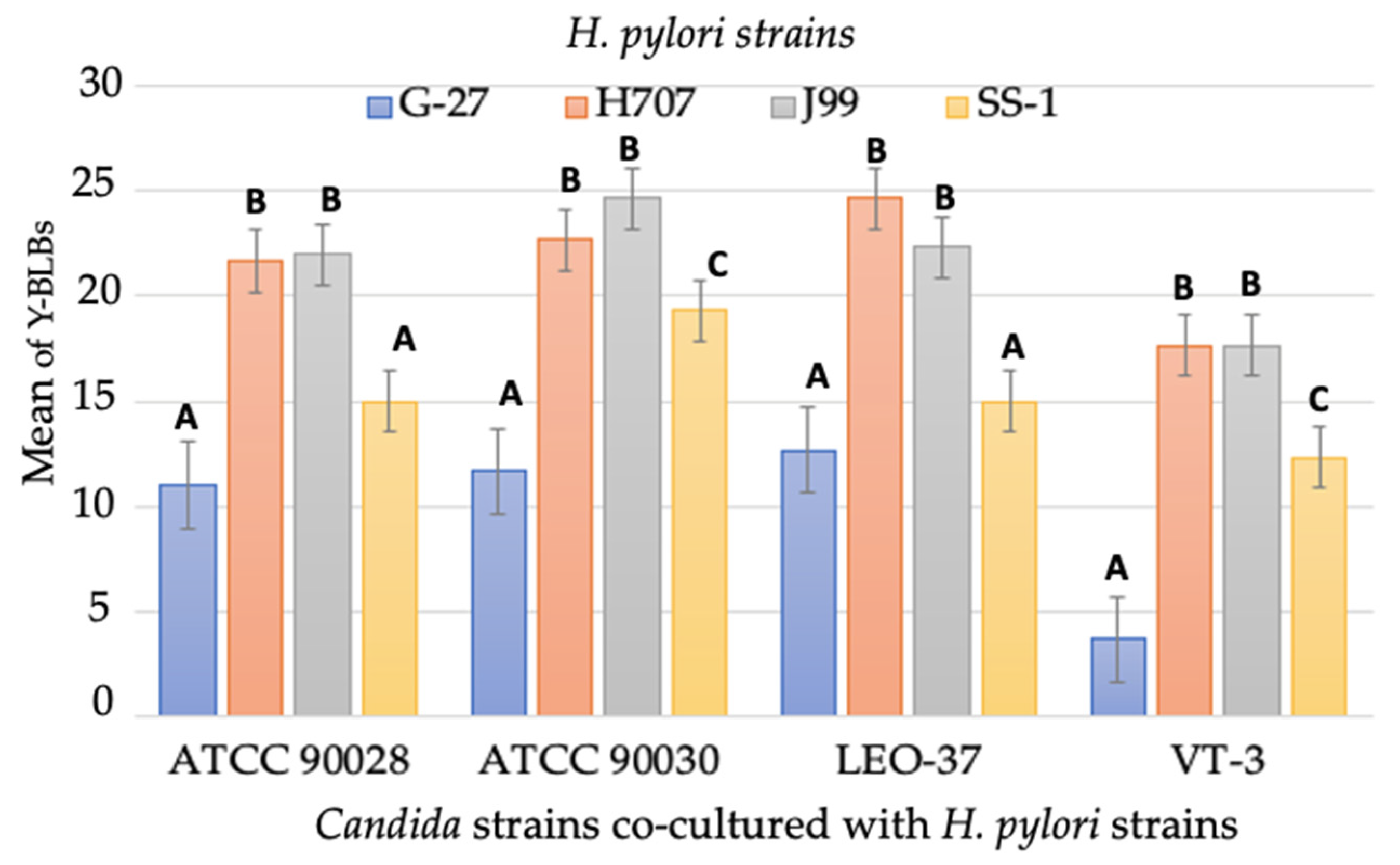
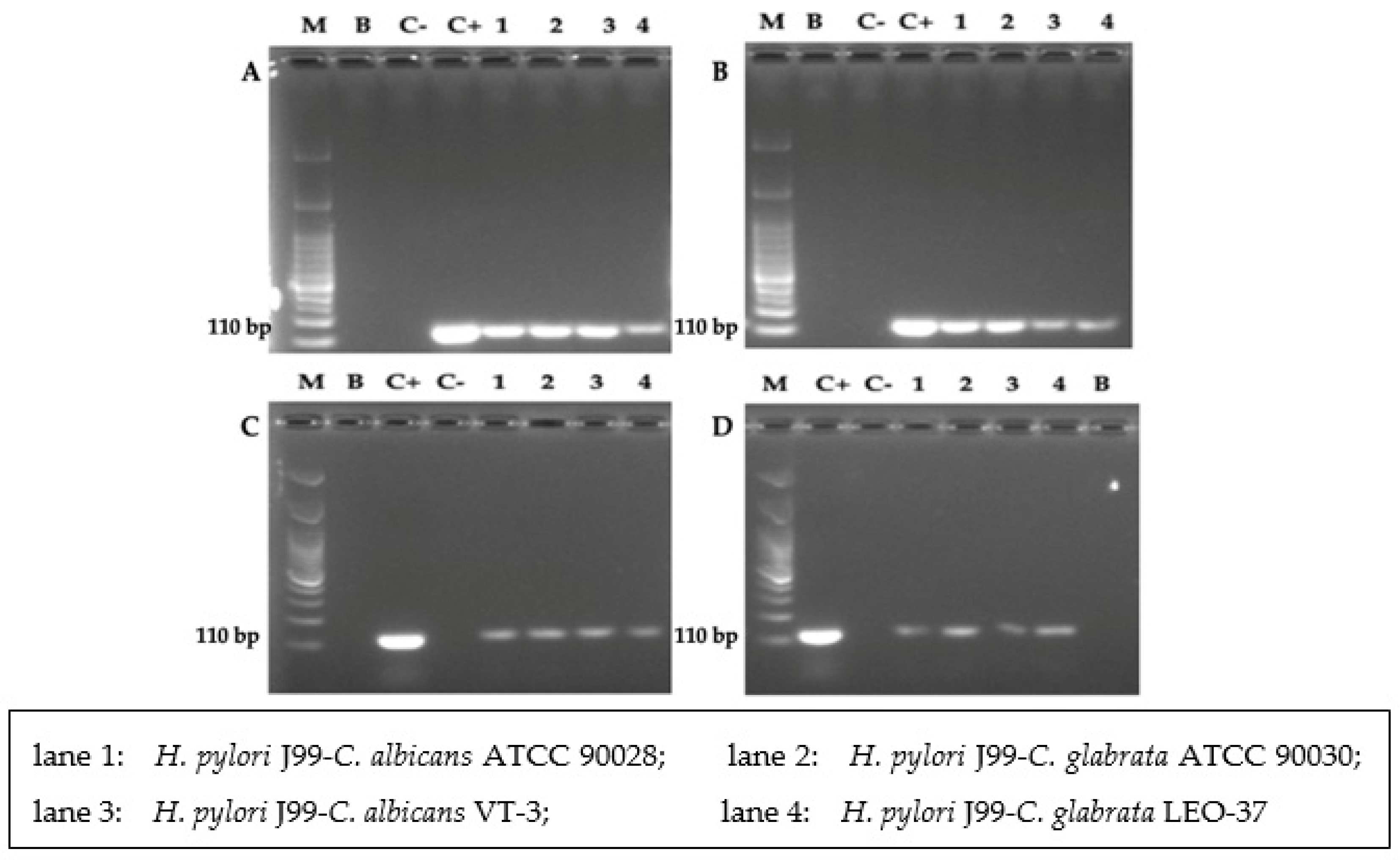
3. Results
3.1. Strain Cultures
3.2. Growth Curves of the Different Strains of H. pylori and Candida at Varying FBS Concentrations or SS
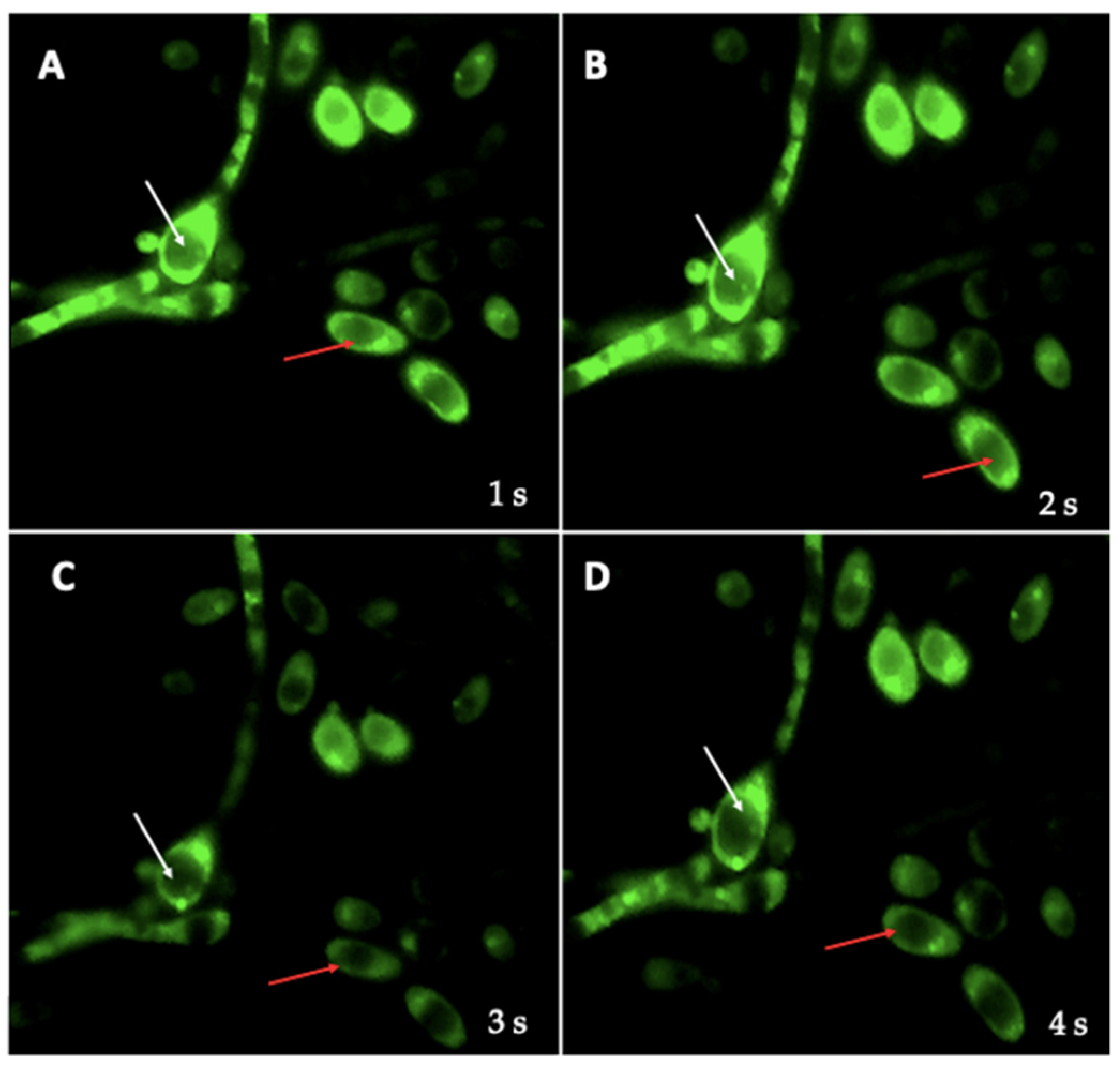
3.3. Search for BLBs within Yeasts of the Genus Candida
3.4. Identification of BLBs in Yeast Using the Fluorescent In Situ Hybridization (FISH) Technique
3.5. Detection of H. pylori 16S rRNA Gene
3.6. Cell Viability Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Burucoa, C.; Axon, A. Epidemiology of Helicobacter pylori infection. Helicobacter 2017, 22 (Suppl. S1), e12403. [Google Scholar] [CrossRef]
- Sjomina, O.; Pavlova, J.; Niv, Y.; Leja, M. Epidemiology of Helicobacter pylori infection. Helicobacter 2018, 23 (Suppl. S1), e12514. [Google Scholar] [CrossRef]
- Sachs, G.; Scott, D. Helicobacter pylori: Eradication or Preservation. F1000 Med. Rep. 2012, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Den Hollander, W.J.; Holster, I.L.; den Hoed, C.M.; Capelle, L.G.; Tang, T.J.; Anten, M.-P.; Prytz-Berset, I.; Witteman, E.M.; ter Borg, F.; Hartog, G.d.; et al. Surveillance of premalignant gastric lesions: A multicentre prospective cohort study from low incidence regions. Gut 2019, 68, 585. [Google Scholar] [CrossRef] [PubMed]
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; di Pietro, M.; Uedo, N.; Bhandari, P.; Pritchard, D.M.; Kuipers, E.J.; et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef]
- Schistosomes, I.A.R.C. Liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr. Eval. Carcinog. Risks Hum. 1994, 61, 1–241. [Google Scholar]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Robinson, K.; Atherton, J.C. The Spectrum of Helicobacter-Mediated Diseases. Annu. Rev. Pathol. 2021, 16, 123–144. [Google Scholar] [CrossRef]
- Tsay, F.W.; Hsu, P.I. H. pylori infection and extra-gastroduodenal diseases. J. Biomed. Sci. 2018, 25, 65. [Google Scholar] [CrossRef]
- Blanchard, T.G.; Nedrud, J.G. Laboratory maintenance of helicobacter species. Curr. Protoc. Microbiol. 2006. [Google Scholar] [CrossRef]
