Use of PRP, PRF and CGF in Periodontal Regeneration and Facial Rejuvenation—A Narrative Review
Abstract
Simple Summary
Abstract
1. Introduction
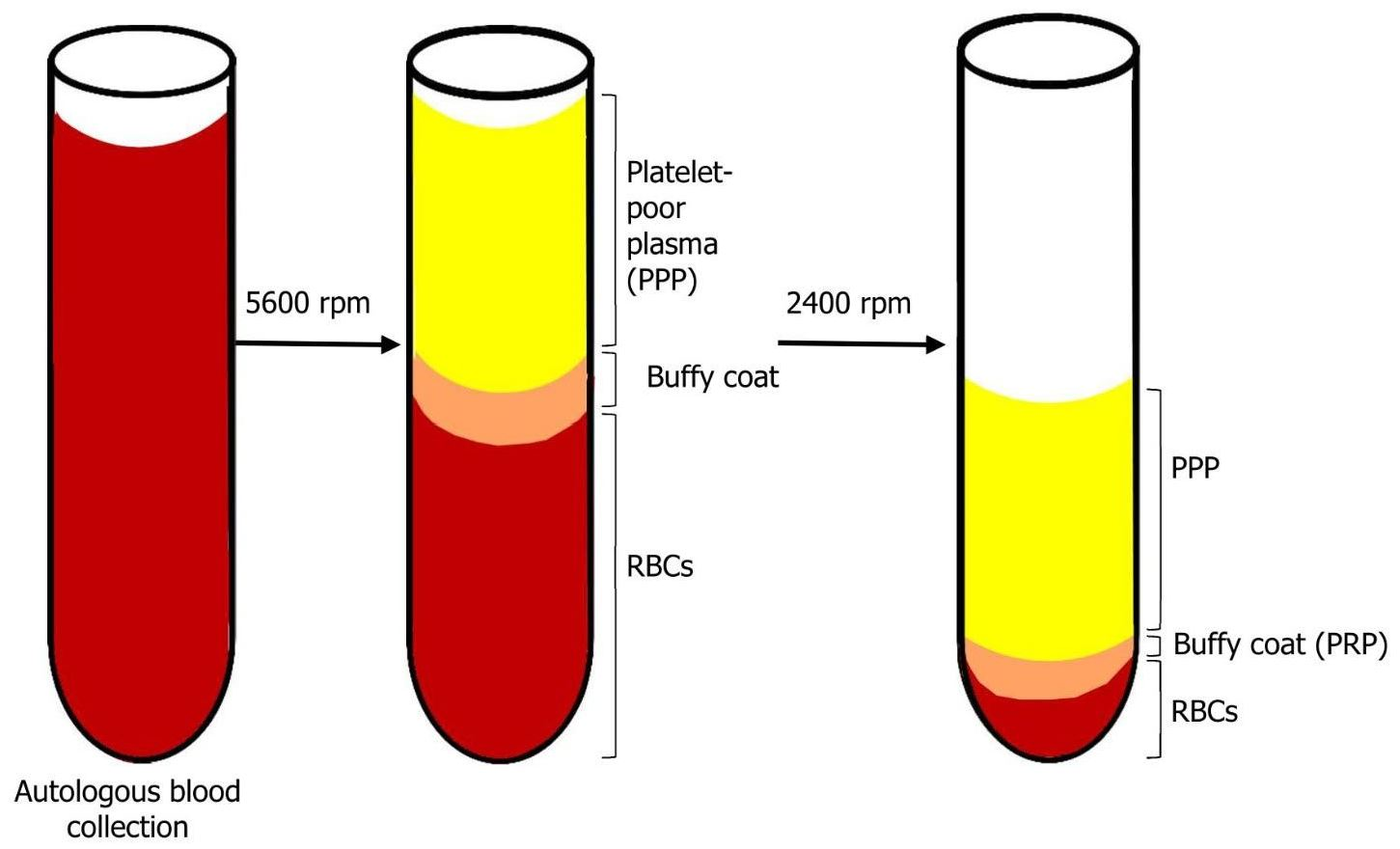
1.1. Platelet-Rich Plasma (PRP)
1.2. The Technique
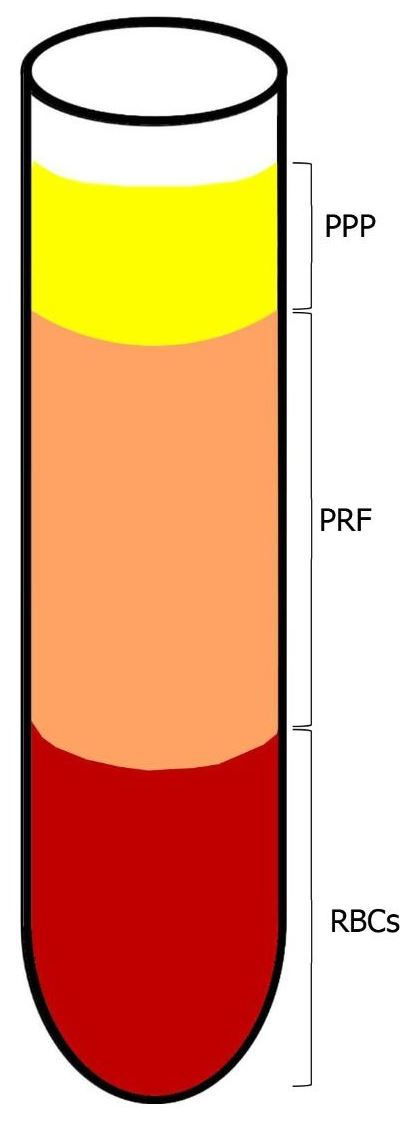
1.3. Platelet-Rich Fibrin (PRF)—The Second Generation of Platelet Concentrates
1.4. The Technique
1.5. Injectable PRF
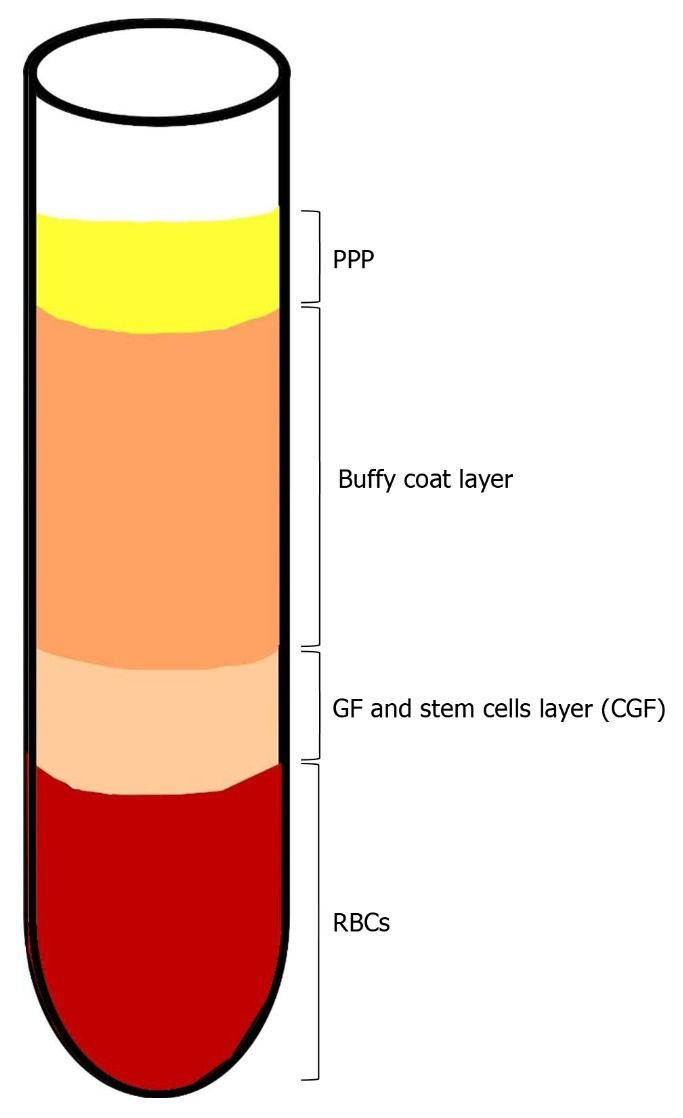
1.6. Concentrated Growth Factor (CGF): The Newest Platelet Concentrate
1.7. The Technique
1.8. Periodontal Regeneration
1.9. Facial Rejuvenation
2. Materials and Methods
- Study design: randomized controlled trials (RCTs), cohort studies, cross-sectional studies only in the English language;
- Population: only studies on humans, with a minimum sample size of 10 patients and no restriction in terms of patient ages;
- Intervention: regenerative periodontal surgery on interproximal bony defects (IBD) and furcation defects (FD);
- Types of outcome: probing pocket depth recovery (PPDR) and clinical attachment level gain (CALG).
- Absence of baseline data before periodontal surgery;
- Patients with systemic diseases or craniofacial anomalies;
- No training in oral hygiene;
- Follow-up < 6 months.
- Study design: randomized controlled trials (RCTs), cohort studies, cross-sectional studies, case reports and case series only in the English language;
- Population: only studies on humans; for CGF, studies on animals were included;
- Intervention: facial skin rejuvenation, facial wrinkles, atrophic acne scars;
- Types of outcome: clinical or histologic evaluation.
- Hair and nail restoration;
- Patients with alopecia;
- Review articles.
3. Results
3.1. PRP
3.2. PRF
3.3. CGF
3.4. Facial Rejuvenation
4. Discussion
4.1. Periodontal Regeneration
4.2. Facial Rejuvenation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cakir, S.; Gultekin, B.A.; Karabagli, M.; Yilmaz, T.E.; Cakir, E.; Guzel, E.E.; Yalcin, S.; Mortellaro, C.; Mijiritsky, E. Histological Evaluation of the Effects of Growth Factors in a Fibrin Network on Bone Regeneration. J. Craniofacial Surg. 2019, 30, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; An, N.; Ouyang, X. Quantification of growth factors in different platelet concentrates. Platelets 2017, 28, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.; Roberts, W. Incorporating facial rejuvenation into the dental practice. Dent. Today 2010, 29, 29. [Google Scholar]
- Kraft, D.C.E.; Bindslev, D.A.; Melsen, B.; Klein-Nulend, J. Human dental pulp cells exhibit bone cell-like responsiveness to fluid shear stress. Cytotherapy 2011, 13, 214–226. [Google Scholar] [CrossRef]
- Marrelli, M.; Codispoti, B.; Shelton, R.M.; Scheven, B.A.; Cooper, P.R.; Tatullo, M.; Paduano, F. Dental Pulp Stem Cell Mechanoresponsiveness: Effects of Mechanical Stimuli on Dental Pulp Stem Cell Behavior. Front. Physiol. 2018, 9, 1685. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Loison-Robert, L.S.; Tassin, M.; Bonte, E.; Berbar, T.; Isaac, J.; Berdal, A.; Simon, S.; Fournier, B.P.J. In vitro effects of two silicate-based materials, Biodentine and BioRoot RCS, on dental pulp stem cells in models of reactionary and reparative dentinogenesis. PLoS ONE 2018, 13, e0190014. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Falisi, G.; Rastelli, C.; Palmieri, F.; Gargari, M.; Zavan, B.; Paduano, F.; Benagiano, V. Mechanical influence of tissue culture plates and extracellular matrix on mesenchymal stem cell behavior: A topical review. Int. J. Immunopathol. Pharmacol. 2015, 29, 3–8. [Google Scholar] [CrossRef]
- Spagnuolo, G.; Codispoti, B.; Marrelli, M.; Rengo, C.; Rengo, S.; Tatullo, M. Commitment of Oral-Derived Stem Cells in Dental and Maxillofacial Applications. Dent. J. 2018, 6, 72. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Paduano, F. The Regenerative Medicine in Oral and Maxillofacial Surgery: The Most Important Innovations in the Clinical Application of Mesenchymal Stem Cells. Int. J. Med. Sci. 2015, 12, 72–77. [Google Scholar] [CrossRef]
- Ballini, A.; Boccaccio, A.; Saini, R.; Van Pham, P.; Tatullo, M. Dental-Derived Stem Cells and Their Secretome and Interactions with Bioscaffolds/Biomaterials in Regenerative Medicine: From the In Vitro Research to Translational Applications. Stem Cells Int. 2017, 2017, 1–3. [Google Scholar] [CrossRef]
- Tomar, G.B.; Srivastava, R.K.; Gupta, N.; Barhanpurkar, A.P.; Pote, S.T.; Jhaveri, H.M.; Mishra, G.C.; Wani, M.R. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem. Biophys. Res. Commun. 2010, 393, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Whitman, D.H.; Berry, R.L.; Green, D.M. Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J. Oral Maxillofac. Surg. 1997, 55, 1294–1299. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e37–e44. [Google Scholar] [CrossRef]
