Synovial Fluid Fatty Acid Profiles Are Differently Altered by Inflammatory Joint Pathologies in the Shoulder and Knee Joints
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Sampling
2.2. Fatty Acid Analysis
2.3. Statistical Analyses
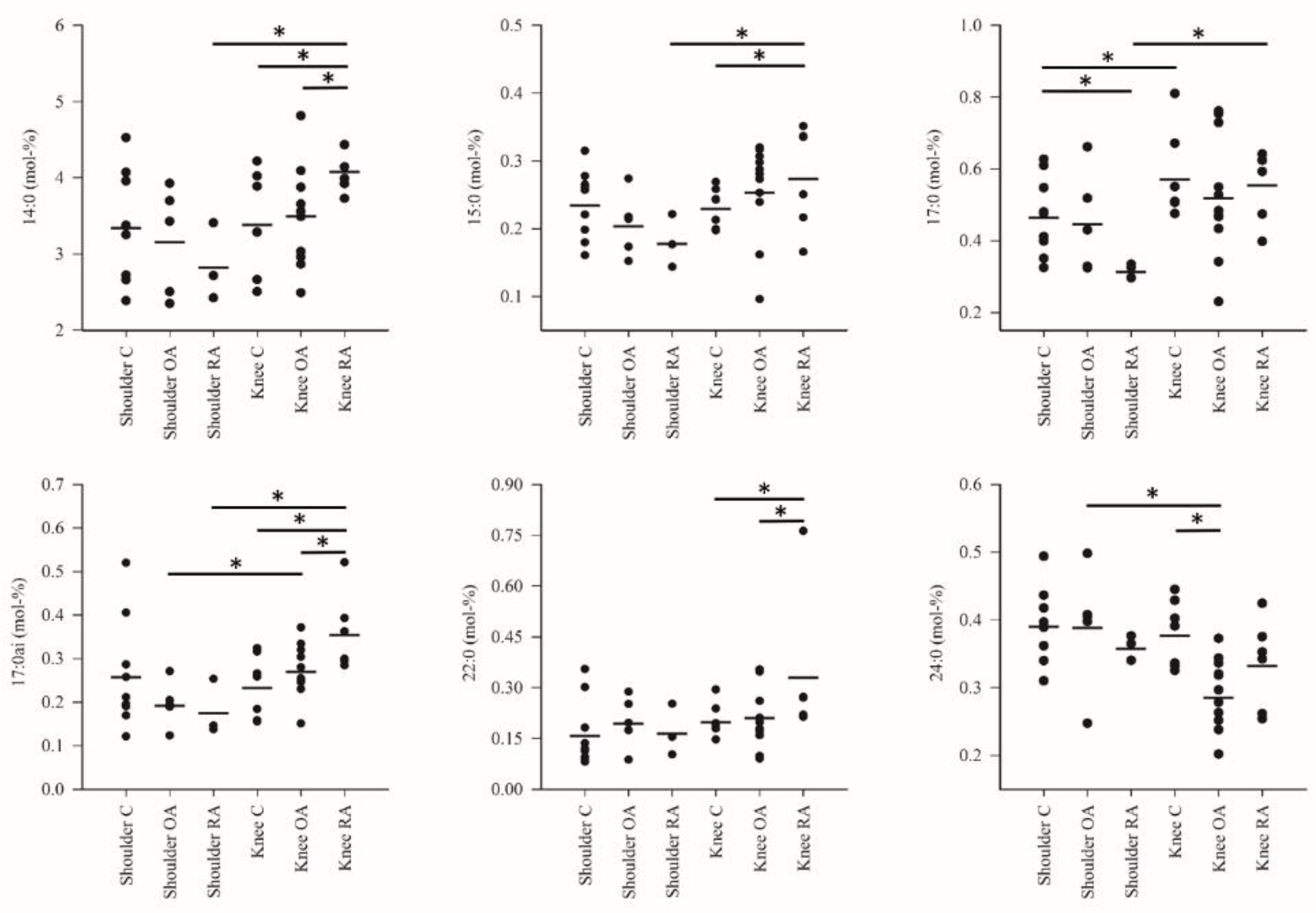
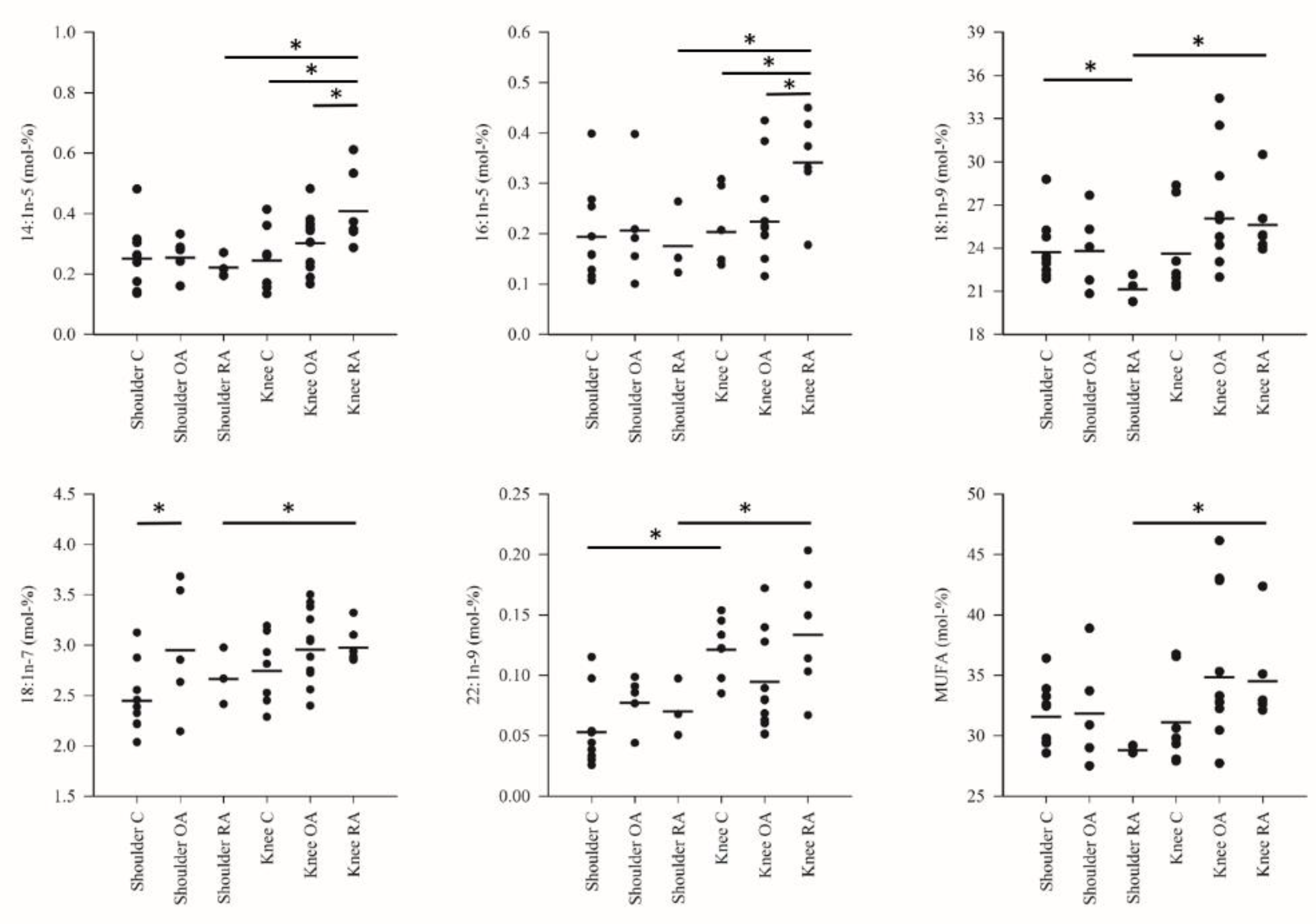
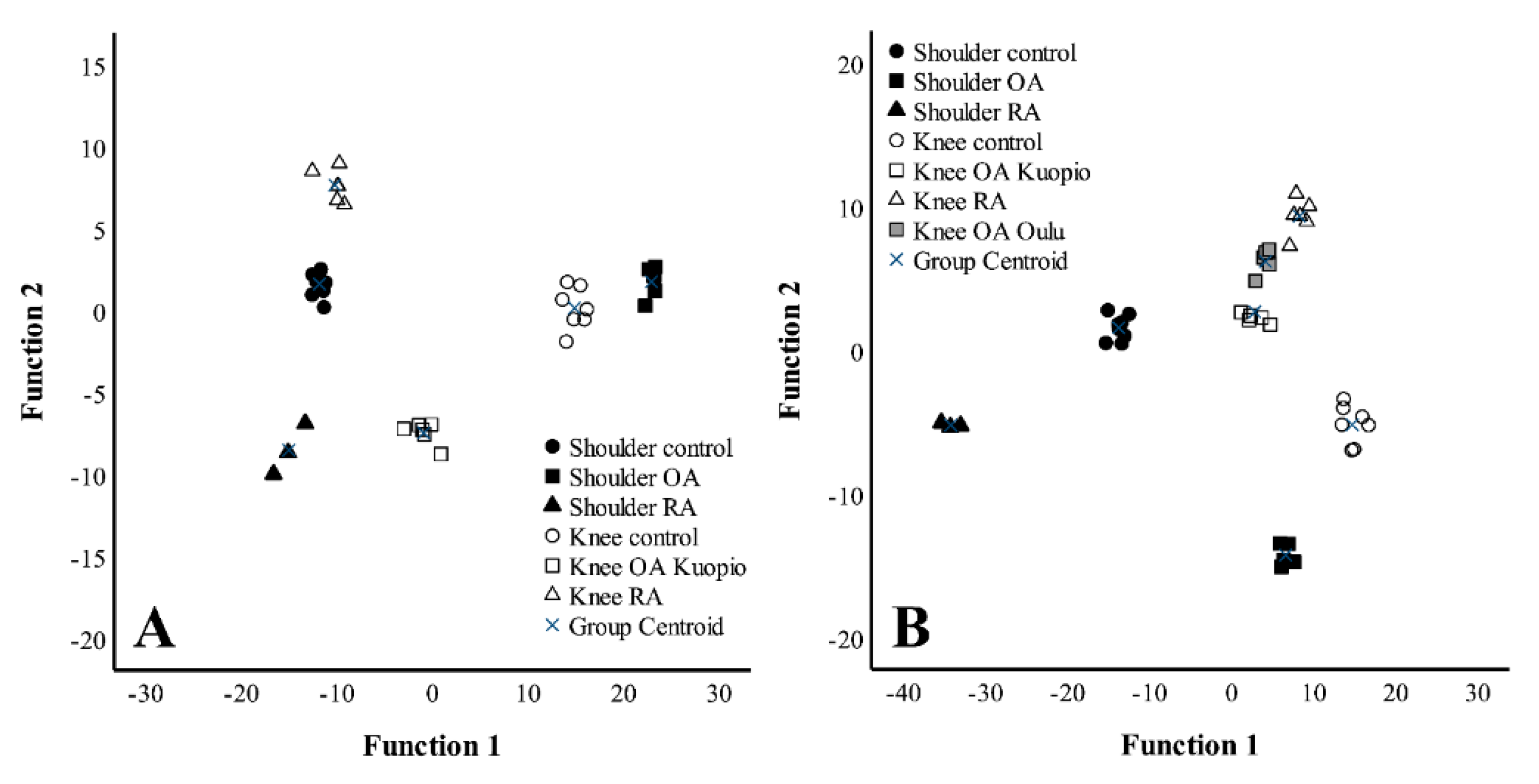
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Frommer, K.W.; Schäffler, A.; Rehart, S.; Lehr, A.; Müller-Ladner, U.; Neumann, E. Free fatty acids: Potential proinflammatory mediators in rheumatic diseases. Ann. Rheum. Dis. 2015, 74, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Frommer, K.W.; Hasseli, R.; Schäffler, A.; Lange, U.; Rehart, S.; Steinmeyer, J.; Rickert, M.; Sarter, K.; Zaiss, M.M.; Culmsee, C.; et al. Free fatty acids in bone pathophysiology of rheumatic diseases. Front. Immunol. 2019, 10, 2757. [Google Scholar] [CrossRef] [PubMed]
- Jónasdóttir, H.S.; Brouwers, H.; Kwekkeboom, J.C.; van der Linden, H.M.J.; Huizinga, T.; Kloppenburg, M.; Toes, R.E.M.; Giera, M.; Ioan-Facsinay, A. Targeted lipidomics reveals activation of resolution pathways in knee osteoarthritis in humans. Osteoarthr. Cartil. 2017, 25, 1150–1160. [Google Scholar] [CrossRef]
- Guilak, F. Biomechanical factors in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2011, 25, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Boden, G. Obesity and free fatty acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Lippiello, L.; Walsh, T.; Fienhold, M. The association of lipid abnormalities with tissue pathology in human osteoarthritic articular cartilage. Metabolism 1991, 40, 571–576. [Google Scholar] [CrossRef]
- Navarro, E.; Esteve, M.; Olivé, A.; Klaassen, J.; Cabré, E.; Tena, X.; Fernández-Bañares, F.; Pastor, C.; Gassull, M.A. Abnormal fatty acid pattern in rheumatoid arthritis. A rationale for treatment with marine and botanical lipids. J. Rheumatol. 2000, 27, 298–303. [Google Scholar]
- Humphries, J.M.; Kuliwaba, J.S.; Gibson, R.J.; Fazzalari, N.L. In situ fatty acid profile of femoral cancellous subchondral bone in osteoarthritic and fragility fracture females: Implications for bone remodelling. Bone 2012, 51, 218–223. [Google Scholar] [CrossRef]