- Testerman, T.L.; McGee, D.J.; Mobley, H.L. Helicobacter pylori growth and urease detection in the chemically defined medium Ham’s F-12 nutrient mixture. J. Clin. Microbiol. 2001, 39, 3842–3850. [Google Scholar] [CrossRef] [PubMed]
- Bayona-Rojas, M. Microbiological conditions for culturing Helicobacter Pylori. Rev. Colomb. Gastroenterol. 2013, 28, 94–99. [Google Scholar]
- Chen, C.; Pande, K.; French, S.D.; Tuch, B.B.; Noble, S.M. An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe 2011, 10, 118–135. [Google Scholar] [CrossRef]
- Merrell, D.S.; Thompson, L.J.; Kim, C.C.; Mitchell, H.; Tompkins, L.S.; Lee, A.; Falkow, S. Growth phase-dependent response of Helicobacter pylori to iron starvation. Infect. Immun. 2003, 71, 6510–6525. [Google Scholar] [CrossRef] [PubMed]
- Cover, T.L. Perspectives on methodology for in vitro culture of Helicobacter pylori. Methods Mol. Biol. 2012, 921, 11–15. [Google Scholar] [CrossRef]
- Pich, O.Q.; Merrell, D.S. The ferric uptake regulator of Helicobacter pylori: A critical player in the battle for iron and colonization of the stomach. Future Microbiol. 2013, 8, 725–738. [Google Scholar] [CrossRef]
- El Batawi, H.Y.; Venkatachalam, T.; Francis, A.; Abujabal, R.; Shehadat, S.A. Dental Caries-A Hiding Niche for Helicobacter pylori in Children. J. Clin. Pediatr. Dent. 2020, 44, 90–94. [Google Scholar] [CrossRef]
- Kadota, T.; Hamada, M.; Nomura, R.; Ogaya, Y.; Okawa, R.; Uzawa, N.; Nakano, K. Distribution of Helicobacter pylori and Periodontopathic Bacterial Species in the Oral Cavity. Biomedicines 2020, 8, 161. [Google Scholar] [CrossRef]
- Iwai, K.; Watanabe, I.; Yamamoto, T.; Kuriyama, N.; Matsui, D.; Nomura, R.; Ogaya, Y.; Oseko, F.; Adachi, K.; Takizawa, S.; et al. Association between Helicobacter pylori infection and dental pulp reservoirs in Japanese adults. BMC Oral Health 2019, 19, 267. [Google Scholar] [CrossRef]
- Junqueira, A.C.M.; Ratan, A.; Acerbi, E.; Drautz-Moses, D.I.; Premkrishnan, B.N.V.; Costea, P.I.; Linz, B.; Purbojati, R.W.; Paulo, D.F.; Gaultier, N.E.; et al. The microbiomes of blowflies and houseflies as bacterial transmission reservoirs. Sci. Rep. 2017, 7, 16324. [Google Scholar] [CrossRef]