- Sacco, L. Lecture. In International Academy of Implant Prosthesis and Osteoconnection; Lecture: Sersale, Italy, 2006. [Google Scholar]
- Fang, D.; Long, Z.; Hou, J. Clinical Application of Concentrated Growth Factor Fibrin Combined with Bone Repair Materials in Jaw Defects. J. Oral Maxillofac. Surg. 2020, 78, 882–892. [Google Scholar] [CrossRef]
- Wroblewski, A.P.; Mejia, H.A.; Wright, V.J. Application of Platelet-Rich Plasma to Enhance Tissue Repair. Oper. Tech. Orthop. 2010, 20, 98–105. [Google Scholar] [CrossRef]
- E Marx, R.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; E Strauss, J.; Georgeff, K.R. Platelet-rich plasma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Marques, F.P.; Ingham, S.J.M.; Forgas, A.; Franciozi, C.E.D.S.; Sasaki, P.H.; Abdalla, R.J. A manual method to obtain platelet rich plasma. Acta Ortopédica Bras. 2014, 22, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.R.; Sándor, G.K. Use of fibrin glue in maxillofacial surgery. J. Otolaryngol. 1998, 27, 107–112. [Google Scholar]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e51–e55. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e56–e60. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Ghanaati, S.; Choukroun, J. Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry? Clin. Oral Investig. 2017, 21, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Miron, R.J.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Choukroun, J. Optimized Platelet-Rich Fibrin with the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J. Periodontol. 2017, 88, 112–121. [Google Scholar] [CrossRef]
- Wang, L.; Wan, M.; Li, Z.; Zhong, N.; Liang, D.; Ge, L. A comparative study of the effects of concentrated growth factors in two different forms on osteogenesis in vitro. Mol. Med. Rep. 2019, 20, 1039–1048. [Google Scholar] [CrossRef]
- Doğan Şeyma, B.; Dede, F.O.; Balli, U.; Atalay, E.N.; Durmuşlar, M.C. Concentrated Growth Factor in the Treatment of Adjacent Multiple Gingival Recessions: A Split-Mouth Randomized Clinical Trial. J. Clin. Periodontol. 2015, 42, 868–875. [Google Scholar] [CrossRef]
- Kornman, K.S. Mapping the Pathogenesis of Periodontitis: A New Look. J. Periodontol. 2008, 79, 1560–1568. [Google Scholar] [CrossRef]
- Smiley, C.J.; Tracy, S.L.; Abt, E.; Michalowicz, B.S.; John, M.T.; Gunsolley, J.; Cobb, C.M.; Rossmann, J.; Harrel, S.K.; Forrest, J.L.; et al. Evidence-based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J. Am. Dent. Assoc. 2015, 146, 525–535. [Google Scholar] [CrossRef]
- Lindhe, J.; Schallhorn, R.; Bowers, G.; Garrett, S.; Becker, B.; Cortellini, P.; Ferris, R.; Karring, T.; McClain, P.; O’Neal, R.; et al. Periodontal Regeneration around Natural Teeth. J. Am. Dent. Assoc. 1998, 129, 43. [Google Scholar] [CrossRef]
- Gottlow, J.; Nyman, S.; Karring, T.; Lindhe, J. New attachment formation as the result of controlled tissue regeneration. J. Clin. Periodontol. 1984, 11, 494–503. [Google Scholar] [CrossRef]
- Larsson, L.; Decker, A.; Nibali, L.; Pilipchuk, S.; Berglundh, T.; Giannobile, W. Regenerative Medicine for Periodontal and Peri-implant Diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef]
- Kim, D.H.; Je, Y.J.; Kim, C.D.; Lee, Y.H.; Seo, Y.J.; Lee, J.H. Can Platelet-rich Plasma Be Used for Skin Rejuvenation? Evaluation of Effects of Platelet-rich Plasma on Human Dermal Fibroblast. Ann. Dermatol. 2011, 23, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Rophael, J.A.; Craft, R.O.; Palmer, J.A.; Hussey, A.J.; Thomas, G.P.; Morrison, W.A.; Penington, A.J.; Mitchell, G.M. Angiogenic Growth Factor Synergism in a Murine Tissue Engineering Model of Angiogenesis and Adipogenesis. Am. J. Pathol. 2007, 171, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.; Miron, J.C. Platelet Rich Fibrin in Regenerative Dentistry: Biological Background and Clinical Indications; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Hu, Y.; Jiang, Y.; Wang, M.; Tian, W.; Wang, H. Concentrated Growth Factor Enhanced Fat Graft Survival: A Comparative Study. Dermatol. Surg. 2018, 44, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Trejo, P.M.; Weltman, R.L. Treatment of Intrabony Defects with Bovine-Derived Xenograft Alone and in Combination with Platelet-Rich Plasma: A Randomized Clinical Trial. J. Periodontol. 2004, 75, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Camargo, P.M.; Lekovic, V.; Weinlaender, M.; Vasilic, N.; Madzarevic, M.; Kenney, E.B. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J. Periodontal Res. 2002, 37, 300–306. [Google Scholar] [CrossRef]
- Leković, V.; Camargo, P.M.; Weinlaender, M.; Vasilic, N.; Kenney, E.B. Comparison of Platelet-Rich Plasma, Bovine Porous Bone Mineral, and Guided Tissue Regeneration Versus Platelet-Rich Plasma and Bovine Porous Bone Mineral in the Treatment of Intrabony Defects: A Reentry Study. J. Periodontol. 2002, 73, 198–205. [Google Scholar] [CrossRef]