- Mustonen, A.-M.; Käkelä, R.; Lehenkari, P.; Huhtakangas, J.; Turunen, S.; Joukainen, A.; Kääriäinen, T.; Paakkonen, T.; Kröger, H.; Nieminen, P. Distinct fatty acid signatures in infrapatellar fat pad and synovial fluid of patients with osteoarthritis versus rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 124. [Google Scholar] [CrossRef]
- Van de Vyver, A.; Clockaerts, S.; van de Lest, C.H.A.; Wei, W.; Verhaar, J.; Van Osch, G.J.V.M.; Bastiaansen-Jenniskens, Y.M. Synovial fluid fatty acid profiles differ between osteoarthritis and healthy patients. Cartilage 2020, 11, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Toyoshima, S.; Miki, Y.; Taketomi, Y.; Ito, M.; Lee, H.; Saito, S.; Murakami, M.; Okayama, Y. Activation of inflammation and resolution pathways of lipid mediators in synovial fluid from patients with severe rheumatoid arthritis compared with severe osteoarthritis. Asia Pac. Allergy 2020, 10, e21. [Google Scholar] [CrossRef]
- Harasymowicz, N.S.; Dicks, A.; Wu, C.-L.; Guilak, F. Physiologic and pathologic effects of dietary free fatty acids on cells of the joint. Ann. N. Y. Acad. Sci. 2019, 1440, 36–53. [Google Scholar] [CrossRef]
- Alvarez-Garcia, O.; Rogers, N.H.; Smith, R.G.; Lotz, M.K. Palmitate has proapoptotic and proinflammatory effects on articular cartilage and synergizes with interleukin-1. Arthritis Rheumatol. 2014, 66, 1779–1788. [Google Scholar] [CrossRef]
- Nazli, S.A.; Loeser, R.F.; Chubinskaya, S.; Willey, J.S.; Yammani, R.R. High fat-diet and saturated fatty acid palmitate inhibits IGF-1 function in chondrocytes. Osteoarthr. Cartil. 2017, 25, 1516–1521. [Google Scholar] [CrossRef]
- Sekar, S.; Shafie, S.R.; Prasadam, I.; Crawford, R.; Panchal, S.K.; Brown, L.; Xiao, Y. Saturated fatty acids induce development of both metabolic syndrome and osteoarthritis in rats. Sci. Rep. 2017, 7, 46457. [Google Scholar] [CrossRef] [PubMed]
- Zainal, Z.; Longman, A.J.; Hurst, S.; Duggan, K.; Caterson, B.; Hughes, C.E.; Harwood, J.L. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthr. Cartil. 2009, 17, 896–905. [Google Scholar] [CrossRef]