- Castillo, M.; Bernabe, L.; Castaneda, C.A.; Chavez, I.; Ruiz, E.; Barreda, F.; Valdivia, D.; Suarez, N.; Nieves, J.; Dias-Neto, E.; et al. Helicobacter Pylori Detected in Tap Water of Peruvian Patients with Gastric Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 3193–3196. [Google Scholar] [CrossRef] [PubMed]
- Mashak, Z.; Jafariaskari, S.; Alavi, I.; Sakhaei Shahreza, M.; Safarpoor Dehkordi, F. Phenotypic and Genotypic Assessment of Antibiotic Resistance and Genotyping of vacA, cagA, iceA, oipA, cagE, and babA2 Alleles of Helicobacter pylori Bacteria Isolated from Raw Meat. Infect. Drug Resist. 2020, 13, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Elhariri, M.; Hamza, D.; Elhelw, R.; Hamza, E. Occurrence of cagA + vacA s1a m1 i1 Helicobacter pylori in farm animals in Egypt and ability to survive in experimentally contaminated UHT milk. Sci. Rep. 2018, 8, 14260. [Google Scholar] [CrossRef] [PubMed]
- Can, F.; Karahan, C.; Dolapci, I.; Demirbilek, M.; Tekeli, A.; Arslan, H. Urease activity and urea gene sequencing of coccoid forms of H. pylori induced by different factors. Curr. Microbiol. 2008, 56, 150–155. [Google Scholar] [CrossRef]
- Nilsson, H.O.; Blom, J.; Abu-Al-Soud, W.; Ljungh, A.A.; Andersen, L.P.; Wadström, T. Effect of cold starvation, acid stress, and nutrients on metabolic activity of Helicobacter pylori. Appl. Environ. Microbiol. 2002, 68, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Sun, Y.; Wang, N.; Yu, H.; Zhou, Y.; Chen, C.; Jia, J. Changes of proteome components of Helicobacter pylori biofilms induced by serum starvation. Mol. Med. Rep. 2013, 8, 1761–1766. [Google Scholar] [CrossRef]
- Santus, W.; Devlin, J.R.; Behnsen, J. Crossing Kingdoms: How the Mycobiota and Fungal-Bacterial Interactions Impact Host Health and Disease. Infect. Immun. 2021, 89, e00620–e00648. [Google Scholar] [CrossRef]
- Al-Rusan, R.M.; Darwazeh, A.M.G.; Lataifeh, I.M. The relationship of Candida colonization of the oral and vaginal mucosae of mothers and oral mucosae of their newborns at birth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 459–463. [Google Scholar] [CrossRef]
- Linda, A.W.-F.; Walker, M.W.; Richard, J.H.; Pfaller, M.A.; James, E.; Ferguson, J.E., II; Wenzel, R.P.; Leigh, G.D. Vertical and Horizontal Transmission of Unique Candida Species to Premature Newborns. Clin. Infect. Dis. 1996, 22, 803–808. [Google Scholar]