- Harnack, L.; Boedeker, R.H.; Kurtulus, I.; Boehm, S.; Gonzales, J.; Meyle, J. Use of platelet-rich plasma in periodontal surgery—a prospective randomised double blind clinical trial. Clin. Oral Investig. 2008, 13, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Döri, F.; Húszár, T.; Nikolidakis, D.; Arweiler, N.B.; Gera, I.; Sculean, A. Effect of Platelet-Rich Plasma on the Healing of Intrabony Defects Treated with an Anorganic Bovine Bone Mineral and Expanded Polytetrafluoroethylene Membranes. J. Periodontol. 2007, 78, 983–990. [Google Scholar] [CrossRef]
- Döri, F.; Kovács, V.; Arweiler, N.B.; Húszár, T.; Gera, I.; Nikolidakis, D.; Sculean, A. Effect of Platelet-Rich Plasma on the Healing of Intrabony Defects Treated with an Anorganic Bovine Bone Mineral: A Pilot Study. J. Periodontol. 2009, 80, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, M.; Aspriello, S.D.; Rubini, C.; Ferrante, L.; Procaccini, M. Treatment of Periodontal Intrabony Defects with Demineralized Freeze-Dried Bone Allograft in Combination with Platelet-Rich Plasma: A Comparative Clinical Trial. J. Periodontol. 2008, 79, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Karp, J.M.; Sarraf, F.; Shoichet, M.S.; Davies, J.E. Fibrin-filled scaffolds for bone-tissue engineering: Anin vivo study. J. Biomed. Mater. Res. 2004, 71, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Nowzari, H.; Rich, S.K. Risk of Prion Disease Transmission through Bovine-Derived Bone Substitutes: A Systematic Review. Clin. Implant. Dent. Relat. Res. 2011, 15, 645–653. [Google Scholar] [CrossRef]
- Landesberg, R.; Moses, M.; Karpatkin, M. Risks of using platelet rich plasma gel. J. Oral Maxillofac. Surg. 1998, 56, 1116–1117. [Google Scholar] [CrossRef]
- Lekovic, V.; Milinkovic, I.; Aleksic, Z.; Jankovic, S.; Stankovic, P.; Kenney, E.B.; Camargo, P.M. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J. Periodontal Res. 2012, 47, 409–417. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Pai, S.; Garg, G.; Devi, P.; Shetty, S.K. A randomized clinical trial of autologous platelet-rich plasma in the treatment of mandibular degree II furcation defects. J. Clin. Periodontol. 2009, 36, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pradeep, A. Treatment of 3-Wall Intrabony Defects in Patients with Chronic Periodontitis with Autologous Platelet-Rich Fibrin: A Randomized Controlled Clinical Trial. J. Periodontol. 2011, 82, 1705–1712. [Google Scholar] [CrossRef]
- Thorat, M.; Pradeep, A.R.; Pallavi, B. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: A controlled clinical trial. J. Clin. Periodontol. 2011, 38, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Gaekwad, S.S.; Gujjari, S.K.; Veerendra Kumar, S.C.; Veerendra Kumar, S.C. Platelet-Rich Fibrin in Regeneration of Intrabony Defects: A Randomized Controlled Trial. J. Periodontol. 2017, 88, 1192–1199. [Google Scholar] [CrossRef]
- Bajaj, P.; Agarwal, E.; Rao, N.S.; Naik, S.B.; Pradeep, A.; Kalra, N.; Priyanka, N.; Kumari, M. Autologous Platelet-Rich Fibrin in the Treatment of 3-Wall Intrabony Defects in Aggressive Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pradeep, A.R. Autologous Platelet-Rich Fibrin in the Treatment of Mandibular Degree II Furcation Defects: A Randomized Clinical Trial. J. Periodontol. 2011, 82, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Arabacı, T.; Kose, O.; Albayrak, M.; Cicek, Y.; Kizildag, A.; Arabaci, T. Advantages of Autologous Platelet-Rich Fibrin Membrane on Gingival Crevicular Fluid Growth Factor Levels and Periodontal Healing: A Randomized Split-Mouth Clinical Study. J. Periodontol. 2017, 88, 771–777. [Google Scholar] [CrossRef]
- Turkal, H.A.; Demirer, S.; Dolgun, A.B.; Keceli, H.G.; Turkal, H.A. Evaluation of the adjunctive effect of platelet-rich fibrin to enamel matrix derivative in the treatment of intrabony defects. Six-month results of a randomized, split-mouth, controlled clinical study. J. Clin. Periodontol. 2016, 43, 955–964. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Bajaj, P.; Rao, N.S.; Agarwal, E.; Naik, S.B. Platelet-Rich Fibrin Combined with a Porous Hydroxyapatite Graft for the Treatment of 3-Wall Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 1288–1296. [Google Scholar] [CrossRef]
- Pradeep, A.; Rao, N.S.; Agarwal, E.; Bajaj, P.; Kumari, M.; Naik, S.B. Comparative Evaluation of Autologous Platelet-Rich Fibrin and Platelet-Rich Plasma in the Treatment of 3-Wall Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2012, 83, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Pradeep, A.R.; Agarwal, E.; Rao, N.S.; Naik, S.B.; Priyanka, N.; Kalra, N. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of mandibular degree II furcation defects: A randomized controlled clinical trial. J. Periodontal Res. 2013, 48, 573–581. [Google Scholar] [CrossRef]
