GP88/PGRN Serum Levels Are Associated with Prognosis for Oral Squamous Cell Carcinoma Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Preanalytics
2.3. GP88 EIA
2.4. Statistics
3. Results
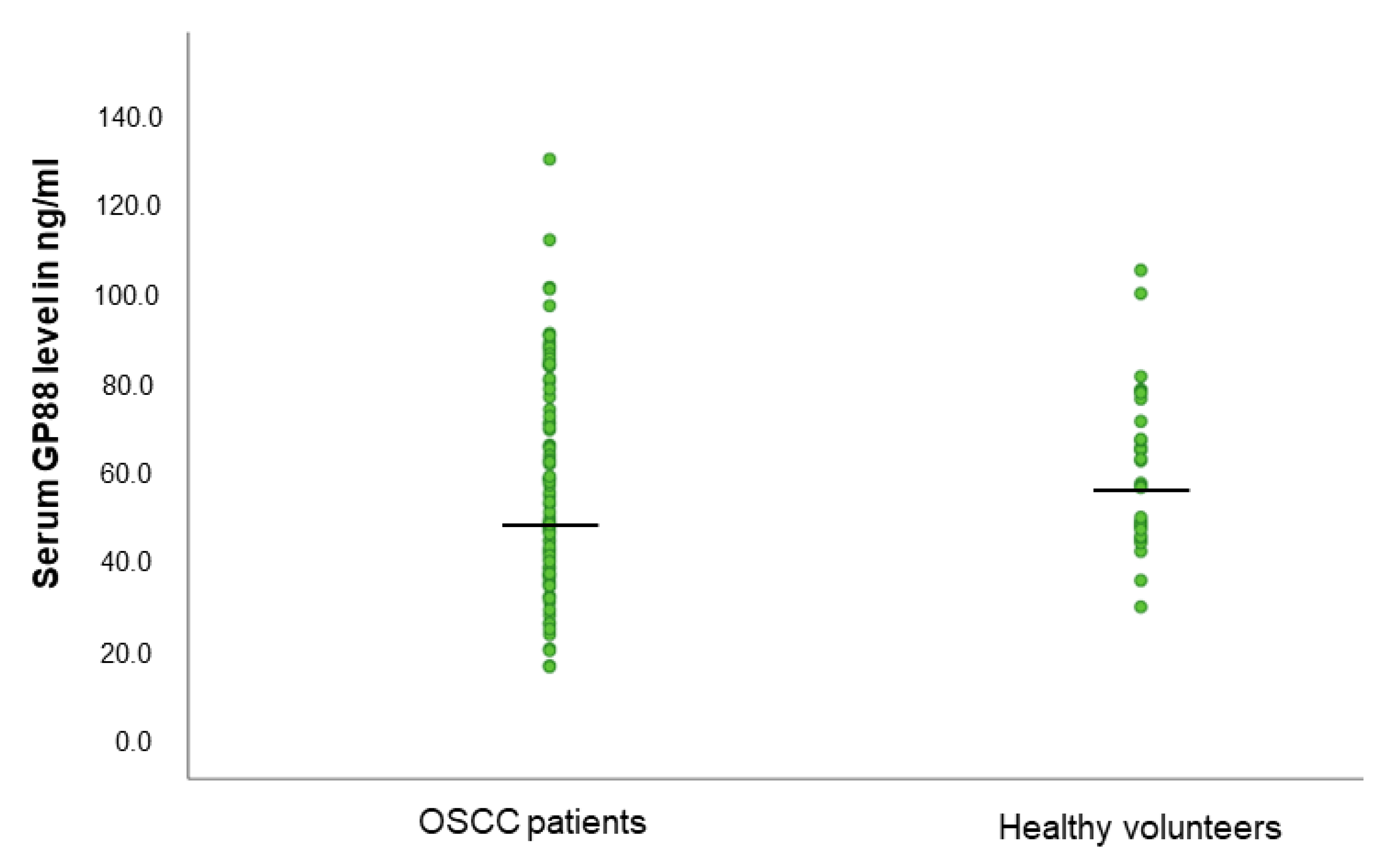
3.1. GP88 Levels Are Not Different in the Serum of OSCC Patients and Healthy Volunteers
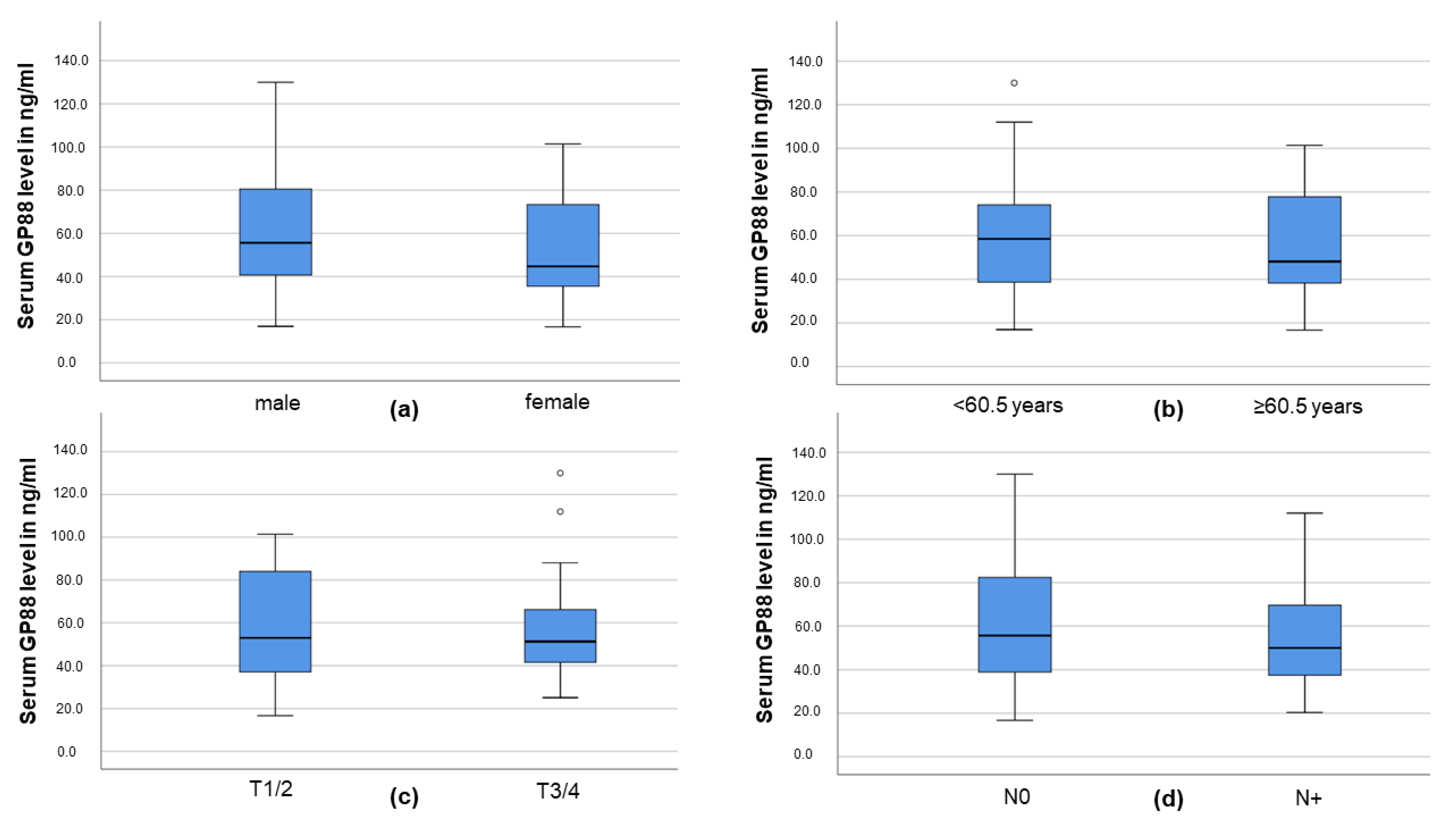
3.2. Serum GP88 Level and Demographic and Clinicopathological Parameters
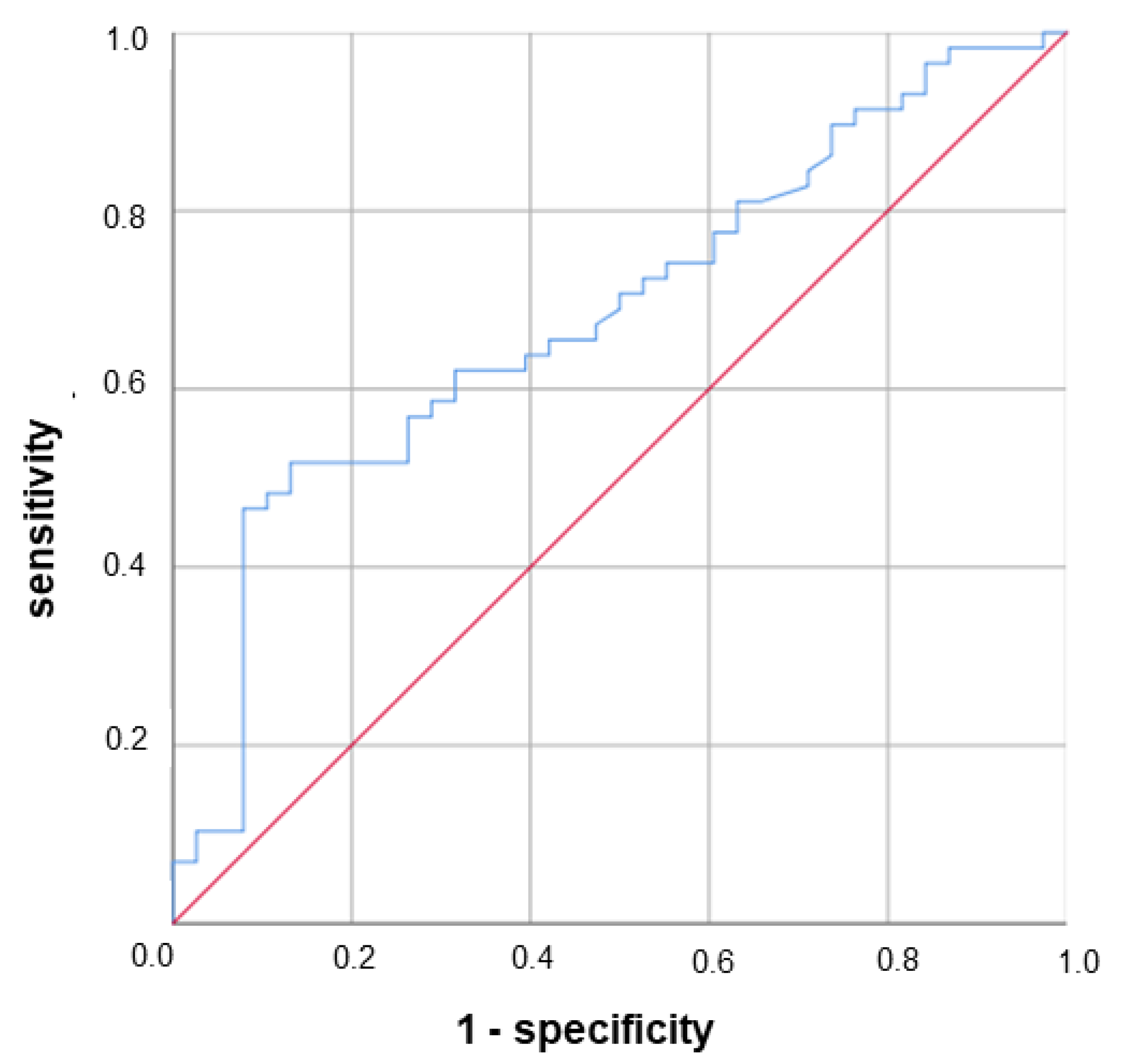
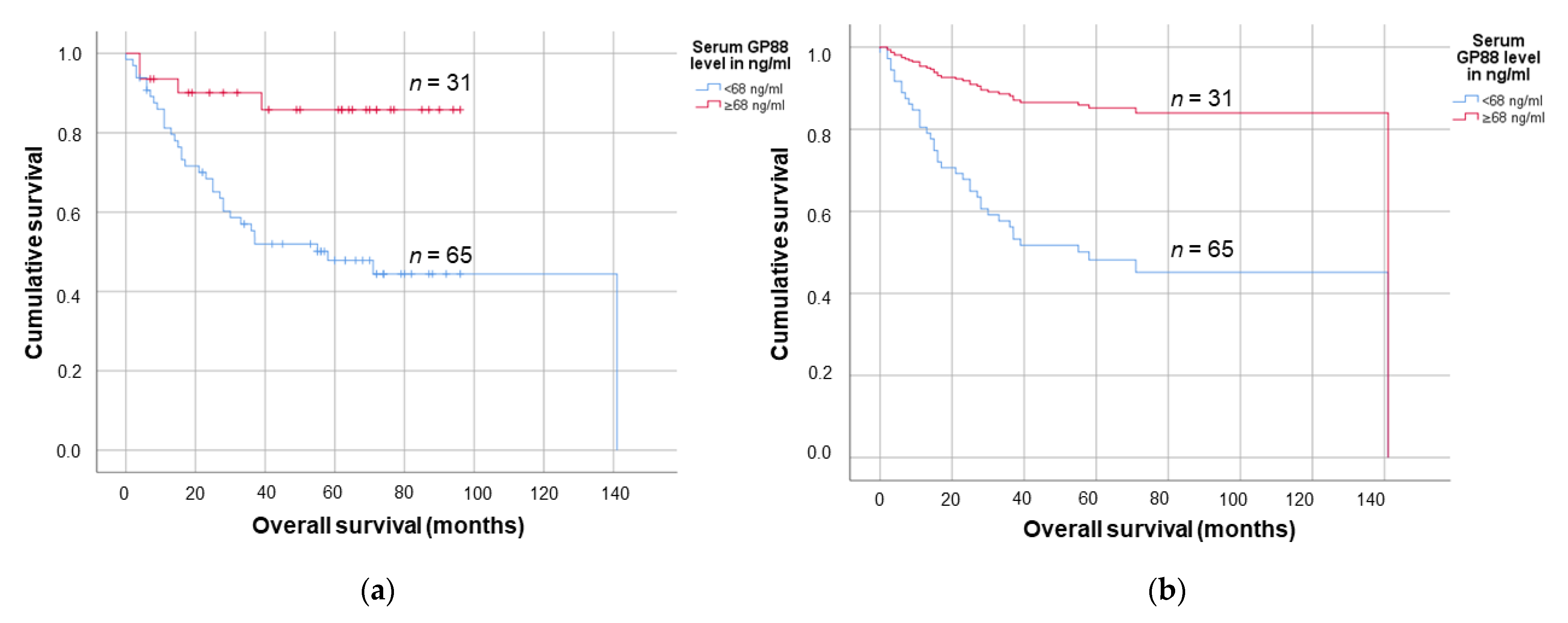
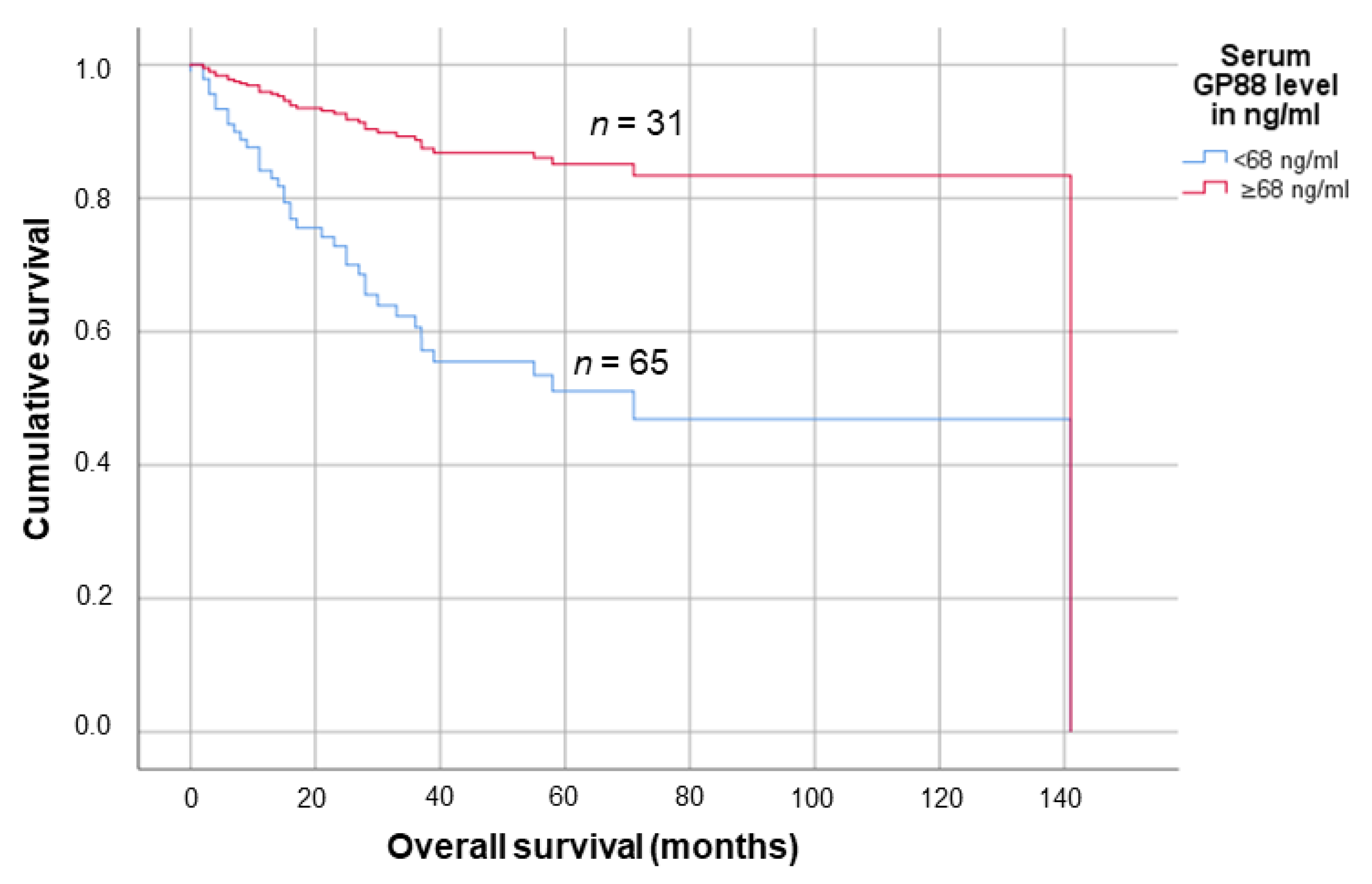
3.3. Lower Serum GP88 Levels Are Significantly Associated with Worsened Survival
3.4. Lower Serum GP88 Levels Are a Negative Prognostic Marker in Elderly OSCC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Zizzari, V.L.; Zara, S.; Tetè, G.; Vinci, R.; Gherlone, E.; Cataldi, A. Biologic and clinical aspects of integration of different bone substitutes in oral surgery: A literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Probst, F.A.; Fliefel, R.; Burian, E.; Probst, M.; Eddicks, M.; Cornelsen, M.; Riedl, C.; Seitz, H.; Aszódi, A.; Schieker, M.; et al. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds. Sci. Rep. 2020, 10, 2062. [Google Scholar] [CrossRef] [PubMed]
- Gherlone, E.F.; Capparé, P.; Tecco, S.; Polizzi, E.; Pantaleo, G.; Gastaldi, G.; Grusovin, M.G. Implant Prosthetic Rehabilitation in Controlled HIV-Positive Patients: A Prospective Longitudinal Study with 1-Year Follow-Up. Clin. Implant Dent. Relat. Res. 2016, 18, 725–734. [Google Scholar] [CrossRef]
- Fliefel, R.; Ehrenfeld, M.; Otto, S. Induced pluripotent stem cells (iPSCs) as a new source of bone in reconstructive surgery: A systematic review and meta-analysis of preclinical studies. J. Tissue Eng. Regen. Med. 2018, 12, 1780–1797. [Google Scholar] [CrossRef]
- Capparè, P.; Tetè, G.; Sberna, M.T.; Panina-Bordignon, P. The Emerging Role of Stem Cells in Regenerative Dentistry. Curr. Gene Ther. 2020, 20, 259–268. [Google Scholar] [CrossRef]
- Eckert, A.W.; Wickenhauser, C.; Salins, P.C.; Kappler, M.; Bukur, J.; Seliger, B. Clinical relevance of the tumor microenvironment and immune escape of oral squamous cell carcinoma. J. Transl. Med. 2016, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Serrero, G.; Hicks, D. Measurement of Circulating Progranulin (PGRN/GP88/GEP) by Enzyme-Linked Immunosorbent Assay and Application in Human Diseases. Methods Mol. Biol. 2018, 1806, 95–105. [Google Scholar] [CrossRef]