- Caron, J.P.; Gandy, J.C.; Brown, J.L.; Sordillo, L.M. Omega-3 fatty acids and docosahexaenoic acid oxymetabolites modulate the inflammatory response of equine recombinant interleukin1β-stimulated equine synoviocytes. Prostaglandins Other Lipid Mediat. 2019, 142, 1–8. [Google Scholar] [CrossRef]
- Bao, M.; Zhang, K.; Wei, Y.; Hua, W.; Gao, Y.; Li, X.; Ye, L. Therapeutic potentials and modulatory mechanisms of fatty acids in bone. Cell Prolif. 2020, 53, e12735. [Google Scholar] [CrossRef] [PubMed]
- Tchetina, E.V.; Di Battista, J.A.; Zukor, D.J.; Antoniou, J.; Poole, A.R. Prostaglandin PGE2 at very low concentrations suppresses collagen cleavage in cultured human osteoarthritic articular cartilage: This involves a decrease in expression of proinflammatory genes, collagenases and COL10A1, a gene linked to chondrocyte hypertrophy. Arthritis Res. Ther. 2007, 9, R75. [Google Scholar] [CrossRef] [PubMed]
- Shibata-Nozaki, T.; Ito, H.; Mitomi, H.; Akaogi, J.; Komagata, T.; Kanaji, T.; Maruyama, T.; Mori, T.; Nomoto, S.; Ozaki, S.; et al. Endogenous prostaglandin E2 inhibits aberrant overgrowth of rheumatoid synovial tissue and the development of osteoclast activity through EP4 receptor. Arthritis Rheum. 2011, 63, 2595–2605. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Singh, G.K.; Zhang, F.; Wang, P.; Liu, W.; Zhong, L.; Yang, L. Comparative study of normal and rheumatoid arthritis fibroblast-like synoviocytes proliferation under cyclic mechanical stretch: Role of prostaglandin E2. Connect. Tissue Res. 2012, 53, 246–254. [Google Scholar] [CrossRef]
- Adler, N.; Schoeniger, A.; Fuhrmann, H. Polyunsaturated fatty acids influence inflammatory markers in a cellular model for canine osteoarthritis. J. Anim. Physiol. Anim. Nutr. 2018, 102, e623–e632. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Driban, J.B.; Xu, C.; Lapane, K.L.; McAlindon, T.E.; Eaton, C.B. Dietary fat intake and radiographic progression of knee osteoarthritis: Data from the Osteoarthritis Initiative. Arthritis Care Res. 2017, 69, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Kimmerling, K.A.; Little, D.; Guilak, F. Serum and synovial fluid lipidomic profiles predict obesity-associated oste-oarthritis, synovitis, and wound repair. Sci. Rep. 2017, 7, 44315. [Google Scholar] [CrossRef] [PubMed]