- He, L.; Lin, Y.; Hu, X.; Zhang, Y.; Wu, H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 707–713. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, J.; Sun, Q.; Yan, S.; Wang, W.; Yang, P.; Song, A. One-Year Results Evaluating the Effects of Concentrated Growth Factors on the Healing of Intrabony Defects Treated with or without Bone Substitute in Chronic Periodontitis. Med. Sci. Monit. 2019, 25, 4384–4389. [Google Scholar] [CrossRef]
- Sohn, D.-S.; Heo, J.-U.; Kwak, D.-H.; Kim, D.-E.; Kim, J.-M.; Moon, J.-W.; Lee, J.-H.; Park, I.-S. Bone Regeneration in the Maxillary Sinus Using an Autologous Fibrin-Rich Block with Concentrated Growth Factors Alone. Implant. Dent. 2011, 20, 389–395. [Google Scholar] [CrossRef]
- Cameli, N.; Mariano, M.; Cordone, I.; Abril, E.; Masi, S.; Foddai, M.L. Autologous Pure Platelet-Rich Plasma Dermal Injections for Facial Skin Rejuvenation: Clinical, Instrumental, and Flow Cytometry Assessment. Dermatol. Surg. 2017, 43, 826–835. [Google Scholar] [CrossRef]
- Gawdat, H.I.; Hegazy, R.A.; Fawzy, M.M.; Fathy, M. Autologous Platelet Rich Plasma: Topical Versus Intradermal After Fractional Ablative Carbon Dioxide Laser Treatment of Atrophic Acne Scars. Dermatol. Surg. 2014, 40, 152–161. [Google Scholar] [CrossRef]
- Sclafani, A.P. Platelet-rich fibrin matrix for improvement of deep nasolabial folds. J. Cosmet. Dermatol. 2010, 9, 66–71. [Google Scholar] [CrossRef]
- Hassan, H.; Quinlan, D.J.; Ghanem, A. Injectable platelet-rich fibrin for facial rejuvenation: A prospective, single-center study. J. Cosmet. Dermatol. 2020, 19, 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, C.; Frustaci, I.; Armellin, E.; Condò, R.; Arcuri, C.; Cerroni, L. Autologous blood preparations rich in platelets, fibrin and growth factors. ORAL Implantol. 2015, 8, 96–113. [Google Scholar]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.-Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant. Dent. 2016, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, A.H.; Belal, S.M. Clinical and Radiographic Evaluation of Socket Preservation Using Autologous Concentrated Growth Factors Enriched Bone Graft Matrix (Sticky Bone): A Case Report. EC Dent. Sci. 2016, 5, 1128–1135. [Google Scholar]
- Shyu, S.-S.; Fu, E.; Shen, E.-C. Clinical and Microcomputed Topography Evaluation of the Concentrated Growth Factors as a Sole Material in a Cystic Bony Defect in Alveolar Bone Followed by Dental Implantation. Implant. Dent. 2016, 25, 707–714. [Google Scholar] [CrossRef]
- Kim, J.-M.; Sohn, D.-S.; Bae, M.-S.; Moon, J.-W.; Lee, J.-H.; Park, I.-S. Flapless Transcrestal Sinus Augmentation Using Hydrodynamic Piezoelectric Internal Sinus Elevation with Autologous Concentrated Growth Factors Alone. Implant. Dent. 2014, 23, 168–174. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, Z.; Zheng, D.; Lin, P.; Cai, Y.; Hong, S.; Lai, Y.; Wu, D. Inlay osteotome sinus floor elevation with concentrated growth factor application and simultaneous short implant placement in severely atrophic maxilla. Sci. Rep. 2016, 6, 27348. [Google Scholar] [CrossRef]
- Tadic, A.; Puskar, T.; Petronijevic, B. Application of fibrin rich blocks with concentrated growth factors in pre-implant augmentation procedures. Med. Pregl. 2014, 67, 177–180. [Google Scholar] [CrossRef]
- Gheno, E.; Palermo, A.; Rodella, L.F.; Buffoli, B. The effectiveness of the use of xenogeneic bone blocks mixed with autologous Concentrated Growth Factors (CGF) in bone regeneration techniques: A case series. J. Osseointegration 2014, 6, 37–42. [Google Scholar] [CrossRef]
- Akcan, S.K.; Ünsal, B. Gingival recession treatment with concentrated growth factor membrane: A comparative clinical trial. J. Appl. Oral. Sci. 2020, 28, e20190236. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, T.S. Concentrated Growth Factor Membrane—A Novel Barrier for Accelerated Repair of Gingival Defect Along with Sliding Flap Technique. Int. J. Curr. Res. Rev. 2016, 8, 1–5. [Google Scholar]
- Li, X.; Yang, H.; Zhang, Z.; Yan, Z.; Lv, H.; Zhang, Y.; Wu, B. Concentrated growth factor exudate enhances the proliferation of human periodontal ligament cells in the presence of TNF α. Mol. Med. Rep. 2018, 19, 943–950. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Yu, L.; Zhou, J.; Zheng, D.; Zhang, B. Effect of Concentrated Growth Factor (CGF) on the Promotion of Osteogenesis in Bone Marrow Stromal Cells (BMSC) in vivo. Sci. Rep. 2018, 8, 5876. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; Wang, Z. A comparative study of the effect of Bio-Oss® in combination with concentrated growth factors or bone marrow-derived mesenchymal stem cells in canine sinus grafting. J. Oral Pathol. Med. 2016, 46, 528–536. [Google Scholar] [CrossRef]
- Sohn, D.-S.; Huang, B.; Kim, J.; Park, W.E.; Park, C.C. Utilization of Autologous Concentrated Growth Factors (CGF) Enriched Bone Graft Matrix (Sticky Bone) and CGF-Enriched Fibrin Membrane in Implant Dentistry. J. Implant Adv. Clin. Dent. 2015, 7, 17–29. [Google Scholar]