- Tkaczuk, K.R.; Yue, B.; Zhan, M.; Tait, N.; Yarlagadda, L.; Dai, H.; Serrero, G. Increased Circulating Level of the Survival Factor GP88 (Progranulin) in the Serum of Breast Cancer Patients When Compared to Healthy Subjects. Breast Cancer 2011, 5, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tkaczuk, K.H.R.; Hawkins, D.; Yue, B.; Hicks, D.; Tait, N.; Serrero, G. Association of Serum Progranulin Levels with Disease Progression, Therapy Response and Survival in Patients with Metastatic Breast Cancer. Clin. Breast Cancer 2020, 20, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.H.; Park, C.-Y.; Lee, E.S.; Ro, J.; Oh, S.W. Progranulin as a prognostic biomarker for breast cancer recurrence in patients who had hormone receptor-positive tumors: A cohort study. PLoS ONE 2012, 7, e39880. [Google Scholar] [CrossRef] [PubMed]
- Edelman, M.J.; Feliciano, J.; Yue, B.; Bejarano, P.; Ioffe, O.; Reisman, D.; Hawkins, D.; Gai, Q.; Hicks, D.; Serrero, G. GP88 (progranulin): A novel tissue and circulating biomarker for non-small cell lung carcinoma. Hum. Pathol. 2014, 45, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Greither, T.; Fischer, K.; Theil, G.; Marcou, M.; Holzhausen, H.-J.; Weigelt, K.; Serrero, G.; Hicks, D.; Yue, B.; Fornara, P.; et al. Expression of GP88 (progranulin) in serum of prostate cancer patients is associated with Gleason scores and overall survival. Cancer Manag. Res. 2018, 10, 4173–4180. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-X.; Kinch, M.S.; Kiener, P.A.; Langermann, S.; Serrero, G.; Le, S.; Corvera, J.; Sweeney, C.J.; Li, L.; Zhang, S.; et al. PC cell-derived growth factor expression in prostatic intraepithelial neoplasia and prostatic adenocarcinoma. Clin. Cancer Res. 2004, 10, 1333–1337. [Google Scholar] [CrossRef]
- Sleegers, K.; Brouwers, N.; van Damme, P.; Engelborghs, S.; Gijselinck, I.; van der Zee, J.; Peeters, K.; Mattheijssens, M.; Cruts, M.; Vandenberghe, R.; et al. Serum biomarker for progranulin-associated frontotemporal lobar degeneration. Ann. Neurol. 2009, 65, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Schofield, E.C.; Halliday, G.M.; Kwok, J.; Loy, C.; Double, K.L.; Hodges, J.R. Low serum progranulin predicts the presence of mutations: A prospective study. J. Alzheimers Dis. 2010, 22, 981–984. [Google Scholar] [CrossRef]