- Navarini, L.; Afeltra, A.; Gallo Afflitto, G.; Margiotta, D.P.E. Polyunsaturated fatty acids: Any role in rheumatoid arthritis? Lipids Health Dis. 2017, 16, 197. [Google Scholar] [CrossRef]
- Hall, M.C. The Locomotor System: Functional Anatomy; Charles C. Thomas: Springfield, IL, USA, 1965. [Google Scholar]
- Veeger, H.E.J.; van der Helm, F.C.T. Shoulder function: The perfect compromise between mobility and stability. J. Biomech. 2007, 40, 2119–2129. [Google Scholar] [CrossRef]
- Chen, A.L.; Joseph, T.N.; Zuckerman, J.D. Rheumatoid arthritis of the shoulder. J. Am. Acad. Orthop. Surg. 2003, 11, 12–24. [Google Scholar] [CrossRef]
- Chillemi, C.; Franceschini, V. Shoulder osteoarthritis. Arthritis 2013, 2013, 370231. [Google Scholar] [CrossRef]
- Fox, J.A.; Cole, B.J.; Romeo, A.A.; Meininger, A.K.; Glenn, R.E., Jr.; Bicos, J.; Hayden, J.K.; Dorow, C.B. Articular cartilage thickness of the humeral head: An anatomic study. Orthopedics 2008, 31, 216. [Google Scholar] [CrossRef]
- Chubinskaya, S.; Cotter, E.J.; Frank, R.M.; Hakimiyan, A.A.; Yanke, A.B.; Cole, B.J. Biologic characteristics of shoulder articular cartilage in comparison to knee and ankle articular cartilage from individual donors. Cartilage 2019. [Google Scholar] [CrossRef]
- Bollet, A.J.; Handy, J.R.; Sturgill, B.C. Chondroitin sulfate concentration and protein-polysaccharide composition of articular cartilage in osteoarthritis. J. Clin. Investig. 1963, 42, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, A.; Flatow, E.L.; Roth, N.; Saed-Nejad, F.; Bigliani, L.U. Biochemical markers in synovial fluid identify early osteoarthritis of the glenohumeral joint. Clin. Orthop. Relat. Res. 1996, 330, 45–53. [Google Scholar] [CrossRef]
- Gierman, L.M.; Wopereis, S.; van El, B.; Verheij, E.R.; Werff-van der Vat, B.J.C.; Bastiaansen-Jenniskens, Y.M.; van Osch, G.J.V.M.; Kloppenburg, M.; Stojanovic-Susulic, V.; Huizinga, T.W.J.; et al. Metabolic profiling reveals differences in concentrations of oxylipins and fatty acids secreted by the infrapatellar fat pad of donors with end-stage osteoarthritis and normal donors. Arthritis Rheum. 2013, 65, 2606–2614. [Google Scholar] [CrossRef]