- Koyuncu, B.Ö.; Çelik, K.I.; Yüce, M.Ö.; Günbay, T.; Çömlekoğlu, M.E. The role of concentrated growth factor on implant stability: A preliminary study. J. Stomatol. Oral. Maxillofac. Surg. 2020, 121, 363–367. [Google Scholar] [CrossRef]
- Isler, S.C.; Soysal, F.; Ceyhanlı, T.; Bakırarar, B.; Unsal, B. Regenerative surgical treatment of peri-implantitis using either a collagen membrane or concentrated growth factor: A 12-month randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2018, 20, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Samadi, P.; Sheykhhasan, M.; Khoshinani, H.M. The Use of Platelet-Rich Plasma in Aesthetic and Regenerative Medicine: A Comprehensive Review. Aesthetic Plast. Surg. 2019, 43, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Mayes, A.E.; Murray, P.G.; A Gunn, D.; Tomlin, C.C.; Catt, S.D.; Wen, Y.B.; Zhou, L.P.; Wang, H.Q.; Catt, M.; Granger, S.P. Ageing appearance in China: Biophysical profile of facial skin and its relationship to perceived age. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, F.; Liborio, F.; Barone, S.; Antonelli, A.; Buffone, C.; Fortunato, L.; Giudice, A. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: A pilot study. Clin. Oral Investig. 2021, 1–9. [Google Scholar] [CrossRef]
- Trink, A.; Sorbellini, E.; Bezzola, P.; Rodella, L.F.; Ramot, Y.; Rezzani, R.; Rinaldi, F. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br. J. Dermatol. 2013, 169, 690–694. [Google Scholar] [CrossRef]

| First Author | Participants | Methods | Treatment | Parameters | Follow-Up (Months) | Results (mm) | Author’s Conclusions |
|---|---|---|---|---|---|---|---|
| PRP | |||||||
| Camargo et al. [39] | 18 split-mouth design | 5600 rpm/10% trisodium citrate solution | IBD | PPDR | 6 | GTR 3.58 ± 0.94 PRP/BDX/GTR 4.95 ± 0.85 | PRP and BDX provide an added regenerative effect to GTR in promoting the clinical resolution of intrabony defects in patients with severe periodontitis. |
| CALG | GTR 2.55 ± 1.22 PRP/BDX/GTR 4.34 ± 1.32 | ||||||
| Lekovic et al. [40] | 21 split-mouth design | 5600 rpm/10% trisodium citrate solution | IBD | PPDR | 6 | PRP/BDX 3.96 ± 0.98 PRP/BDX/GTR 4.2 ± 0.90 | Combinations of PRP/BPBM/GTR and PRP/BPBM are effective in the treatment of intrabony defects present in patients with advanced chronic periodontitis. The GTR adds no clinical benefit to PRP/BPBM. |
| CALG | PRP/BDX 3.81 ± 0.74 PRP/BDX/GTR 4.14 ± 0.81 | ||||||
| Hanna et al. [38] | 13 RCT, split-mouth design | SmartPReP, 10% CaCl2 mixed with 1000 U.S. units of topical thrombin | IBD | PPDR | 6 | BDX 2.53 PRP/BDX 3.54 | The addition of PRP to BDX for the treatment of intabony defects demonstrated good clinical results with respect to the use of graft alone. |
| CALG | BDX 2.31 PRP/BDX 3.15 | ||||||
| Dori et al. [42] | 30 Parallel design study | Curasan PRP kit 2400 rpm/10 min-3600 rpm for 15 min | IBD | PPDR | 12 | BDX/GTR 5.5 ± 2.4 PRP/BDX/GTR 5.5 ± 2.2 | Regenerative surgery with both PRP/BDX/GTR and BDX/GTR, significant PD reductions and CAL gains were found; the use of PRP failed to improve the results obtained with BDX/GTR. |
| CALG | BDX/GTR 4.6 ± 2.4 PRP/BDX/GTR 4.5 ± 2.0 | ||||||
| Piemontese et al. [44] | 60 RCT, double-masked | SmartPReP, 10% CaCl2 mixed with 1000 U.S. units of topical thrombin | IBD | PPDR | 12 | DFDBA 2.6 ± 2.2 DFDBA/PRP 4.3 ± 1.7 | The combination of PRP and DFDBA led to a significantly greater clinical improvement in intrabony defects compared to DFDBA with saline. No statistically significant differences were observed in the hard tissue response between the two groups. |
| CALG | DFDBA 2.3 ± 2.4 DFDBA/PRP 3.5 ± 2.1 | ||||||
| Harnack et al. [41] | 22 Prospective RCT, split-mouth | Curasan PRP kit-2400 rpm/10 min-3600 rpm for 15 min | IBD | PPDR | 6 | HA 0.28 HA/PRP 0.8 |
PRP did not improve the results achieved with β-TCP alone in the treatment. |
| CALG | HA 0.13 HA/PRP 0.4 | ||||||
| Dori et al. [43] | 30 parallel-arm design RCT | Curasan PRP kit-2400 rpm/10 min-3600 rpm for 15 min | IBD | PPDR | 12 | BDX 5.3 ± 1.7 BDX/PRP 5.2 ± 1.6 | The use of PRP failed to improve the results obtained with BDX alone. |
| CALG | BDX 4.6 ± 1.6 BDX/PRP 4.6 ± 1.7 | ||||||
| PRF | |||||||
| Pradeep et al. [50] | 20 Prospective RCT, split-mouth | Su et al. | FD | PPDR | 6 | OFD 0.8 ± 1.31 OFD/PRF 2.3 ± 1.41 | A statistically significant difference was observed in all the clinical and radiographic parameters at the sites treated with PRP as compared with those with OFD. However, all the furcation defects retained their degree II status. |
| CALG | OFD 0.1 ± 1.10 OFD/PRF 2.5 ± 1.64 | ||||||