- Youn, B.-S.; Bang, S.-I.; Klöting, N.; Park, J.W.; Lee, N.; Oh, J.-E.; Pi, K.-B.; Lee, T.H.; Ruschke, K.; Fasshauer, M.; et al. Serum progranulin concentrations may be associated with macrophage infiltration into omental adipose tissue. Diabetes 2009, 58, 627–636. [Google Scholar] [CrossRef]
- Matsubara, T.; Mita, A.; Minami, K.; Hosooka, T.; Kitazawa, S.; Takahashi, K.; Tamori, Y.; Yokoi, N.; Watanabe, M.; Matsuo, E.-I.; et al. PGRN is a key adipokine mediating high fat diet-induced insulin resistance and obesity through IL-6 in adipose tissue. Cell Metab. 2012, 15, 38–50. [Google Scholar] [CrossRef]
- Tanaka, A.; Tsukamoto, H.; Mitoma, H.; Kiyohara, C.; Ueda, N.; Ayano, M.; Ohta, S.-i.; Inoue, Y.; Arinobu, Y.; Niiro, H.; et al. Serum progranulin levels are elevated in patients with systemic lupus erythematosus, reflecting disease activity. Arthritis Res. Ther. 2012, 14, R244. [Google Scholar] [CrossRef]
- Qiu, F.; Song, L.; Ding, F.; Liu, H.; Shu, Q.; Yang, N.; Liu, W.; Li, X. Expression level of the growth factor progranulin is related with development of systemic lupus erythematosus. Diagn. Pathol. 2013, 8, 88. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Takemura, M.; Serrero, G.; Hayashi, J.; Yue, B.; Tsuboi, A.; Kubo, H.; Mitsuhashi, T.; Mannami, K.; Sato, M.; et al. Increased serum GP88 (Progranulin) concentrations in rheumatoid arthritis. Inflammation 2014, 37, 1806–1813. [Google Scholar] [CrossRef]
- Cerezo, L.A.; Kuklová, M.; Hulejová, H.; Vernerová, Z.; Kaspříková, N.; Veigl, D.; Pavelka, K.; Vencovský, J.; Šenolt, L. Progranulin Is Associated with Disease Activity in Patients with Rheumatoid Arthritis. Mediat. Inflamm. 2015, 2015, 740357. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, G.; Crabb, J.W.; Serrero, G. Purification of an autocrine growth factor homologous with mouse epithelin precursor from a highly tumorigenic cell line. J. Biol. Chem. 1993, 268, 10863–10869. [Google Scholar] [CrossRef]
- He, Z.; Bateman, A. Progranulin gene expression regulates epithelial cell growth and promotes tumor growth in vivo. Cancer Res. 1999, 59, 3222–3229. [Google Scholar] [PubMed]
- Lu, R.; Serrero, G. Inhibition of PC cell-derived growth factor (PCDGF, epithelin/granulin precursor) expression by antisense PCDGF cDNA transfection inhibits tumorigenicity of the human breast carcinoma cell line MDA-MB-468. Proc. Natl. Acad. Sci. USA 2000, 97, 3993–3998. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.T.; Wong, S.Y.; Leung, K.L.; Chen, X.; So, S.; Ng, I.O.; Fan, S.T. Granulin-epithelin precursor overexpression promotes growth and invasion of hepatocellular carcinoma. Clin. Cancer Res. 2004, 10, 7629–7636. [Google Scholar] [CrossRef] [PubMed]
