Simple Summary
Growth factors play a vital role in cell proliferation, migration, differentiation and angiogenesis. Autologous platelet concentrates which contain high levels of growth factors are used in many fields of dentistry. The current review is designed to provide clinical information regarding the use of three autologous platelet concentrates techniques in periodontal regeneration and facial rejuvenation: platelet-rich plasma, platelet-rich fibrin and concentrated growth factor techniques. The aim is to provide the clinician with an up-to-date overview of autologous platelet concentrates evolution over the past decade, clinical indications for use and advantages and limitations of each technique. This article was written in clinical orientation and is designed to provide clinicians with reliable and useful information applicable to their clinical work. Overall, platelet-rich plasma is mainly used in cases of hard and soft tissue procedures, while platelet-rich fibrin is used in gingival recession and treatment of furcation and intrabony defects; concentrated growth factor is mainly used in bone regeneration. In the field of facial rejuvenation, the use of platelet-rich plasma promotes tissue remodeling in aged skin and may be used as an adjuvant treatment to lasers; platelet-rich fibrin holds significant potential for stimulated dermal augmentation, and concentrated growth factor treatment could improve the survival and quality of fat grafts.
Abstract
Growth factors (GFs) play a vital role in cell proliferation, migration, differentiation and angiogenesis. Autologous platelet concentrates (APCs) which contain high levels of GFs make them especially suitable for periodontal regeneration and facial rejuvenation. The main generations of APCs presented are platelet-rich plasma (PRP), platelet-rich fibrin (PRF) and concentrated growth factor (CGF) techniques. The purpose of this review is to provide the clinician with an overview of APCs’ evolution over the past decade in order to give reliable and useful information to be used in clinical work. This review summarizes the most interesting and novel articles published between 1997 and 2020. Electronic and manual searches were conducted in the following databases: Pubmed, Scopus, Cochrane Library and Embase. The following keywords were used: growth factors, VEGF, TGF-b1, PRP, PRF, CGF and periodontal regeneration and/or facial rejuvenation. A total of 73 articles were finally included. The review then addresses the uses of the three different techniques in the two disciplines, as well as the advantages and limitations of each technique. Overall, PRP is mainly used in cases of hard and soft tissue procedures, while PRF is used in gingival recession and the treatment of furcation and intrabony defects; CGF is mainly used in bone regeneration.
1. Introduction
The use of platelets for regenerative medicine has increased in recent years. Platelets, which contain growth factors, play major roles in cell migration, proliferation, differentiation and angiogenesis and are associated with the tissue regeneration process. Autologous platelet concentrates (APCs) are produced by the centrifugation of venous blood at different speeds and the use or non-use of thrombin and anticoagulant. As a result of these processing protocols, a fibrin clot is formed that contains platelets and leukocytes [1]. The main generations of APCs are platelet-rich plasma (PRP), platelet-rich fibrin (PRF) and concentrated growth factor (CGF). The efficacy of platelet concentrates in promoting wound healing and tissue regeneration has been at the center of scientific interest over the past few decades. Platelets include growth factors (GFs) such as basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), transforming growth factor β-1 (TGF-β1) and platelet-derived growth factor-BB (PDGF-BB). There are significant differences in the amounts of GFs produced using the three different APC techniques (CGF, PRF and PRP). PRF and CGF produce significantly more GFs during the procedure as compared to PRP. The levels of bFGF in CGF and PRF are significantly higher than in PRP. However, the levels of the other growth factors abovementioned do not differ significantly among the different APCs [2]. The current narrative review sought to address the increasing usage of APCs in the dentistry, with special attention given to periodontal regeneration and facial rejuvenation, with the latter receiving increasing attention from dentists worldwide [3].
There is also a mechanical impact of biomaterials on cell regeneration, repair and homeostasis. As previously described by Kraft et al., dental pulp stem cells (DPSCs) have the potential to repair and generate teeth and bone [4]. DPSCs can differentiate to odontoblastic-like cells and enable regeneration. Odontoblasts and DPSCs serve as key regulators for dentin formation and its repair due to their ability to sense fluid shear stress, such as the biomechanical forces that occur due to trauma over the cell surface [5,6].
The term “dentinogenesis” refers to the complex dentin pulp which responds to external mechanical stress and repairs itself by forming dentin [7]. The pulp senses the external physical and chemical changes by a signal transduction pathway and then differentiates and forms odontoblastic-like cells, which enable regeneration [8].
Furthermore, stem cells in the oral cavity express mesenchymal cell-like features and are attracting interest due to the fact that they can be easily isolated from teeth extracted due to irreversible periodontitis or for orthodontic reasons and also because of the large amount of cells that can be produced [9,10]. Different kinds of stem cells in the oral cavity can differentiate to different lineages of cells [11]. For example, gingival mesenchymal stem cells can easily differentiate and proliferate, even better than bone marrow stem cells [6,12]. Periodontal ligament stem cells (PDLSCs) can differentiate into adipocytes, collagen-forming cells and cementoblast-like cells. After transplantation, these cells have shown an ability to generate a cementum/PDL-like structure and enable the repair of the periodontal tissue. Hence, these cells have the potential to repair tissues destroyed by periodontal diseases [13]. Stem cells from human exfoliated deciduous teeth have the ability to differentiate into a variety of cell types, such as adipocytes, odobtoblasts and neural cells. These cells have the ability to generate dentin and induce bone formation [14]. From the above, it can be concluded that there are various mechanisms that allow the tissues to maintain homeostasis and regeneration, including biomaterials, mechanical forces exerted on the tooth that stimulate tooth repair, GFs and key signaling pathways.
The current review will discuss the main APCs: PRP, PRF and CGF. PRP, which was first described by Whitmen et al., is prepared by the centrifugation of autologous whole blood together with thrombin and calcium chloride, to form a “platelet gel” [15]. The second generation of APCs, PRF, was developed by Choukroun and described first by Dohan et al. The preparation of PRF does not require the addition of any exogenous material [16]. The newest APC, CGF, was first defined by Sacco. CGF is produced in a manner similar to that used to produce PRF but involves different centrifugation speeds [17]. In the current review, the use of these three techniques in periodontal regeneration and facial rejuvenation will be discussed, taking into consideration the advantages and disadvantages of each technique.
1.1. Platelet-Rich Plasma (PRP)
In 1997, Whitman et al. published the first article on the use of platelet-rich plasma (PRP), the first generation of APCs, in oral and maxillofacial surgery. During the preparation of PRP, xenogeneic thrombin and anticoagulant were added. This technique uses exogenous materials and might cause an immunologic and infectious response, making its use controversial [15,18].