- Salmanian, A.-H.; Siavoshi, F.; Beyrami, Z.; Latifi-Navid, S.; Tavakolian, A.; Sadjadi, A. Foodborne Yeasts Serve as Reservoirs of Helicobacter Pylori. J. Food Saf. 2012, 32, 152–160. [Google Scholar] [CrossRef]
- Salmanian, A.H.; Siavoshi, F.; Akbari, F.; Afshari, A.; Malekzadeh, R. Yeast of the oral cavity is the reservoir of Heliobacter pylori. J. Oral Pathol. Med. 2008, 37, 324–328. [Google Scholar] [CrossRef]
- Siavoshi, F.; Salmanian, A.H.; Akbari, F.; Malekzadeh, R.; Massarrat, S. Detection of Helicobacter pylori-specific genes in the oral yeast. Helicobacter 2005, 10, 318–322. [Google Scholar] [CrossRef]
- Matamala-Valdés, L.; Sánchez-Alonzo, K.; Parra, C.; Sáez, K.; Aguayo-Reyes, A.; García, A. Detection of intracellular Helicobacter pylori in Candida. SPP from neonate oral swabs. Rev. Assoc. Med. Bras. (1992) 2018, 64, 928–935. [Google Scholar] [CrossRef]
- Sánchez-Alonzo, K.; Matamala-Valdés, L.; Parra-Sepúlveda, C.; Bernasconi, H.; Campos, V.L.; Smith, C.T.; Sáez, K.; García-Cancino, A. Intracellular Presence of Helicobacter pylori and Its Virulence-Associated Genotypes within the Vaginal Yeast of Term Pregnant Women. Microorganisms 2021, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alonzo, K.; Parra-Sepúlveda, C.; Vergara, L.; Bernasconi, H.; García-Cancino, A. Detection of Helicobacter pylori in oral yeasts from students of a Chilean university. Rev. Assoc. Med. Bras. (1992) 2020, 66, 1509–1514. [Google Scholar] [CrossRef]
- Siavoshi, F.; Taghikhani, A.; Malekzadeh, R.; Sarrafnejad, A.; Kashanian, M.; Jamal, A.S.; Saniee, P.; Sadeghi, S.; Sharifi, A.H. The role of mother’s oral and vaginal yeasts in transmission of Helicobacter pylori to neonates. Arch. Iran. Med. 2013, 16, 288–294. [Google Scholar]
- Siavoshi, F.; Saniee, P. Vacuoles of Candida yeast as a specialized niche for Helicobacter pylori. World J. Gastroenterol. 2014, 20, 5263–5273. [Google Scholar] [CrossRef]