- Abrhale, T.; Brodie, A.; Sabnis, G.; Macedo, L.; Tian, C.; Yue, B.; Serrero, G. GP88 (PC-Cell Derived Growth Factor, progranulin) stimulates proliferation and confers letrozole resistance to aromatase overexpressing breast cancer cells. BMC Cancer 2011, 11, 231. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Liu, J.; Jiang, W.; Wen, D.; Xue, H. Effect of Progranulin on Migration and Invasion of Human Colon Cancer Cells. J. Coll. Physicians Surg. Pak. 2018, 28, 607–611. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.-L.; Dong, T.-T.; Shen, Y.-H.; Guo, X.-S.; Liu, C.-Y.; Liu, J.; Zhang, P.; Li, J.; Sun, Y.-P. Progranulin promotes colorectal cancer proliferation and angiogenesis through TNFR2/Akt and ERK signaling pathways. Am. J. Cancer Res. 2015, 5, 3085–3097. [Google Scholar]
- Kojima, Y.; Ono, K.; Inoue, K.; Takagi, Y.; Kikuta, K.-i.; Nishimura, M.; Yoshida, Y.; Nakashima, Y.; Matsumae, H.; Furukawa, Y.; et al. Progranulin expression in advanced human atherosclerotic plaque. Atherosclerosis 2009, 206, 102–108. [Google Scholar] [CrossRef]
- Yang, D.; Li, R.; Wang, H.; Wang, J.; Han, L.; Pan, L.; Li, X.; Kong, Q.; Wang, G.; Su, X. Clinical implications of progranulin in gastric cancer and its regulation via a positive feedback loop involving AKT and ERK signaling pathways. Mol. Med. Rep. 2017, 16, 9685–9691. [Google Scholar] [CrossRef]
- Ong, C.H.P.; Bateman, A. Progranulin (granulin-epithelin precursor, PC-cell derived growth factor, acrogranin) in proliferation and tumorigenesis. Histol. Histopathol. 2003, 18, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Li, C.; Zhao, T.; Li, D.; Yang, L.; Zhang, B. LncRNA H19/miR-29b-3p/PGRN Axis Promoted Epithelial-Mesenchymal Transition of Colorectal Cancer Cells by Acting on Wnt Signaling. Mol. Cells 2018, 41, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Tangkeangsirisin, W.; Serrero, G. PC cell-derived growth factor (PCDGF/GP88, progranulin) stimulates migration, invasiveness and VEGF expression in breast cancer cells. Carcinogenesis 2004, 25, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Bandey, I.; Chiou, S.-H.; Huang, A.-P.; Tsai, J.-C.; Tu, P.-H. Progranulin promotes Temozolomide resistance of glioblastoma by orchestrating DNA repair and tumor stemness. Oncogene 2015, 34, 1853–1864. [Google Scholar] [CrossRef]