- Connor, W.E.; Lin, D.S.; Colvis, C. Differential mobilization of fatty acids from adipose tissue. J. Lipid Res. 1996, 37, 290–298. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, J.; Kim, J.; Ahn, J.K.; Cha, H.-S.; Kim, K.H. Metabolite profiles of synovial fluid change with the radiographic severity of knee osteoarthritis. Jt. Bone Spine 2017, 84, 605–610. [Google Scholar] [CrossRef]
- Plumb, M.S.; Aspden, R.M. High levels of fat and (n-6) fatty acids in cancellous bone in osteoarthritis. Lipids Health Dis. 2004, 3, 12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baker, K.R.; Matthan, N.R.; Lichtenstein, A.H.; Niu, J.; Guermazi, A.; Roemer, F.; Grainger, A.; Nevitt, M.C.; Clancy, M.; Lewis, C.E.; et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: The MOST study. Osteoarthr. Cartil. 2012, 20, 382–387. [Google Scholar] [CrossRef]
- Hurst, S.; Rees, S.G.; Randerson, P.F.; Caterson, B.; Harwood, J.L. Contrasting effects of n-3 and n-6 fatty acids on cyclooxygenase-2 in model systems for arthritis. Lipids 2009, 44, 889–896. [Google Scholar] [CrossRef]
- Bastiaansen-Jenniskens, Y.M.; Siawash, M.; van de Lest, C.H.A.; Verhaar, J.A.N.; Kloppenburg, M.; Zuurmond, A.-M.; Stojanovic-Susulic, V.; Van Osch, G.J.V.M.; Clockaerts, S. Monounsaturated and saturated, but not n-6 polyunsaturated fatty acids decrease cartilage destruction under inflammatory conditions: A preliminary study. Cartilage 2013, 4, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kawahito, Y.; Kondo, M.; Tsubouchi, Y.; Hashiramoto, A.; Bishop-Bailey, D.; Inoue, K.-i.; Kohno, M.; Yamada, R.; Hla, T.; Sano, H. 15-deoxy-Δ12,14-PGJ2 induces synoviocyte apoptosis and suppresses adjuvant-induced arthritis in rats. J. Clin. Investig. 2000, 106, 189–197. [Google Scholar] [CrossRef]
- Sodin-Semrl, S.; Taddeo, B.; Tseng, D.; Varga, J.; Fiore, S. Lipoxin A4 inhibits IL-1β-induced IL-6, IL-8, and matrix metallo-proteinase-3 production in human synovial fibroblasts and enhances synthesis of tissue inhibitors of metalloproteinases. J. Immunol. 