PRP plays a vital role in wound healing. The wound-healing process can be divided into three stages: biochemical activation, cellular activation and cellular response. First, there is a conversion of the mechanical injury into biochemical signals. This cascade is triggered by the Hageman factor in the serum. As a result of the disruption of microcirculation, the plasma comes into contact with tissue proteins and the basement membrane, activating the Hageman factor and platelets. The clotting cascade enables fibrin to facilitate homeostasis, and it activates thrombin. Thrombin, calcium chloride and ADP trigger the activation of platelets, leading to the release of alpha granules from platelets, with the subsequent secretion of a large variety of growth and differentiation factors [19].
The complement cascade also includes the release of substances that are important for wound repair. During this process, bradykinin is produced, which causes vasodilatation and the activation of plasminogen to produce plasmin, which degrades the fibrin. The fibrin degradation causes monocyte migration and vasodilatation. The third stage is the cellular response. In this stage, GFs are released from platelets. These GFs signal the local epithelial and mesenchymal cells to migrate, divide and enhance the synthesis of the collagen matrix. The platelet count in PRP is 338% of the platelet count of the whole blood [20]. PRP enhances bone deposition and the quality of bone regeneration during bone augmentation as GFs from autologous blood are delivered to the treatment site [20]. Moreover, platelet and GF concentrations in PRP are, on average, 3‒5 times higher in PRP than in peripheral blood.
1.2. The Technique
In the pre-operative period, 450 mL blood is collected in a sterile centrifuge tube, containing citrate–phosphate–dextrose solution (as anticoagulant). First, it is centrifugated (Medtronic Electromedic, Elmd-500 Autotransfusion system, Parker, CO, USA) at 5600 rpm. The result of this stage is the separation into two layers: first layer—platelet-poor plasma (PPP); second layer—red blood cells (RBCs) and buffy coat, which contains platelets and white blood cells (WBCs) (see Figure 1). Only the layer of RBCs and buffy coat then continues to the second stage of separation. The second centrifugation period is processed at 2400 rpm in order to separate the buffy coat into PRP and residual RBCs. When the surgeon needs to use the PRP, thrombin is dissolved in 10 mL 10% calcium chloride in a sterile cup. Then, 7 mL PRP and 2 mL air are aspirated into a 10 mL syringe with a 14 gauge catheter. Then, a 1 mL mixture of thrombin + calcium chloride is aspirated into the syringe. Within 5–30 s, the thrombin enables the polymerization of fibrin into a insoluble gel, platelet degranulation and the release of GFs and cytokines. The gel is injected to the desirable site. It should be noted that there is a difference in platelet quantity: platelet and WBC count is higher in younger people and higher in females compared to males [15,21,22].
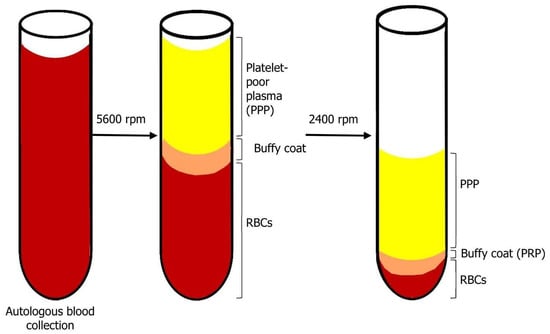
Figure 1.
Blood centrifugation after collection. After the first centrifugation period, there is a separation of two layers: on top—platelet-poor plasma (PPP), on bottom—red blood cells (RBCs) and buffy coat. The products of the second centrifugation period are: top—PPP; bottom—buffy coat (PRP) and residual RBCs.
1.3. Platelet-Rich Fibrin (PRF)—The Second Generation of Platelet Concentrates
In 2000, Choukroun et al. reported on a new alternative to PRP: platelet-rich fibrin (PRF) [16,23]. This biomaterial was developed for use in oral and maxillofacial surgical procedures. The application of PRF is different from PRP and does not require use of any anticoagulant or thrombin, only centrifuged autologous blood. Fibrin is an insoluble molecule that is the activated form of fibrinogen, a soluble molecule by thrombin, factor XIII, calcium ions and fibronectin. Fibrin is part of the last stage in the coagulation cascade. The fibrin molecule plays a vital role during coagulation. This molecule is found in platelet alpha granules and in plasma. Fibrin becomes a biological adhesive that enables the stabilization of the initial platelet cluster during coagulation. The fibrin network is the first to reach the injured tissue. The regeneration capacity of PRF is due to its angiogenesis potential, which can be explained by the 3D fibrin matrix that can carry, at the same time, cytokines and GFs such as VEGF, IGF, TGF-β1 and PDGF. As previously described, these factors play vital roles in the regeneration process. Furthermore, during angiogenesis, endothelial cells express αvβ3 integrin, which enables the interaction between the endothelial cells and fibrin, fibronectin and vitronectin. Moreover, PRF can also recruit stem cells from the circulating blood. Chouckroun et al. reported an immunological benefit of PRF. These benefits could explain why, while using this technique, there are fewer post-operation infections [23,24].
1.4. The Technique
As described by Chouckroun et al., IV blood is collected in 10 mL tubes with no anti-coagulant addition; it is then centrifugated at 3000 rpm for 10 min. At the end of the procedure, three layers are obtained: 1. Bottom—RBC layer; 2. Middle—Fibrin clot layer (PRF); 3. Top—serum layer (PPP) (see Figure 2). As mentioned above, there is no anticoagulant addition. Hence, the coagulation process starts immediately when the blood comes into contact with the glass tube [16].
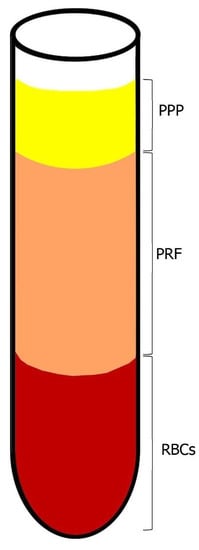
Figure 2.
Blood centrifugation after collection. The layers after centrifugation period are: on bottom—RBCs, middle layer—fibrin clot layer (PRF) and on top—PPP.
1.5. Injectable PRF
Miron et al. published a modification to PRF: a liquid formulation of PRF injectable PRF (i-PRF) with no use of anticoagulants. As compared to PRP, after 10 days, i-PRF released higher levels of GFs such as IGF-1, EGF, PDGF-AA/AB. Furthermore, i-PRF induced the highest fibroblast migration, while PRP induced higher levels of cell proliferation [25]. Fujioka-Kobayashi et al. noted that modification to centrifugation speed and time influence GF release. As centrifugation speed decreases, GF and leukocyte release from the PRF clot is increased [26].
1.6. Concentrated Growth Factor (CGF): The Newest Platelet Concentrate
In 2006, Sacco [26] reported on the newest platelet concentrate—CGF. CGF is produced in a manner that is similar to that used to produce PRF, but it involves a different centrifuge speed (Medifuge, Silfradent, Italy). CGF contains GFs such as VEGF, PDGF, IGF-I and TGF-β1. Compared to PRF, CGF contains a denser and richer GF‒fibrin matrix. Furthermore, CGF has a 3D fibrin network in which growth factors are closely bound to one another. This provides the slow release of growth factors, which helps with wound healing [18,27].