- Sánchez-Alonzo, K.; Parra-Sepúlveda, C.; Vega, S.; Bernasconi, H.; Campos, V.L.; Smith, C.T.; Sáez, K.; García-Cancino, A. In Vitro Incorporation of Helicobacter pylori into Candida albicans Caused by Acidic pH Stress. Pathogens 2020, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Böckelmann, U.; Manz, W.; Neu, T.R.; Szewzyk, U. Investigation of lotic microbial aggregates by a combined technique of fluorescent in situ hybridization and lectin-binding-analysis. J. Microbiol. Methods 2002, 49, 75–87. [Google Scholar] [CrossRef]
- Rüssmann, H.; Kempf, V.A.; Koletzko, S.; Heesemann, J.; Autenrieth, I.B. Comparison of fluorescent in situ hybridization and conventional culturing for detection of Helicobacter pylori in gastric biopsy specimens. J. Clin. Microbiol. 2001, 39, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Senkovich, O.; Ceaser, S.; McGee, D.J.; Testerman, T.L. Unique host iron utilization mechanisms of Helicobacter pylori revealed with iron-deficient chemically defined media. Infect. Immun. 2010, 78, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Gulec, S.; Anderson, G.J.; Collins, J.F. Mechanistic and regulatory aspects of intestinal iron absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G397–G409. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.-l.; Cheng, D.-d.; Xu, W.-t.; Lu, N.-h. Adhesion and Invasion of Gastric Mucosa Epithelial Cells by Helicobacter pylori. Front. Cell. Infect. Microbiol. 2016, 6, 159. [Google Scholar] [CrossRef]
- Posselt, G.; Crabtree, J.E.; Wessler, S. Proteolysis in Helicobacter pylori-Induced Gastric Cancer. Toxins 2017, 9, 134. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Coleman, W.G.; Yang, Z.; Yang, Y.; Hodgson, N.; Chen, F.; Jin, D.J. Regulation of cell growth during serum starvation and bacterial survival in macrophages by the bifunctional enzyme SpoT in Helicobacter pylori. J. Bacteriol. 2008, 190, 8025–8032. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.H.; Gaynor, E.C. Helicobacter pylori Initiates the Stringent Response upon Nutrient and pH Downshift. J. Bacteriol. 2006, 188, 3726–3729. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Hu, X.; Ling, J.; Du, Y.; Liu, J.; Liu, H.; Peng, Z. Candida albicans survival and biofilm formation under starvation conditions. Int. Endod. J. 2013, 46, 62–70. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Rodrigues, M.E.; Silva, S.; Henriques, M. Candida glabrata Biofilms: How Far Have We Come? J. Fungi 2017, 3, 11. [Google Scholar] [CrossRef]