2000, 164, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, H.; von Hegedus, J.; Toes, R.; Kloppenburg, M.; Ioan-Facsinay, A. Lipid mediators of inflammation in rheumatoid arthritis and osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2015, 29, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, A.; Ma, L.; Yu, H.; Zhang, L.; Meng, H.; Cui, Y.; Yu, F.; Yang, B. Docosahexenoic acid treatment ameliorates cartilage degeneration via a p38 MAPK-dependent mechanism. Int. J. Mol. Med. 2016, 37, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Linos, A.; Kaklamani, V.G.; Kaklamani, E.; Koumantaki, Y.; Giziaki, E.; Papazoglou, S.; Mantzoros, C.S. Dietary factors in relation to rheumatoid arthritis: A role for olive oil and cooked vegetables? Am. J. Clin. Nutr. 1999, 70, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Sugioka, Y.; Tada, M.; Okano, T.; Mamoto, K.; Inui, K.; Habu, D.; Koike, T. Monounsaturated fatty acids might be key factors in the Mediterranean diet that suppress rheumatoid arthritis disease activity: The TOMORROW study. Clin. Nutr. 2018, 37, 675–680. [Google Scholar] [CrossRef]
- Lee, S.W.; Rho, J.H.; Lee, S.Y.; Chung, W.T.; Oh, Y.J.; Kim, J.H.; Yoo, S.H.; Kwon, W.Y.; Bae, J.Y.; Seo, S.Y.; et al. Dietary fat-associated osteoarthritic chondrocytes gain resistance to lipotoxicity through PKCK2/STAMP2/FSP27. Bone Res. 2018, 6, 20. [Google Scholar] [CrossRef]
- Djoussé, L.; Matsumoto, C.; Hanson, N.Q.; Weir, N.L.; Tsai, M.Y.; Gaziano, J.M. Plasma cis-vaccenic acid and risk of heart failure with antecedent coronary heart disease in male physicians. Clin. Nutr. 2014, 33, 478–482. [Google Scholar] [CrossRef]
- Bruderlein, H.; Daniel, R.; Boismenu, D.; Julien, N.; Couture, F. Fatty acid profiles of serum phospholipids in patients suffering rheumatoid arthritis. Prog. Lipid Res. 1981, 20, 625–631. [Google Scholar] [CrossRef]
- Jacobsson, L.; Lindgärde, F.; Manthorpe, R.; Åkesson, B. Correlation of fatty acid composition of adipose tissue lipids and serum phosphatidylcholine and serum concentrations of micronutrients with disease duration in rheumatoid arthritis. Ann. Rheum. Dis. 1990, 49, 901–905. [Google Scholar] [CrossRef]
- de Pablo, P.; Romaguera, D.; Fisk, H.L.; Calder, P.C.; Quirke, A.-M.; Cartwright, A.J.; Panico, S.; Mattiello, A.; Gavrila, D.; Navarro, C.; et al. High erythrocyte levels of the n-6 polyunsaturated fatty acid linoleic acid are associated with lower risk of subsequent rheumatoid arthritis in a southern European nested case–control study. Ann. Rheum. Dis. 2018, 77, 981–987. [Google Scholar] [CrossRef]