- Cheung, P.F.Y.; Cheng, C.K.C.; Wong, N.C.L.; Ho, J.C.Y.; Yip, C.W.; Lui, V.C.H.; Cheung, A.N.Y.; Fan, S.T.; Cheung, S.T. Granulin-epithelin precursor is an oncofetal protein defining hepatic cancer stem cells. PLoS ONE 2011, 6, e28246. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hayashi, J.; Serrero, G. PC cell-derived growth factor confers resistance to dexamethasone and promotes tumorigenesis in human multiple myeloma. Clin. Cancer Res. 2006, 12, 49–56. [Google Scholar] [CrossRef]
- Pizarro, G.O.; Zhou, X.C.; Koch, A.; Gharib, M.; Raval, S.; Bible, K.; Jones, M.B. Prosurvival function of the granulin-epithelin precursor is important in tumor progression and chemoresponse. Int. J. Cancer 2007, 120, 2339–2343. [Google Scholar] [CrossRef]
- Zhu, J.; Nathan, C.; Jin, W.; Sim, D.; Ashcroft, G.S.; Wahl, S.M.; Lacomis, L.; Erdjument-Bromage, H.; Tempst, P.; Wright, C.D.; et al. Conversion of proepithelin to epithelins: Roles of SLPI and elastase in host defense and wound repair. Cell 2002, 111, 867–878. [Google Scholar] [CrossRef]
- Yin, F.; Banerjee, R.; Thomas, B.; Zhou, P.; Qian, L.; Jia, T.; Ma, X.; Ma, Y.; Iadecola, C.; Beal, M.F.; et al. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J. Exp. Med. 2010, 207, 117–128. [Google Scholar] [CrossRef]
- Eckert, A.W.; Horter, S.; Bethmann, D.; Kotrba, J.; Kaune, T.; Rot, S.; Bache, M.; Bilkenroth, U.; Reich, W.; Greither, T.; et al. Investigation of the Prognostic Role of Carbonic Anhydrase 9 (CAIX) of the Cellular mRNA/Protein Level or Soluble CAIX Protein in Patients with Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 375. [Google Scholar] [CrossRef]
- Rot, S.; Kaune, T.; Taubert, H.; Greither, T.; Kotrba, J.; Güttler, A.; Wichmann, H.; Bilkenroth, U.; Wienke, A.; Al-Nawas, B.; et al. Prognostic impact of mRNA levels of LGR5 transcript variants in OSCC patients. BMC Cancer 2019, 19, 155. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Goto, N.; Takemura, M.; Yamasuge, W.; Yabe, K.; Takami, T.; Miyazaki, T.; Takeuchi, T.; Shiraki, M.; Shimizu, M.; et al. Association between increased serum GP88 (progranulin) concentrations and prognosis in patients with malignant lymphomas. Clin. Chim. Acta 2017, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, G.; Yin, J.; Lin, T.; Zhang, J. Progranulin overexpression predicts overall survival in patients with glioblastoma. Med. Oncol. 2012, 29, 2423–2431. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, N.; Chen, Q.; Qiu, F.; Li, X. Upregulated expression level of the growth factor, progranulin, is associated with the development of primary Sjögren’s syndrome. Exp. Ther. Med. 2014, 8, 1643–1647. [Google Scholar] [CrossRef]