1.7. The Technique
As described by Bozkurt et al., IV blood is collected in two 10 mL glass-coated plastic tubes with no anticoagulant addition. The tubes are immediately centrifuged (Medifuge, Silfradent, S. Sofia, Italy) in the following manner: 30’’ acceleration, 2’ 2700 rpm, 4’ 2400 rpm, 4’ 2700 rpm, 3’ 3000 rpm and 36’’ deceleration until end. At the end of the procedure, four layers are obtained from bottom to top: RBC layer, GF and stem cell layer (CGF), Buffy coat layer, serum layer (PPP) (see Figure 3). Then, the CGF layer is separated using sterile surgical scissors. The CGF clot is then squeezed in a special box at a thickness of 1 mm. The CGF is then placed over the target site [28].
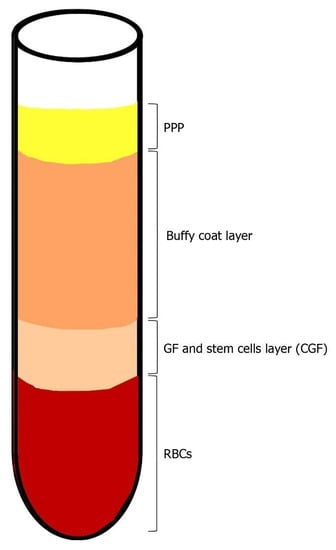
Figure 3.
Blood centrifugation after collection. At the end of the centrifugation period, four layers are obtained: 1. Bottom—RBC layer; 2. GF and stem cell layer (CGF); 3. Buffy coat layer; 4. Top—serum layer (PPP).
1.8. Periodontal Regeneration
The cementum, gingiva, periodontal ligament (PDL) and alveolar bone serve as the tooth-supporting tissues (periodontium). Periodontitis is a chronic multifactorial inflammatory disease which is primarily characterized by the destruction of alveolar bone and tooth-supporting connective tissue, which is manifested by a loss of clinical attachment, presence of periodontal pocketing and bleeding on probing [29]. Lack of treatment may lead to tooth loss. Scaling and root planning (SRP) is an initial treatment for periodontitis, enabling plaque removal and local inflammation control [30]. These therapies cannot provide reattachment of the periodontium tissues to teeth. The aim of periodontal regeneration is to regenerate the tooth-supporting tissues: to form new bone, cementum and supportive PDL in order to provide optimal structure and function [31]. As previously described by Gottlow et al., periodontal regeneration is based on guided tissue regeneration, which enables selected cell populations to reach the target site together with barriers in order to prevent the migration of epithelial cells to the regenerating site. As a result, PDL cells can migrate and enable connective tissue attachment and regeneration [32] As previously described by Larsson et al., recent advances in this field include the use of diverse biomaterials, GFs, stem cells and bone replacement grafts [33]. The current narrative review will discuss the use of three APC techniques in the field of periodontology: treatment of gingival recession, furcation defects and intrabony defects.
1.9. Facial Rejuvenation
In recent years, there has been an increase in the use of the techniques in the field of skin rejuvenation due to the high concentration of growth factors in platelets. APCs are used in facial rejuvenation of the periorbital area and wrinkles, the treatment of acne scars and in lip augmentation. Kim et al. demonstrated that PRP stimulates dermal fibroblast proliferation, collagen synthesis and matrix metalloproteinase expression, thereby aiding facial rejuvenation [34]. There are facial rejuvenation procedures that use combined PRP and fat-grafting procedures to achieve facial volume. Rophael et al. indicated that the angiogenesis benefit of PRP (which contains GFs) may explain the mechanism underlying the fat graft retention, especially during the ischemic period after fat injection [35]. Choukroun and Miron indicated that PRF can be utilized for lip augmentation and to treat both acne scars and wrinkles [36]. Wang et al. reported improved results following CGF injections used to treat periorbital wrinkles, as evaluated at 3 months after treatment [37].
This narrative review is designed to provide the clinician with an overview of APC evolution over the past decade. The uses of the different methods will be elaborated, to provide the clinician with reliable and useful information for clinical implementation and to consider novel uses of the three techniques in the fields of periodontal regeneration and facial rejuvenation. To the best of our knowledge, this is the first time in the literature that a narrative review article is devoted to discussing the uses of the three APC methods in the areas of periodontal regeneration and skin rejuvenation, summarizing the indications for using each method, as well as advantages and limitations, and presenting the reader with the most up-to-date information, mostly collected over the last decade.
2. Materials and Methods
The Pubmed, Cochrane Library, Scopus and Embase databases were searched from January 1997 to December 2020 to find published studies on the effects of different autologous platelet concentrates on periodontal regeneration and facial rejuvenation. (see Figure 4) The keywords used in the preliminary search were as follows: “VEGF”, “TGF-b1”, “PRP”, “PRF”, “CGF”, AND “periodontal regeneration” or “facial rejuvenation”. The selection included all studies presented in the English language that investigated the effect of autologous platelet concentrates on periodontal regeneration and facial rejuvenation. The review process, including search and selection (identification, screening, eligibility of included studies), was performed according to the PRISMA criteria. In the selection process, all articles were selected by abstract and title; abstracts were initially read by two independent researchers to identify potentially eligible full-text papers. All authors discussed and agreed upon which articles met the inclusion criteria and which articles to exclude.
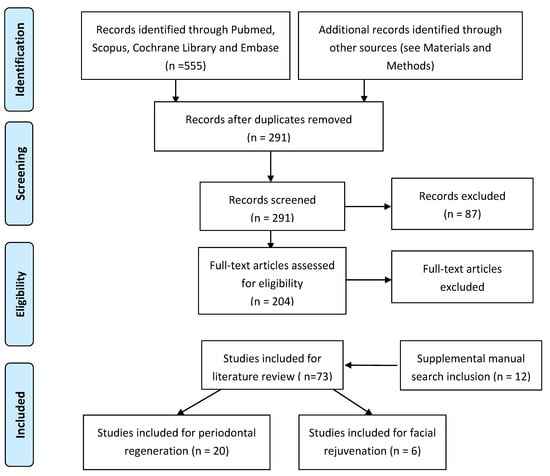
Figure 4.
Flowchart of the search strategy.
The following criteria were applied for APCs in periodontal regeneration:
Inclusion criteria:
- Study design: randomized controlled trials (RCTs), cohort studies, cross-sectional studies only in the English language;
- Population: only studies on humans, with a minimum sample size of 10 patients and no restriction in terms of patient ages;
- Intervention: regenerative periodontal surgery on interproximal bony defects (IBD) and furcation defects (FD);
- Types of outcome: probing pocket depth recovery (PPDR) and clinical attachment level gain (CALG).