- He, M.; van Wijk, E.; Berger, R.; Wang, M.; Strassburg, K.; Schoeman, J.C.; Vreeken, R.J.; van Wietmarschen, H.; Harms, A.C.; Kobayashi, M.; et al. Collagen induced arthritis in DBA/1J mice associates with oxylipin changes in plasma. Mediat. Inflamm. 2015, 2015, 543541. [Google Scholar] [CrossRef] [PubMed]
- Loef, M.; Ioan-Facsinay, A.; Mook-Kanamori, D.O.; van Dijk, K.W.; de Mutsert, R.; Kloppenburg, M.; Rosendaal, F.R. The association of plasma fatty acids with hand and knee osteoarthritis: The NEO study. Osteoarthr. Cartil. 2020, 28, 223–230. [Google Scholar] [CrossRef]
- Chan, M.M.-Y.; Moore, A.R. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J. Immunol. 2010, 184, 6418–6426. [Google Scholar] [CrossRef]
- Prete, P.E.; Gurakar-Osborne, A.; Kashyap, M.L. Synovial fluid lipids and apolipoproteins: A contemporary perspective. Biorheology 1995, 32, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sluzalska, K.D.; Liebisch, G.; Lochnit, G.; Ishaque, B.; Hackstein, H.; Schmitz, G.; Rickert, M.; Steinmeyer, J. Interleukin-1β affects the phospholipid biosynthesis of fibroblast-like synoviocytes from human osteoarthritic knee joints. Osteoarthr. Cartil. 2017, 25, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.L.; Gregg, J.R.; Nixon, J.E.; Schumacher, H.R. Lipid composition of the tissues of human knee joints. I. Observations in normal joints (articular cartilage, meniscus, ligaments, synovial fluid, synovium, intra-articular fat pad and bone marrow). Clin. Orthop. Relat. Res. 1979, 143, 260–265. [Google Scholar]
- Kosinska, M.K.; Liebisch, G.; Lochnit, G.; Wilhelm, J.; Klein, H.; Kaesser, U.; Lasczkowski, G.; Rickert, M.; Schmitz, G.; Steinmeyer, J. A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum. 2013, 65, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.B.; Dodge, J.T. Composition of phospholipids and of phospholipid fatty acids of human plasma. J. Lipid Res. 1967, 8, 676–681. [Google Scholar] [CrossRef]