| Sharma et al. [55] | 18 Prospective RCT, split-mouth | Choukroun et al. 3000 rpm 10 min | FD | PPDR | 9 | OFD 2.89 ± 0.68 OFD/PRF 4.06 ± 0.42 | Significant improvement with autologous PRF implies its role as a regenerative material in the treatment of furcation defects. |
| CALG | OFD 1.28 ± 0.46 OFD/PRF 2.33 ± 0.49 | ||||||
| Sharma et al. [51] | 35 (56 sites) longitudinal interventional study | Choukroun et al. 3000 rpm 10 min | IBD | PPDR | 9 | OFD 3.21 ± 1.64 OFD/PRF 4.55 ± 1.87 | There was greater PPD reduction, CAL gain and bone fill at sites treated with PRF with conventional open-flap debridement compared to conventional open-flap debridement alone. |
| CALG | OFD 2.77 ± 1.44 OFD/PRF 3.31 ± 1.76 | ||||||
| Thorat et al. [52] | 32 CCT | Choukroun et al. 400 g 12 min | IBD | PPDR | 9 | OFD 3.56 ± 1.09 OFD/PRF 4.69 ± 1.45 | There was greater reduction in PD, more CAL gain and greater intrabony defect fill at sites treated with PRF than the open-flap debridement alone. |
| CALG | OFD 2.13 ± 1.71 OFD/PRF 4.13 ± 1.63 | ||||||
| Lekovic et al. [49] | 17 Prospective RCT, split-mouth | 1000 g 10 min | IBD | PPDR | 6 | PRF 3.29 ± 0.70 PRF/BDX 4.38 ± 0.80 | PRF can improve clinical parameters associated with human intrabony periodontal defects, and BDX has the ability to augment the effects of PRF in reducing pocket depth, improving clinical attachment levels and promoting defect fill. |
| CALG | PRF 2.18 ± 0.71 PRF/BDX 3.76 ± 0.77 | ||||||
| Aydemir et al. [57] | 28 RCT, split-mouth | 400 g 10 min | IBD | PPDR | 6 | EMD 3.88 ± 1.26 PRF/EMD 4.00 ± 1.38 | Both therapies resulted in significant clinical improvement in IBD treatment. Addition of PRF did not improve the clinical and radiographic outcomes. |
| CALG | EMD 3.29 ± 1.30 PRF/EMD 3.42 ± 1.28 | ||||||
| Arabaci et al. [56] | 26 RCT, split-mouth design | 2800 rpm Tunalı et al. | IBD | PPDR | 9 | OFD 2.84 ± 0.97 OFD/PRF 3.54 ± 1.11 | PRF membrane combined with OFD provides significantly higher GCF concentrations of angiogenic biomarkers for 2 to 4 weeks and better periodontal healing in terms of conventional flap sites. |
| CALG | OFD 2.22 ± 0.75 OFD + PRF 2.88 ± 1.03 | ||||||
| Patel et al. [53] | 26 RCT, split-mouth design | Choukroun et al. 3000 rpm 10 min | IBD | PPDR | 12 | OFD 2.40 ± 0.84 OFD/PRF 3.80 ± 1.48 | The adjunctive use of PRF with conventional OFD may be usefully used in the treatment of intrabony defects. |
| CALG | OFD 2.10 ± 0.74 OFD + PRF 3.70 ± 0.67 | ||||||
| Pradeep et al. [58] | 57 (90) RCT | Choukroun et al. 3000 rpm 10 min | IBD | PPDR | 9 | OFD 2.97 ± 0.93 OFD/PRF 3.90 ± 1.09 OFD/PRF/HA 4.27 ± 0.98 | Treatment of IBD with PRF results in significant improvements in clinical parameters compared to baseline. When added to PRF, HA increases the regenerative effects observed with PRF in the treatment of 3-wall IBDs. |
| CALG | OFD 2.67 ± 1.09 OFD/PRF 3.03 ± 1.16 OFD/PRF/HA 3.67 ± 1.03 | ||||||
| Bajaj et al. [54] | 17 (44 sites) RCT | Choukroun et al. 3000 rpm 10 min | IBD | PPDR | 9 | OFD 2.14 ± 1.26 OFD/PRF 3.14 ± 1.26 | There is greater bone fill at sites treated with PRF with conventional OFD than conventional OFD alone. |
| CALG | OFD 1.59 ± 1.01 OFD/PRF 2.66 ± 1.07 | ||||||
| PRP vs. PRF | |||||||
| Pradeep et al. [59] | 50 (90 sites) RCT | PRP: 5600 rpm/10% trisodium citrate solution PRF:3000 rpm 10 min | IBD | PPDR | 6 | OFD 2.97 ± 0.93 OFD + PRP 3.77 ± 1.03 OFD + PRF 3.77 ± 1.19 | Within the limits of the present study, there was similar PD reduction, CAL gain and bone fill at sites treated with PRF or PRP with conventional open-flap debridement. Because PRF is less time-consuming and less technique-sensitive, it may seem a better treatment option than PRP. |
| CALG | OFD 2.83 ± 0.91 OFD + PRP 2.93 ± 1.08 OFD + PRF 3.17 ± 1.29 | ||||||
| Bajaj et al. [60] | 37 (72 sites) RCT | PRP: 5600 rpm/10% trisodium citrate solution PRF: Choukroun et al. 400 g 12 min | FD | PPDR | 9 | OFD 1.58 ± 1.02 OFD + PRP 3.92 ± 0.93 OFD + PRF 4.29 ± 1.04 | All clinical and radiographic parameters showed statistically significant improvement at both the test sites (PRF with OFD and PRP with OFD) compared to those with OFD alone. The use of autologous PRF or PRP was effective in the treatment of furcation defects with uneventful healing of sites. |
| CALG | OFD 1.37 ± 0.58 OFD + PRP 2.71 ± 1.04 OFD + PRF 2.87 ± 0.85 | ||||||
| CGF | |||||||
| Xu et al. [62] | 54 (120 sites) RCT | Acceleration for 30 s, 2700 rpm for 2 min, 2400 rpm for 4 min, 2700 rpm for 4 min, 3000 rpm for 3 min, deceleration for 36 s | IBD | PPDR |
OFD 1.55 ± 0.93
OFD/CGF 2.45 ± 0.76 OFD/BDX 3.72 ± 0.90 OFD/BDX/CGF 4.36 ± 1.03 | CGF reduced periodontal intrabony defect depth and, when mixed with Bio-Oss, CGF showed better results in the early period and the effect was more stable. | |
| CALG |
OFD 2.36 ± 0.92
OFD/CGF 3.09 ± 1.14 OFD/BDX 4.18 ± 1.08 OFD/BDX/CGF 4.45 ± 1.13 | ||||||
| Clinical Applications | Advantages | Limitations |
|---|---|---|
| PRP | ||