Exclusion criteria:
- Absence of baseline data before periodontal surgery;
- Patients with systemic diseases or craniofacial anomalies;
- No training in oral hygiene;
- Follow-up < 6 months.
The following criteria were applied for APCs in facial rejuvenation:
Inclusion criteria:
- Study design: randomized controlled trials (RCTs), cohort studies, cross-sectional studies, case reports and case series only in the English language;
- Population: only studies on humans; for CGF, studies on animals were included;
- Intervention: facial skin rejuvenation, facial wrinkles, atrophic acne scars;
- Types of outcome: clinical or histologic evaluation.
Exclusion criteria:
- Hair and nail restoration;
- Patients with alopecia;
- Review articles.
For the manual search, we selected six journals (Journal of Periodontology, Periodontology 2000, Journal of Clinical Periodontology, Annals of Dermatology, Aesthetic Plastic Surgery, Journal of Cosmetic Dermatology). In addition, the references of the selected articles were evaluated to find additional publications by manual searches.
3. Results
The aim of the present study was to evaluate the state of the art in the use of autologous platelet concentrates in periodontal regeneration and facial rejuvenation. The electronic search identified 741 references (see Figure 4). Citations that were not connected with the topic were rejected and duplicates eliminated. Titles and abstracts were selected according to the inclusion and exclusion criteria; the articles that presented at least one inclusion criteria and no exclusion criteria in the abstract were kept. After evaluation, 204 records were screened on the basis of titles and abstracts, and full texts were read and analyzed. A manual search was performed to find supplementary articles. Consequently, 12 new titles were considered, and 131 that did not have appropriate full texts were excluded at this stage. In total, 73 articles were included for the literary review on APCs; of these, 20 were included in the review on periodontal regeneration and six studies on facial rejuvenation.
3.1. PRP
Seven clinical studies on PRP use for infrabony defects were included in the current review (see Table 1). Some studies found that over a period of 6 months, the addition of PRP to a bovine-derived xenograft (BDX) improved the clinical periodontal response. Hanna et al. demonstrated good clinical results in terms of probing depth and clinical attachment loss, in comparison to the use of a graft alone [38]. The combination of PRP and BDX is effective in infrabony defects as compared to GTR alone [39,40]. However, PRP did not provide clinical benefit with β-TCP in the treatment of infrabony defects in patients with periodontitis [41]. Furthermore, at 12 months, the use of PRP failed to improve the results obtained with BDX [42,43]. Piemontese et al. [44] have shown that the addition of PRP to demineralized freeze-dried bone allografts (DFDBA) is effective in pocket depth (PD) reduction and clinical attachment level (CAL) gain. As previously described by Kobayashi et al., at earlier time points, PRP provides more rapid delivery of growth factors, as compared to PRF [45]. Moreover, the addition of PRP to bone autografts and allografts has been shown to induce dense matured bone with organized trabeculae [18] and the use of PRP increases bone deposition and improves the quality of bone for augmentation of edentulous sites to aid future implant placement [20]. Wroblewski et al. have also demonstrated that PRP facilitates graft placement and stability [19]. However, there are also a few limitations to the use of PRP. Thrombin inhibits cell migration during bone repair [46]. As the source of thrombin is exogenous (i.e., bovine), there may a risk of transmissible infectious diseases, e.g., bovine spongiform encephalopathy [47]. In addition, there is a risk of coagulopathies. Specifically, the use of bovine thrombin increases the risk of the production of antibodies to factors V and XI, which increases the risk of coagulopathies. The exogenous thrombin can react with the patient’s immune system. The cross-reactivity between the anti-bovine factor V antibody and the endogenous factor V may cause factor-V deficiency after the use of thrombin in PRP. Note that the reaction is dependent on the source, purity and quality of the thrombin used [48]. Another limitation is that, when used in sinus augmentation, PRP requires living cells [20].

Table 1.
Comparison between the 3 APC techniques, PRP, PRF and CGF, in periodontal regeneration.
3.2. PRF
Since 2009, the use of the PRP technique has diminished, as demonstrated in Table 1 by the number of studies published since 2009 regarding the use of PRF. Lekovic et al. [49] tested the use of PRF as an adjunct to BDX, and the clinical parameters were significantly improved. Similar results were also reported when PRF was used only with the open-flap debridement (OFD) procedure [50,51,52,53,54]. A statistically significant difference was observed in terms of regeneration in the treatment of furcation defects by Sharma et al. [55]. PRF combined with OFD provided significantly higher GCF concentrations of angiogenic biomarkers and better periodontal healing in terms of conventional flap sites [56]. The addition of PRF did not improve PPD reduction and CAL gain when added to Emdogain placement [57]. The combination of OFD/PRP/hydroxyapatite (HA) improved the outcome in intrabony defects with respect to PRF alone [58].
Pradeep et al. [59] and Bajaj et al. [60] studied the effects of PRP and PRF in infrabony and furcation defects, respectively. In the first study, similar PD reduction and CAL gain were observed in infrabony defects treated with PRP or PRF, and both of them showed a significant improvement compared to open-flap debridement alone. PRF was slightly more effective than PRP in the treatment of furcation defects. Because PRF is less time-consuming and less technique-sensitive, it may represent a better treatment option than PRP [59]. As described in Table 2, the use of PRF presents a number of advantages: compared to PRP, PRF provides advantages in terms of the relative amount and diversity of cytokines and their release over time [25,61]. As previously described by He et al., the use of PRF is associated with the steady release of GFs over 10 days [61]. As compared to PRP, the use of PRF does not require any anticoagulant, thrombin or blood manipulation. Therefore, this approach provides immunological biocompatibility [25]. The material is easy to prepare and the technique is simple to use. Another advantage is the reduction of patient discomfort during the early stages of wound healing [24]. As compared to PRP, the time and cost of preparation are lower in the PRF technique. However, there are also a few limitations to the use of PRF: Miron et al. noted that, while there have been studies to support the use of PRF for periodontal purposes and soft tissue repair, we still lack sufficient data regarding the effects of PRF on hard tissue repair during bone regeneration [24]. Another limitation is that, since PRF is prepared from autologous blood, only small quantities can be produced at one time and, in order to obtain usable PRF, the preparation process must be quickly completed.

Table 2.
Summary of the the 3 APC techniques, PRP, PRF and CGF—clinical applications, advantages and limitations.