- Delgado, M.L.; Gil, M.L.; Gozalbo, D. Starvation and temperature upshift cause an increase in the enzymatically active cell wall-associated glyceraldehyde-3-phosphate dehydrogenase protein in yeast. FEMS Yeast Res. 2003, 4, 297–303. [Google Scholar] [CrossRef]
- Kumar, K.; Askari, F.; Sahu, M.S.; Kaur, R. Candida glabrata: A Lot More Than Meets the Eye. Microorganisms 2019, 7, 39. [Google Scholar] [CrossRef]
- Ansorg, R.; Schmid, E.N. Adhesion of Helicobacter pylori to yeast cells. Zent. Bakteriol. 1998, 288, 501–508. [Google Scholar] [CrossRef]
- Van Dyck, K.; Viela, F.; Mathelié-Guinlet, M.; Demuyser, L.; Hauben, E.; Jabra-Rizk, M.A.; Vande Velde, G.; Dufrêne, Y.F.; Krom, B.P.; Van Dijck, P. Adhesion of Staphylococcus aureus to Candida albicans During Co-Infection Promotes Bacterial Dissemination Through the Host Immune Response. Front. Cell. Infect. Microbiol. 2020, 10, 624839. [Google Scholar] [CrossRef]
- Shing, S.R.; Ramos, A.R.; Patras, K.A.; Riestra, A.M.; McCabe, S.; Nizet, V.; Coady, A. The Fungal Pathogen Candida albicans Promotes Bladder Colonization of Group B Streptococcus. Front. Cell. Infect. Microbiol. 2020, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Beaussart, A.; Herman, P.; El-Kirat-Chatel, S.; Lipke, P.N.; Kucharíková, S.; Van Dijck, P.; Dufrêne, Y.F. Single-cell force spectroscopy of the medically important Staphylococcus epidermidis-Candida albicans interaction. Nanoscale 2013, 5, 10894–10900. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, L.L.; Oh, S.-H.; Jones, R.; Cota, E. A proposed mechanism for the interaction between the Candida albicans Als3 adhesin and streptococcal cell wall proteins. Front. Microbiol. 2014, 5, 564. [Google Scholar] [CrossRef]
- Dubois, A. Intracellular Helicobacter pylori and gastric carcinogenesis: An “old” frontier worth revisiting. Gastroenterology 2007, 132, 1177–1180. [Google Scholar] [CrossRef]
- Karczewska, E.; Wojtas, I.; Sito, E.; Trojanowska, D.; Budak, A.; Zwolinska-Wcislo, M.; Wilk, A. Assessment of co-existence of Helicobacter pylori and Candida fungi in diseases of the upper gastrointestinal tract. J. Physiol. Pharmacol. 2009, 60 (Suppl. S6), 33–39. [Google Scholar]