- Qi, X.; Guo, H.; Sun, C.; Tian, Y.; Ding, M.; Yang, Y.; Jin, H. Clinical significance of progranulin correlated with serum soluble Oxford 40 ligand in primary Sjögren’s syndrome. Medicine 2020, 99, e19967. [Google Scholar] [CrossRef]
- Han, J.J.; Yu, M.; Houston, N.; Steinberg, S.M.; Kohn, E.C. Progranulin is a potential prognostic biomarker in advanced epithelial ovarian cancers. Gynecol. Oncol. 2011, 120, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.M.; Maurer, M.J.; Goergen, K.M.; Kalli, K.R.; Erskine, C.L.; Behrens, M.D.; Knutson, K.L.; Block, M.S. Utility of progranulin and serum leukocyte protease inhibitor as diagnostic and prognostic biomarkers in ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1730–1735. [Google Scholar] [CrossRef]
- Göbel, M.; Eisele, L.; Möllmann, M.; Hüttmann, A.; Johansson, P.; Scholtysik, R.; Bergmann, M.; Busch, R.; Döhner, H.; Hallek, M.; et al. Progranulin is a novel independent predictor of disease progression and overall survival in chronic lymphocytic leukemia. PLoS ONE 2013, 8, e72107. [Google Scholar] [CrossRef] [PubMed]
- El-Ghammaz, A.M.S.; Azzazi, M.O.; Mostafa, N.; Hegab, H.M.; Mahmoud, A.A. Prognostic significance of serum progranulin level in de novo adult acute lymphoblastic leukemia patients. Clin. Exp. Med. 2020, 20, 269–276. [Google Scholar] [CrossRef]
- Luo, Q.; He, X.; Zheng, Y.; Ning, P.; Xu, Y.; Yang, D.; Shang, Y.; Gao, Z. Elevated progranulin as a novel biomarker to predict poor prognosis in community-acquired pneumonia. J. Infect. 2020, 80, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Dong, T.; Yang, D.; Gao, A.; Luo, J.; Yang, H.; Wang, L. Progranulin promotes lymphangiogenesis through VEGF-C and is an independent risk factor in human esophageal cancers. Hum. Pathol. 2018, 75, 116–124. [Google Scholar] [CrossRef]
- Taghavi, N.; Yazdi, I. Prognostic factors of survival rate in oral squamous cell carcinoma: Clinical, histologic, genetic and molecular concepts. Arch. Iran. Med. 2015, 18, 314–319. [Google Scholar] [PubMed]
- Ong, W.; Zhao, R.; Lui, B.; Tan, W.; Ebrahimi, A.; Clark, J.R.; Soo, K.-C.; Tan, N.-C.; Tan, H.-K.; Iyer, N.G. Prognostic significance of lymph node density in squamous cell carcinoma of the tongue. Head Neck 2016, 38 (Suppl. 1), E859–E866. [Google Scholar] [CrossRef]