| 1. Treatment of periodontal intrabony defects. | 1. Rapid delivery of GFs at earlier time points. | 1. Thrombin inhibits cell migration during bone repair. |
| 2. Maxillary sinus augmentation procedure. | 2. Adding PRP to autografts and xenografts induced organized bone trabecules. | 2. Risk of coagulopathies. |
| 3. Treatment of grade II periodontal furcation defects. | 3. PRP facilitates graft placement and stability. | |
| 4. Facial rejuvenation. | 4. Improving quality of bone for augmentation of edentulous site for future implant placement. | |
| PRF | ||
| 1. Treatment of intrabony defects. | 1. Releasing diversity of cytokines over time as compared to PRP. | 1. Lack of sufficient data regarding the effect of PRF on hard tissue repair. |
| 2. Sinus augmentation procedure with simultaneous implant placement. | 2. Steady release of GFs over 10 days. | 2. Small quantities can be produced at one time. |
| 3. Treatment of grade II periodontal furcation defects. | 3. Biocompatibility—the technique does not require any anticoagulants. | 3. In order to obtain usable PRF, the preparation process must be quickly completed. |
| 4. Coverage of gingival recession. | 4. Easy to prepare and use. | |
| 5. Peri-implant regeneration procedures. | 5. Reduce patients’ discomfort during the early stages of wound healing. | |
| 6. Facial rejuvenation. | 6. Simple protocol as compared to PRP. | |
| CGF | ||
| 1. Surgical correction of periodontal defects. | 1. A simple procedure. | 1. The platelet count in CGF is influenced by blood pH. Changes in blood pH may disturb cell proliferation. |
| 2. Coverage of gingival recession. | 2. Biocompatibility—the technique does not require any anticoagulants. | The duration of CGF preparation and the blood volume may influence the results. |
| 3. Peri-implant regeneration procedures. | 3. Steady release of GFs over 7–10 days. | |
| 4. Facial rejuvenation. | 4. The use of CGF is inexpensive. |
| First Author | Objective | Methods | Results | Author’s Conclusions |
|---|---|---|---|---|
| PRP | ||||
| Kim et al. [34] | The effects of activated platelet-rich plasma (aPRP) and activated platelet-poor plasma (aPPP) on the remodeling of the extracellular matrix. | Platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were prepared using a double-spin method and then activated with thrombin and calcium chloride. | Platelet numbers in PRP increased 9.4-fold over baseline values. aPRP and aPPP both stimulated cell proliferation, with peak proliferation occurring in cells grown in 5% aPRP. Levels of PIP were highest in cells grown in the presence of 5% aPRP. Additionally, aPRP and aPPP increased the expression of type I collagen, MMP-1 protein and mRNA in human dermal fibroblasts. | aPRP and aPPP promote tissue remodeling in aged skin and may be used as adjuvant treatment to lasers. |
| Cameli et al. [64] | To evaluate the efficacy and safety of autologous pure PRP dermal injections on facial skin rejuvenation. | Twelve patients underwent 3 sessions of PRP injection at 1-month intervals. The clinical and instrumental outcomes were evaluated before (T0) and 1 month (T1) after the end of treatment by means of transepidermal water loss, corneometry, Cutometer, Visioscan and Visioface. A flow cytometry characterization of PRP and peripheral blood (PB) samples was performed. | Clinical and patient evaluation showed improvement in skin texture. Skin gross elasticity, skin smoothness parameters, skin barrier function and capacitance were significantly improved. | PRP poor in leukocytes can provide objective improvements in skin biostimulation. Although a pilot study, it may be helpful for future investigations on PRP cellularity. |
| Gawdat et al. [65] | To compare the efficacy and safety of two administration modes of autologous PRP: intradermal injection (ID) and topical application after FCL with that of FCL alone in the treatment of atrophic acne scars. | Thirty patients divided into two groups. Both underwent split-face therapy. Group 1 was administered FCL followed by ID PRP on one side and FCL followed by ID saline on the other. In group 2, one cheek was treated with FCL followed by ID PRP, and the other received FCL followed by topical PRP. Each patient received 3 monthly sessions. The final assessment took place at 6 months. | Combined PRP- and FCL-treated areas had a significantly better response (p = 0.03), fewer side effects and shorter downtime (p = 0.02) than FCL-treated areas, but there were no significant differences in ID- and topical PRP-treated areas in degree of response and downtime (p = 0.10); topically treated areas had significantly lower pain scores. | The combination of topical PRP and FCL as an effective, safe modality in the treatment of atrophic acne scars with shorter downtime than FCL alone and better tolerability than FCL combined with ID PRP. |