3.3. CGF
Regarding CGF, there are limited studies available with relation to periodontal regeneration. Only one study reported the effect of CGF in infrabony defects. The authors concluded that CGF can enhance bone regeneration and reduce the depth of periodontal intrabony defects. When combined with a xenograft, CGF might be a superior scaffolding material [62]. CGF presents number of advantages. The use of CGF involves a simple and inexpensive procedure [63]. As mentioned above regarding the use of PRF, the use of CGF requires no exogenous additions, such as thrombin or bovine calcium chloride. Therefore, the probability of cross-contamination is low. As mentioned above regarding PRF, the use of CGF is associated with the steady release of growth factors over 7–10 days. There are also some limitations to the use of CGF: for example, the platelet count in CGF is influenced by the blood pH [63]. Moreover, changes in the blood pH may disturb cell proliferation. Moreover, the duration of CGF preparation and the blood volume may influence the results.
3.4. Facial Rejuvenation
As mentioned in Table 3, PRP enhances rejuvenation by inducing collagen synthesis and dermal fibroblast proliferation. Facial aging is caused by extracellular matrix (ECM) alterations and poor fibroblast proliferation. PRP is a more natural treatment for skin rejuvenation and is also used to treat acne scarring and alopecia. There is a specific need for long-term, follow-up studies to evaluate whether the benefits of PRP persist over time. PRP application (even as a single application) could be considered as an effective and safe procedure for the rejuvenation of facial skin. There is a need for further research to clarify the specific biological and clinical effects of this procedure [64,65,66]. Further studies will be needed to determine if platelet concentrates are a valid aid in dermatology and if they can be considered as an alternative to or support for other therapies.

Table 3.
Comparison between the 3 APC techniques, PRP, PRF and CGF, in facial rejuvenation.
4. Discussion
The current narrative review includes RCT studies, clinical studies, cross-sectional studies, case reports and case series. The aim was to provide clinicians with up-to-date information about the use of PRP, PRF and CGF in dentistry, including the clinical indications, advantages and limitations of each technique. This article has a clinical orientation and was written in a manner designed to provide clinicians with reliable and useful information applicable to their work and interest in novel uses of the three techniques in the field of periodontal regeneration and facial rejuvenation.
4.1. Periodontal Regeneration
Periodontal regeneration, following destructive episodes of periodontal disease, must involve not only the affected alveolar bone but also the periodontal ligament and the root cementum [68].
The three techniques described in this article differ in GF amounts [2]. Mazuki et al. found that PRF and CGF preparations contained significant amounts of growth factors as compared to PRP. Moreover, these two preparations are more capable of inducing angiogenesis and, as a result, increase the wound-healing regeneration as compared to PRP [69]. The three techniques are also different in the use of thrombin and calcium chloride (PRP) or non-use of them and in the complexity of the preparation protocol—PRP preparation requires two stages of centrifugation, while PRF and CGF are simpler and require only one stage.
As previously described in Table 1, Hanna et al. demonstrated good clinical results in terms of probing depth and clinical attachment loss, in comparison to the use of a graft alone [38]. The combination of PRP and BDX is effective in infrabony defects as compared to GTR alone [39,40]. Furthermore, at 12 months, the use of PRP failed to improve the results obtained with BDX [42,43]. These results might be affected by the different follow-up periods or due to differences in the study design (split-mouth vs. parallel design).
Since 2009, the use of the PRP technique has diminished, as demonstrated in Table 1 by the number of studies published since 2009 regarding the use of PRF. Lekovic et al. tested the use of PRF as an adjunct to BDX, and the clinical parameters were significantly improved [49]. Similar results were also reported when PRF was used only with the open-flap debridement (OFD) procedure [50,51,52,53,54]. A statistically significant difference was observed in terms of regeneration in the treatment of furcation defects by Sharma et al. [55]. Pradeep et al. and Bajaj et al. studied the effects of PRP and PRF in infrabony and furcation defects, respectively [59,60]. In the first study, similar PD reduction and CAL gain were observed in infrabony defects treated with PRP or PRF, and both of them showed a significant improvement compared to open-flap debridement alone. PRF was slightly more effective than PRP in the treatment of furcation defects.
Regarding CGF, there are limited studies available in relation to periodontal regeneration. Its use has been proposed in dentistry for various situations, ranging from the filling of extraction sockets [70] to the filling of a cavity after cystectomy [71], sinus augmentation procedures [63,72,73], simple GBR procedures [74,75] or as a membrane support in recession coverage [28,76,77,78]. It was demonstrated to possess the capacity to accelerate new bone formation [79]. Moreover, it can be used alone or with autologous bone particles or biomaterials [75,80]. Bozkurt et al. reported that using CGF, together with coronally advanced flap (CAF), to treat maxillary gingival recession led to significantly wider keratinized gingiva and thicker gingiva, as compared to CAF, 6 months after the procedure [28]. No significant differences between the treatments were noted for recession depth, complete root coverage or mean root coverage. Connective tissue graft (CTG) is superior to CGF for enhancing keratinized tissue thickness and width during surgical root coverage procedures [76]. CGF is widely used in implant surgery. The use of CGF led to increased implant stability and osteo-integration [81,82] and may be preferable due to decreased postoperative pain [82]. In a 12-month RCT, Isler et al. compared the use of a collagen membrane with the use of CGF in the regenerative surgical treatment of peri-implantitis. They found that both approaches yielded significant improvements in both clinical and radiograph assessments [83]. The use of sticky bone in maxillary sinus augmentation procedures has provided new bone formation and predictable clinical results. It was also evident in cone beam computer tomography (CBCT) [73]. Only one study reported the effect of CGF in infrabony defects. The authors concluded that CGF can enhance bone regeneration and reduce the depth of periodontal intrabony defects. When combined with a xenograft, CGF might be a superior scaffolding material [62].
4.2. Facial Rejuvenation
In recent years, there has been increased use of the techniques presented in the field of facial rejuvenation. The added value of using these techniques is the ability to deliver a high amount of GF to the scar, acne, periorbital fine line and pigmentation target areas. The alternatives for facial rejuvenation are hyaluronic acid injections and fat grafting. The former is expensive and its effects last only up to 12 months, and the latter can cause unpredictable swelling. The periorbital region is an area of the face that enables us to estimate a person’s age and has aesthetic and beauty aspects. This area is prone to pigmentation, wrinkles, erythema, xerosis, decreased skin elasticity and melanosis, all of which corelate to the person’s age. PRP enhances rejuvenation by inducing collagen synthesis and dermal fibroblast proliferation. Facial aging is caused by extracellular matrix (ECM) alterations and poor fibroblast proliferation.
Samadi et al. published a review regarding the multiple clinical uses of PRP for aesthetic and regenerative medicine [84]. Mayes et al. sought to identify the most influential parameters for assessing a woman’s age and found that wrinkles and hyperpigmentation have the strongest relationships with perceived age [85]. PRP is a more natural treatment for skin rejuvenation and is also used to treat acne scarring and alopecia. Kim et al. demonstrated that PRP stimulates dermal fibroblast proliferation and collagen synthesis [34].