- Tang, W.; Lu, Y.; Tian, Q.-Y.; Zhang, Y.; Guo, F.-J.; Liu, G.-Y.; Syed, N.M.; Lai, Y.; Lin, E.A.; Kong, L.; et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 2011, 332, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zuo, Z.; Chen, Q.; Li, J.; Tang, W.; Yang, P. Progranulin is highly expressed in patients with chronic periodontitis and protects against experimental periodontitis in rats. J. Periodontol. 2018, 89, 1418–1427. [Google Scholar] [CrossRef]
- Lee, L.T.; Wong, Y.K.; Hsiao, H.Y.; Wang, Y.W.; Chan, M.Y.; Chang, K.W. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2018, 47, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, G.; Nandan, S.R.K.; Kulkarni, P.G. Salivary Tumour Necrosis Factor-α as a Biomarker in Oral Leukoplakia and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2019, 20, 2087–2093. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Wang, C.-J.; Xie, K.; Lei, M.; Chai, Y.-S.; Xu, F.; Lin, S.-H. Progranulin Improves Acute Lung Injury through Regulating the Differentiation of Regulatory T Cells and Interleukin-10 Immunomodulation to Promote Macrophage Polarization. Mediat. Inflamm. 2020, 2020, 9704327. [Google Scholar] [CrossRef]
- Jian, J.; Konopka, J.; Liu, C. Insights into the role of progranulin in immunity, infection, and inflammation. J. Leukoc. Biol. 2013, 93, 199–208. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Grimm, W.A.; Garner, W.L.; Qin, L.; Travis, T.; Tan, N.; Han, Y.-P. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-alpha through bone morphogenic protein-2. Am. J. Pathol. 2010, 176, 2247–2258. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, J.; Tang, Y.; Liang, X. Inflammation linking EMT and cancer stem cells. Oral Oncol. 2012, 48, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Dikova, V.R.; Principe, S.; Bagan, J.V. Salivary inflammatory proteins in patients with oral potentially malignant disorders. J. Clin. Exp. Dent. 2019, 11, e659–e664. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, H.; Song, A.; Huang, J.; Zhang, Y.; Jin, S.; Li, S.; Zhang, L.; Yang, C.; Yang, P. Progranulin inhibits LPS-induced macrophage M1 polarization via NF-кB and MAPK pathways. BMC Immunol. 2020, 21, 32. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Subgroup | Serum GP88 < 68 ng/mL | Serum GP88 ≥ 68 ng/mL | n | p |
|---|---|---|---|---|---|