- Reshetnyak, V.I.; Reshetnyak, T.M. Significance of dormant forms of Helicobacter pylori in ulcerogenesis. World J. Gastroenterol. 2017, 23, 4867–4878. [Google Scholar] [CrossRef]
- Smits, G.J.; Kapteyn, J.C.; van den Ende, H.; Klis, F.M. Cell wall dynamics in yeast. Curr. Opin. Microbiol. 1999, 2, 348–352. [Google Scholar] [CrossRef]
- Ingle, S.; Kazi, R.; Patil, R.; Zore, G. Proteome analysis of Candida albicans cells undergoing chlamydosporulation. J. Proteins Proteom. 2019, 10, 269–290. [Google Scholar] [CrossRef]
- Saniee, P.; Siavoshi, F.; Nikbakht Broujeni, G.; Khormali, M.; Sarrafnejad, A.; Malekzadeh, R. Localization of H.pylori within the vacuole of Candida yeast by direct immunofluorescence technique. Arch. Iran. Med. 2013, 16, 705–710. [Google Scholar] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Alonzo, K.; Silva-Mieres, F.; Arellano-Arriagada, L.; Parra-Sepúlveda, C.; Bernasconi, H.; Smith, C.T.; Campos, V.L.; García-Cancino, A. Nutrient Deficiency Promotes the Entry of Helicobacter pylori Cells into Candida Yeast Cells. Biology 2021, 10, 426. https://doi.org/10.3390/biology10050426
Sánchez-Alonzo K, Silva-Mieres F, Arellano-Arriagada L, Parra-Sepúlveda C, Bernasconi H, Smith CT, Campos VL, García-Cancino A. Nutrient Deficiency Promotes the Entry of Helicobacter pylori Cells into Candida Yeast Cells. Biology. 2021; 10(5):426. https://doi.org/10.3390/biology10050426
Chicago/Turabian StyleSánchez-Alonzo, Kimberly, Fabiola Silva-Mieres, Luciano Arellano-Arriagada, Cristian Parra-Sepúlveda, Humberto Bernasconi, Carlos T. Smith, Víctor L. Campos, and Apolinaria García-Cancino. 2021. "Nutrient Deficiency Promotes the Entry of Helicobacter pylori Cells into Candida Yeast Cells" Biology 10, no. 5: 426. https://doi.org/10.3390/biology10050426
APA StyleSánchez-Alonzo, K., Silva-Mieres, F., Arellano-Arriagada, L., Parra-Sepúlveda, C., Bernasconi, H., Smith, C. T., Campos, V. L., & García-Cancino, A. (2021). Nutrient Deficiency Promotes the Entry of Helicobacter pylori Cells into Candida Yeast Cells. Biology, 10(5), 426. https://doi.org/10.3390/biology10050426