There are facial rejuvenation procedures that combine PRP and fat grafting to achieve facial volume. Rophael et al. indicated that the angiogenesis benefit from PRP may explain the mechanism underlying fat graft retention, especially during the ischemia period after fat injection [35]. Cameli et al. examined facial skin rejuvenation in 12 patients after three sessions of PRP injections (delivered at 1-month intervals). Their evaluation revealed improvements in skin texture, elasticity and smoothness [64]. The authors concluded that further investigation of this procedure is needed. There is a specific need for long-term, follow-up studies to evaluate whether the benefits of PRP persist over time. PRP application (even a single application) could be considered as an effective and safe procedure for the rejuvenation of facial skin. Liquid injectable PRF can be used instead of PRP for facial rejuvenation. As previously described, PRF does not require the addition of anticoagulants and therefore will not inhibit tissue regeneration. As demonstrated by Choukroun and Miron, PRF can be used for lip augmentation, as well as for the treatment of acne scars and wrinkles [36]. Sclafani et al. examined the efficacy of a single injection of autologous platelet-rich fibrin matrix for the correction of nasolabial fold defects [66]. The results were examined in terms of a wrinkle assessment score and a global aesthetic improvement scale. Digital photographs were taken before and after the procedure. Follow-up evaluations were conducted at 1, 2, 6 and 12 weeks after treatment. The researchers found that treatment with PRF yielded positive results, as compared to other dermal stimulators. The effects appeared quickly and the technique was easy to use. There is a need for further research to clarify the specific biological and clinical effects of this procedure [66]. The regenerative efficacy of CGF is due to the release of GF over a long period of time and its bioactivity advantage. Hu et al. examined the effect of CGF on periorbital wrinkles. They reported on the use of CGF injections to treat periorbital wrinkles. CGF was added to an adipose graft. The results of this treatment were examined in terms of wrinkles, volume and complications at 3 to 6 months after the injection of CGF. Significant results were obtained using CGF [37]. The main studies on facial rejuvenation are reported in Table 3. In addition to their dental indications, APCs may be used as treatment modalities in different medical indications, such as autoimmune diseases, oral lichen planus [86] and alopecia areata [87]. Further studies will be needed to determine whether platelet concentrates are a valid aid in dermatology and if they can be considered as an alternative or support to other therapies.
5. Conclusions
This narrative review is designed to provide clinical information regarding the use of three APC techniques in periodontal regeneration and facial rejuvenation: PRP, PRF and CGF. The advantage of the APCs is the fact that a large amount of GF can be delivered to the target site and encourage angiogenesis and wound healing. PRP is the most established among the techniques reviewed in this article and is used in soft and hard tissue repair. At early time points, PRP provides more rapid delivery of GFs to the target site as compared to PRF and CGF. As a result of the addition of PRP to a bone autograft, dense and mature bone is formed. However, the transmission of infectious diseases and coagulopathies serves as a crucial limitation to the PRP technique and should be taken into consideration. In contrast to PRP, PRF and CGF require only centrifuged autologous blood and therefore provide immunological biocompatibility. PRF is effective mostly for soft tissue repair such as gingival recession coverage and furcation defects and is also used for intrabony defects. CGF is used in oral surgery, mostly for hard tissue regeneration. This narrative review also discussed the use of the three APC techniques in facial rejuvenation. The added value of using these techniques is the ability to deliver a high number of GFs to the target site. The use of PRP promotes tissue remodeling in aged skin and may be used as an adjuvant treatment to lasers. PRF holds significant potential for stimulated dermal augmentation. CGF treatment could improve the survival and quality of fat grafts. As this is a narrative review, this serves as a limitation of this study. Thus, interpretation should be made with caution. Future high-quality studies, e.g., randomized controlled trials, should be conducted; future systematic reviews and meta-analyses regarding the current review topic are also warranted.
Author Contributions
E.M.: conceptualization, methodology, writing—original draft preparation, visualization, supervision, project administration, writing—review and editing. H.D.A.: writing—original draft preparation, visualization, data curation. O.P.: writing—review and editing. M.S.: writing—review and editing. L.C.: writing—review and editing. L.M.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cakir, S.; Gultekin, B.A.; Karabagli, M.; Yilmaz, T.E.; Cakir, E.; Guzel, E.E.; Yalcin, S.; Mortellaro, C.; Mijiritsky, E. Histological Evaluation of the Effects of Growth Factors in a Fibrin Network on Bone Regeneration. J. Craniofacial Surg. 2019, 30, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; An, N.; Ouyang, X. Quantification of growth factors in different platelet concentrates. Platelets 2017, 28, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.; Roberts, W. Incorporating facial rejuvenation into the dental practice. Dent. Today 2010, 29, 29. [Google Scholar]
- Kraft, D.C.E.; Bindslev, D.A.; Melsen, B.; Klein-Nulend, J. Human dental pulp cells exhibit bone cell-like responsiveness to fluid shear stress. Cytotherapy 2011, 13, 214–226. [Google Scholar] [CrossRef]
- Marrelli, M.; Codispoti, B.; Shelton, R.M.; Scheven, B.A.; Cooper, P.R.; Tatullo, M.; Paduano, F. Dental Pulp Stem Cell Mechanoresponsiveness: Effects of Mechanical Stimuli on Dental Pulp Stem Cell Behavior. Front. Physiol. 2018, 9, 1685. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Loison-Robert, L.S.; Tassin, M.; Bonte, E.; Berbar, T.; Isaac, J.; Berdal, A.; Simon, S.; Fournier, B.P.J. In vitro effects of two silicate-based materials, Biodentine and BioRoot RCS, on dental pulp stem cells in models of reactionary and reparative dentinogenesis. PLoS ONE 2018, 13, e0190014. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Falisi, G.; Rastelli, C.; Palmieri, F.; Gargari, M.; Zavan, B.; Paduano, F.; Benagiano, V. Mechanical influence of tissue culture plates and extracellular matrix on mesenchymal stem cell behavior: A topical review. Int. J. Immunopathol. Pharmacol. 2015, 29, 3–8. [Google Scholar] [CrossRef]