| PRF | ||||
| Sclafani et al. [66] | The efficacy of a single injection of autologous platelet-rich fibrin matrix (PRFM) for the correction of deep nasolabial folds (NLFs). | Fifteen adults using a proprietary system (Selphyl; Aesthetic Factors, Inc., Wayne, NJ, USA). Treatment was injected into the dermis and immediate subdermis below the NLFs. Subjects were photographed before and after treatment; NLFs were rated by the treating physician before and after treatment using the Wrinkle Assessment Scale (WAS) and patients rated their appearance at each post-treatment visit using the Global Aesthetic Improvement Scale. Patients were evaluated at 1, 2, 6 and 12 weeks after treatment. | All patients were treated to maximal correction, with a mean reduction in WAS score of 2.12+/−0.56. At 1 week after treatment, this difference was 0.65+/−0.68 but rose to 0.97+/−0.75, 1.08+/−0.59 and 1.13+/−0.72 at 2, 6 and 12 weeks after treatment, respectively (p < 0.001). No patient noted any fibrosis, irregularity, hardness, restricted movement or lumpiness. | PRFM can provide significant long-term diminution of deep NLFs without the use of foreign materials. PRFM holds significant potential for stimulated dermal augmentation. |
| Hassan et al. [67] | This single-center, prospective, uncontrolled study evaluated the efficacy of injectable platelet-rich fibrin (i-PRF) for facial skin rejuvenation using an objective skin analysis system and validated, patient-reported outcome measures. | PRF® PROCESS system technology was used to prepare i-PRP. Eleven female individuals in the study and over 3 months received monthly intradermal injections of i-PRF in 3 facial regions: malar areas (1 mL each side), nasolabial fold (0.5 mL each side) and upper lip skin above the vermilion border (1 mL). The efficacy of the procedures was assessed by objective skin analysis (VISIA®) and a subjective patient-reported outcome (FACE-Q) assessment at baseline and after 3 months. | Improvement in skin surface spots (p = 0.01) and pores (p = 0.03) was seen at 3-month follow-up. Skin texture, wrinkles, ultraviolet spots and porphyrins showed a numerical improvement. FACE-Q scales that measure satisfaction with appearance all showed a significant improvement from baseline, including satisfaction with skin (p = 0.002), satisfaction with facial appearance (p = 0.025), satisfaction with cheeks (p = 0.001), satisfaction with lower face and jawline (p = 0.002) and satisfaction with lips (p = 0.04). No major adverse effects were reported. | A series of three i-PRF injections resulted in significant rejuvenation of the face skin at 3-month follow-up, as shown by improved skin analysis parameters and patient self-assessment scores. |
| CGF vs. PRP vs. PRF | ||||
| Hu et al. [37] | The impact of the new technique, CGF, on fat graft survival, which was compared with platelet-rich plasma (PRP) and platelet-rich fibrin (PRF). | Nude mice receiving fat graft were divided into PRP group, PRF group, CGF group and saline. The grafts were volumetrically and histologically evaluated at 4, 8 and 12 weeks after fat grafting. In vitro growth factor levels in PRP, PRF and CGF were compared using enzyme-linked immunoassay method. Cell count and real-time polymerase chain reaction were used to evaluate the impact of CGF in medium on human adipose-derived stem cell (hADSC) proliferation and vascular differentiation, respectively. | Fat graft weight was significantly higher in the CGF group than those in the other groups, and histologic evaluation revealed greater vascularity, fewer cysts and less fibrosis. Adding CGF to the medium maximally promoted hADSC proliferation and expression of vascular endothelial growth factor and PECAM-1. | CGF treatment improved the survival and quality of fat grafts. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mijiritsky, E.; Assaf, H.D.; Peleg, O.; Shacham, M.; Cerroni, L.; Mangani, L. Use of PRP, PRF and CGF in Periodontal Regeneration and Facial Rejuvenation—A Narrative Review. Biology 2021, 10, 317. https://doi.org/10.3390/biology10040317
Mijiritsky E, Assaf HD, Peleg O, Shacham M, Cerroni L, Mangani L. Use of PRP, PRF and CGF in Periodontal Regeneration and Facial Rejuvenation—A Narrative Review. Biology. 2021; 10(4):317. https://doi.org/10.3390/biology10040317
Chicago/Turabian StyleMijiritsky, Eitan, Haya Drora Assaf, Oren Peleg, Maayan Shacham, Loredana Cerroni, and Luca Mangani. 2021. "Use of PRP, PRF and CGF in Periodontal Regeneration and Facial Rejuvenation—A Narrative Review" Biology 10, no. 4: 317. https://doi.org/10.3390/biology10040317
APA StyleMijiritsky, E., Assaf, H. D., Peleg, O., Shacham, M., Cerroni, L., & Mangani, L. (2021). Use of PRP, PRF and CGF in Periodontal Regeneration and Facial Rejuvenation—A Narrative Review. Biology, 10(4), 317. https://doi.org/10.3390/biology10040317