- McNamara, R.K.; Liu, Y.; Jandacek, R.; Rider, T.; Tso, P. The aging human orbitofrontal cortex: Decreasing polyunsaturated fatty acid composition and associated increases in lipogenic gene expression and stearoyl-CoA desaturase activity. Prostagland. Leukot. Essent. Fatty Acids 2008, 78, 293–304. [Google Scholar] [CrossRef]
- Lohner, S.; Fekete, K.; Marosvölgyi, T.; Decsi, T. Gender differences in the long-chain polyunsaturated fatty acid status: Systematic review of 51 publications. Ann. Nutr. Metab. 2013, 62, 98–112. [Google Scholar] [CrossRef] [PubMed]

| Group | Control | OA | RA | p | p | p |
|---|---|---|---|---|---|---|
| Diagnosis | Location | Interaction | ||||
| Sex (M/F) | 9/7 | 4/13 | 1/8 | 0.053 | ||
| Age | 44 ± 3 | 62 ± 2 * | 71 ± 3 *,† | 0.020 | 0.021 | 0.002 |
| Body mass | 80.2 ± 3.86 | 87.6 ± 4.54 | 65.6 ± 4.57 | 0.170 | 0.926 | 0.592 |
| BMI | 26.8 ± 1.13 | 31.7 ± 1.55 | 25.2 ± 1.09 | 0.700 | 0.509 | 0.925 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustonen, A.-M.; Käkelä, R.; Joukainen, A.; Lehenkari, P.; Jaroma, A.; Kääriäinen, T.; Kröger, H.; Paakkonen, T.; Sihvo, S.P.; Nieminen, P. Synovial Fluid Fatty Acid Profiles Are Differently Altered by Inflammatory Joint Pathologies in the Shoulder and Knee Joints. Biology 2021, 10, 401. https://doi.org/10.3390/biology10050401
Mustonen A-M, Käkelä R, Joukainen A, Lehenkari P, Jaroma A, Kääriäinen T, Kröger H, Paakkonen T, Sihvo SP, Nieminen P. Synovial Fluid Fatty Acid Profiles Are Differently Altered by Inflammatory Joint Pathologies in the Shoulder and Knee Joints. Biology. 2021; 10(5):401. https://doi.org/10.3390/biology10050401
Chicago/Turabian StyleMustonen, Anne-Mari, Reijo Käkelä, Antti Joukainen, Petri Lehenkari, Antti Jaroma, Tommi Kääriäinen, Heikki Kröger, Tommi Paakkonen, Sanna P. Sihvo, and Petteri Nieminen. 2021. "Synovial Fluid Fatty Acid Profiles Are Differently Altered by Inflammatory Joint Pathologies in the Shoulder and Knee Joints" Biology 10, no. 5: 401. https://doi.org/10.3390/biology10050401
APA StyleMustonen, A.-M., Käkelä, R., Joukainen, A., Lehenkari, P., Jaroma, A., Kääriäinen, T., Kröger, H., Paakkonen, T., Sihvo, S. P., & Nieminen, P. (2021). Synovial Fluid Fatty Acid Profiles Are Differently Altered by Inflammatory Joint Pathologies in the Shoulder and Knee Joints. Biology, 10(5), 401. https://doi.org/10.3390/biology10050401