| age | <60.5 | 33 | 16 | 49 | 0.39 |
| ≥60.5 | 32 | 15 | 47 | ||
| sex | male | 46 | 23 | 69 | 0.73 |
| female | 19 | 8 | 27 | ||
| patient status | alive | 31 | 27 | 58 | <0.001 |
| deceased | 34 | 4 | 38 | ||
| T stage | T1 | 12 | 9 | 21 | 0.44 |
| T2 | 22 | 12 | 34 | ||
| T3 | 16 | 4 | 20 | ||
| T4 | 15 | 6 | 21 | ||
| N stage | N0 | 38 | 22 | 60 | 0.50 |
| N1 | 10 | 2 | 12 | ||
| N2 | 16 | 7 | 23 | ||
| N3 | 1 | 0 | 1 | ||
| M | M0 | 64 | 31 | 95 | 0.49 |
| M1 | 1 | 0 | 1 | ||
| Grading | 1 | 10 | 4 | 14 | 0.93 |
| 2 | 40 | 19 | 59 | ||
| 3 | 15 | 8 | 23 | ||
| radiotherapy | no | 43 | 27 | 70 | 0.03 |
| yes | 22 | 4 | 26 | ||
| chemotherapy | no | 54 | 30 | 84 | 0.06 |
| yes | 11 | 1 | 12 |
| OSCC Patients | GP88 Levels | Univariate Cox Regression Analysis | Multivariate Cox Regression Analysis | ||||
|---|---|---|---|---|---|---|---|
| p | RR (95% CI) | n | p | RR (95% CI) | n | ||
| OSCC patients < 60.5 years | Serum GP88 level < 68.0 ng/mL | 0.118 | 3.26 (0.74–14.39) | 33 | 0.261 | 2.4 (0.52–11.24) | 33 |
| Serum GP88 level ≥ 68.0 ng/mL | reference | 16 | reference | 16 | |||
| OSCC patients ≥ 60.5 years | Serum GP88 level < 65.0 ng/mL | 0.019 | 5.74 (1.33–24.72) | 32 | 0.027 | 5.45 (1.22–24.39) | 32 |
| Serum GP88 level ≥ 65.0 ng/mL | reference | 15 | reference | 15 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greither, T.; Steiner, T.; Bache, M.; Serrero, G.; Otto, S.; Taubert, H.; Eckert, A.W.; Kappler, M. GP88/PGRN Serum Levels Are Associated with Prognosis for Oral Squamous Cell Carcinoma Patients. Biology 2021, 10, 400. https://doi.org/10.3390/biology10050400
Greither T, Steiner T, Bache M, Serrero G, Otto S, Taubert H, Eckert AW, Kappler M. GP88/PGRN Serum Levels Are Associated with Prognosis for Oral Squamous Cell Carcinoma Patients. Biology. 2021; 10(5):400. https://doi.org/10.3390/biology10050400
Chicago/Turabian StyleGreither, Thomas, Tina Steiner, Matthias Bache, Ginette Serrero, Sven Otto, Helge Taubert, Alexander W. Eckert, and Matthias Kappler. 2021. "GP88/PGRN Serum Levels Are Associated with Prognosis for Oral Squamous Cell Carcinoma Patients" Biology 10, no. 5: 400. https://doi.org/10.3390/biology10050400
APA StyleGreither, T., Steiner, T., Bache, M., Serrero, G., Otto, S., Taubert, H., Eckert, A. W., & Kappler, M. (2021). GP88/PGRN Serum Levels Are Associated with Prognosis for Oral Squamous Cell Carcinoma Patients. Biology, 10(5), 400. https://doi.org/10.3390/biology10050400