- Spagnuolo, G.; Codispoti, B.; Marrelli, M.; Rengo, C.; Rengo, S.; Tatullo, M. Commitment of Oral-Derived Stem Cells in Dental and Maxillofacial Applications. Dent. J. 2018, 6, 72. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Paduano, F. The Regenerative Medicine in Oral and Maxillofacial Surgery: The Most Important Innovations in the Clinical Application of Mesenchymal Stem Cells. Int. J. Med. Sci. 2015, 12, 72–77. [Google Scholar] [CrossRef]
- Ballini, A.; Boccaccio, A.; Saini, R.; Van Pham, P.; Tatullo, M. Dental-Derived Stem Cells and Their Secretome and Interactions with Bioscaffolds/Biomaterials in Regenerative Medicine: From the In Vitro Research to Translational Applications. Stem Cells Int. 2017, 2017, 1–3. [Google Scholar] [CrossRef]
- Tomar, G.B.; Srivastava, R.K.; Gupta, N.; Barhanpurkar, A.P.; Pote, S.T.; Jhaveri, H.M.; Mishra, G.C.; Wani, M.R. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem. Biophys. Res. Commun. 2010, 393, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Whitman, D.H.; Berry, R.L.; Green, D.M. Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J. Oral Maxillofac. Surg. 1997, 55, 1294–1299. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e37–e44. [Google Scholar] [CrossRef]
- Sacco, L. Lecture. In International Academy of Implant Prosthesis and Osteoconnection; Lecture: Sersale, Italy, 2006. [Google Scholar]
- Fang, D.; Long, Z.; Hou, J. Clinical Application of Concentrated Growth Factor Fibrin Combined with Bone Repair Materials in Jaw Defects. J. Oral Maxillofac. Surg. 2020, 78, 882–892. [Google Scholar] [CrossRef]
- Wroblewski, A.P.; Mejia, H.A.; Wright, V.J. Application of Platelet-Rich Plasma to Enhance Tissue Repair. Oper. Tech. Orthop. 2010, 20, 98–105. [Google Scholar] [CrossRef]
- E Marx, R.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; E Strauss, J.; Georgeff, K.R. Platelet-rich plasma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Marques, F.P.; Ingham, S.J.M.; Forgas, A.; Franciozi, C.E.D.S.; Sasaki, P.H.; Abdalla, R.J. A manual method to obtain platelet rich plasma. Acta Ortopédica Bras. 2014, 22, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.R.; Sándor, G.K. Use of fibrin glue in maxillofacial surgery. J. Otolaryngol. 1998, 27, 107–112. [Google Scholar]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e51–e55. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e56–e60. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Ghanaati, S.; Choukroun, J. Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry? Clin. Oral Investig. 2017, 21, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Miron, R.J.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Choukroun, J. Optimized Platelet-Rich Fibrin with the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J. Periodontol. 2017, 88, 112–121. [Google Scholar] [CrossRef]
- Wang, L.; Wan, M.; Li, Z.; Zhong, N.; Liang, D.; Ge, L. A comparative study of the effects of concentrated growth factors in two different forms on osteogenesis in vitro. Mol. Med. Rep. 2019, 20, 1039–1048. [Google Scholar] [CrossRef]
- Doğan Şeyma, B.; Dede, F.O.; Balli, U.; Atalay, E.N.; Durmuşlar, M.C. Concentrated Growth Factor in the Treatment of Adjacent Multiple Gingival Recessions: A Split-Mouth Randomized Clinical Trial. J. Clin. Periodontol. 2015, 42, 868–875. [Google Scholar] [CrossRef]
- Kornman, K.S. Mapping the Pathogenesis of Periodontitis: A New Look. J. Periodontol. 2008, 79, 1560–1568. [Google Scholar] [CrossRef]
- Smiley, C.J.; Tracy, S.L.; Abt, E.; Michalowicz, B.S.; John, M.T.; Gunsolley, J.; Cobb, C.M.; Rossmann, J.; Harrel, S.K.; Forrest, J.L.; et al. Evidence-based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J. Am. Dent. Assoc. 2015, 146, 525–535. [Google Scholar] [CrossRef]
- Lindhe, J.; Schallhorn, R.; Bowers, G.; Garrett, S.; Becker, B.; Cortellini, P.; Ferris, R.; Karring, T.; McClain, P.; O’Neal, R.; et al. Periodontal Regeneration around Natural Teeth. J. Am. Dent. Assoc. 1998, 129, 43. [Google Scholar] [CrossRef]
- Gottlow, J.; Nyman, S.; Karring, T.; Lindhe, J. New attachment formation as the result of controlled tissue regeneration. J. Clin. Periodontol. 1984, 11, 494–503. [Google Scholar] [CrossRef]
- Larsson, L.; Decker, A.; Nibali, L.; Pilipchuk, S.; Berglundh, T.; Giannobile, W. Regenerative Medicine for Periodontal and Peri-implant Diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef]
- Kim, D.H.; Je, Y.J.; Kim, C.D.; Lee, Y.H.; Seo, Y.J.; Lee, J.H. Can Platelet-rich Plasma Be Used for Skin Rejuvenation? Evaluation of Effects of Platelet-rich Plasma on Human Dermal Fibroblast. Ann. Dermatol. 2011, 23, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Rophael, J.A.; Craft, R.O.; Palmer, J.A.; Hussey, A.J.; Thomas, G.P.; Morrison, W.A.; Penington, A.J.; Mitchell, G.M. Angiogenic Growth Factor Synergism in a Murine Tissue Engineering Model of Angiogenesis and Adipogenesis. Am. J. Pathol. 2007, 171, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.; Miron, J.C. Platelet Rich Fibrin in Regenerative Dentistry: Biological Background and Clinical Indications; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Hu, Y.; Jiang, Y.; Wang, M.; Tian, W.; Wang, H. Concentrated Growth Factor Enhanced Fat Graft Survival: A Comparative Study. Dermatol. Surg. 2018, 44, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Trejo, P.M.; Weltman, R.L. Treatment of Intrabony Defects with Bovine-Derived Xenograft Alone and in Combination with Platelet-Rich Plasma: A Randomized Clinical Trial. J. Periodontol. 2004, 75, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Camargo, P.M.; Lekovic, V.; Weinlaender, M.; Vasilic, N.; Madzarevic, M.; Kenney, E.B. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J. Periodontal Res. 2002, 37, 300–306. [Google Scholar] [CrossRef]
- Leković, V.; Camargo, P.M.; Weinlaender, M.; Vasilic, N.; Kenney, E.B. Comparison of Platelet-Rich Plasma, Bovine Porous Bone Mineral, and Guided Tissue Regeneration Versus Platelet-Rich Plasma and Bovine Porous Bone Mineral in the Treatment of Intrabony Defects: A Reentry Study. J. Periodontol. 2002, 73, 198–205. [Google Scholar] [CrossRef]
- Harnack, L.; Boedeker, R.H.; Kurtulus, I.; Boehm, S.; Gonzales, J.; Meyle, J. Use of platelet-rich plasma in periodontal surgery—a prospective randomised double blind clinical trial. Clin. Oral Investig. 2008, 13, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Döri, F.; Húszár, T.; Nikolidakis, D.; Arweiler, N.B.; Gera, I.; Sculean, A. Effect of Platelet-Rich Plasma on the Healing of Intrabony Defects Treated with an Anorganic Bovine Bone Mineral and Expanded Polytetrafluoroethylene Membranes. J. Periodontol. 2007, 78, 983–990. [Google Scholar] [CrossRef]
- Döri, F.; Kovács, V.; Arweiler, N.B.; Húszár, T.; Gera, I.; Nikolidakis, D.; Sculean, A. Effect of Platelet-Rich Plasma on the Healing of Intrabony Defects Treated with an Anorganic Bovine Bone Mineral: A Pilot Study. J. Periodontol. 2009, 80, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, M.; Aspriello, S.D.; Rubini, C.; Ferrante, L.; Procaccini, M. Treatment of Periodontal Intrabony Defects with Demineralized Freeze-Dried Bone Allograft in Combination with Platelet-Rich Plasma: A Comparative Clinical Trial. J. Periodontol. 2008, 79, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Karp, J.M.; Sarraf, F.; Shoichet, M.S.; Davies, J.E. Fibrin-filled scaffolds for bone-tissue engineering: Anin vivo study. J. Biomed. Mater. Res. 2004, 71, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Nowzari, H.; Rich, S.K. Risk of Prion Disease Transmission through Bovine-Derived Bone Substitutes: A Systematic Review. Clin. Implant. Dent. Relat. Res. 2011, 15, 645–653. [Google Scholar] [CrossRef]
- Landesberg, R.; Moses, M.; Karpatkin, M. Risks of using platelet rich plasma gel. J. Oral Maxillofac. Surg. 1998, 56, 1116–1117. [Google Scholar] [CrossRef]
- Lekovic, V.; Milinkovic, I.; Aleksic, Z.; Jankovic, S.; Stankovic, P.; Kenney, E.B.; Camargo, P.M. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J. Periodontal Res. 2012, 47, 409–417. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Pai, S.; Garg, G.; Devi, P.; Shetty, S.K. A randomized clinical trial of autologous platelet-rich plasma in the treatment of mandibular degree II furcation defects. J. Clin. Periodontol. 2009, 36, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pradeep, A. Treatment of 3-Wall Intrabony Defects in Patients with Chronic Periodontitis with Autologous Platelet-Rich Fibrin: A Randomized Controlled Clinical Trial. J. Periodontol. 2011, 82, 1705–1712. [Google Scholar] [CrossRef]
- Thorat, M.; Pradeep, A.R.; Pallavi, B. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: A controlled clinical trial. J. Clin. Periodontol. 2011, 38, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Gaekwad, S.S.; Gujjari, S.K.; Veerendra Kumar, S.C.; Veerendra Kumar, S.C. Platelet-Rich Fibrin in Regeneration of Intrabony Defects: A Randomized Controlled Trial. J. Periodontol. 2017, 88, 1192–1199. [Google Scholar] [CrossRef]
- Bajaj, P.; Agarwal, E.; Rao, N.S.; Naik, S.B.; Pradeep, A.; Kalra, N.; Priyanka, N.; Kumari, M. Autologous Platelet-Rich Fibrin in the Treatment of 3-Wall Intrabony Defects in Aggressive Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pradeep, A.R. Autologous Platelet-Rich Fibrin in the Treatment of Mandibular Degree II Furcation Defects: A Randomized Clinical Trial. J. Periodontol. 2011, 82, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Arabacı, T.; Kose, O.; Albayrak, M.; Cicek, Y.; Kizildag, A.; Arabaci, T. Advantages of Autologous Platelet-Rich Fibrin Membrane on Gingival Crevicular Fluid Growth Factor Levels and Periodontal Healing: A Randomized Split-Mouth Clinical Study. J. Periodontol. 2017, 88, 771–777. [Google Scholar] [CrossRef]
- Turkal, H.A.; Demirer, S.; Dolgun, A.B.; Keceli, H.G.; Turkal, H.A. Evaluation of the adjunctive effect of platelet-rich fibrin to enamel matrix derivative in the treatment of intrabony defects. Six-month results of a randomized, split-mouth, controlled clinical study. J. Clin. Periodontol. 2016, 43, 955–964. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Bajaj, P.; Rao, N.S.; Agarwal, E.; Naik, S.B. Platelet-Rich Fibrin Combined with a Porous Hydroxyapatite Graft for the Treatment of 3-Wall Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 1288–1296. [Google Scholar] [CrossRef]
- Pradeep, A.; Rao, N.S.; Agarwal, E.; Bajaj, P.; Kumari, M.; Naik, S.B. Comparative Evaluation of Autologous Platelet-Rich Fibrin and Platelet-Rich Plasma in the Treatment of 3-Wall Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2012, 83, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Pradeep, A.R.; Agarwal, E.; Rao, N.S.; Naik, S.B.; Priyanka, N.; Kalra, N. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of mandibular degree II furcation defects: A randomized controlled clinical trial. J. Periodontal Res. 2013, 48, 573–581. [Google Scholar] [CrossRef]
- He, L.; Lin, Y.; Hu, X.; Zhang, Y.; Wu, H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 707–713. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, J.; Sun, Q.; Yan, S.; Wang, W.; Yang, P.; Song, A. One-Year Results Evaluating the Effects of Concentrated Growth Factors on the Healing of Intrabony Defects Treated with or without Bone Substitute in Chronic Periodontitis. Med. Sci. Monit. 2019, 25, 4384–4389. [Google Scholar] [CrossRef]
- Sohn, D.-S.; Heo, J.-U.; Kwak, D.-H.; Kim, D.-E.; Kim, J.-M.; Moon, J.-W.; Lee, J.-H.; Park, I.-S. Bone Regeneration in the Maxillary Sinus Using an Autologous Fibrin-Rich Block with Concentrated Growth Factors Alone. Implant. Dent. 2011, 20, 389–395. [Google Scholar] [CrossRef]
- Cameli, N.; Mariano, M.; Cordone, I.; Abril, E.; Masi, S.; Foddai, M.L. Autologous Pure Platelet-Rich Plasma Dermal Injections for Facial Skin Rejuvenation: Clinical, Instrumental, and Flow Cytometry Assessment. Dermatol. Surg. 2017, 43, 826–835. [Google Scholar] [CrossRef]
- Gawdat, H.I.; Hegazy, R.A.; Fawzy, M.M.; Fathy, M. Autologous Platelet Rich Plasma: Topical Versus Intradermal After Fractional Ablative Carbon Dioxide Laser Treatment of Atrophic Acne Scars. Dermatol. Surg. 2014, 40, 152–161. [Google Scholar] [CrossRef]
- Sclafani, A.P. Platelet-rich fibrin matrix for improvement of deep nasolabial folds. J. Cosmet. Dermatol. 2010, 9, 66–71. [Google Scholar] [CrossRef]
- Hassan, H.; Quinlan, D.J.; Ghanem, A. Injectable platelet-rich fibrin for facial rejuvenation: A prospective, single-center study. J. Cosmet. Dermatol. 2020, 19, 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, C.; Frustaci, I.; Armellin, E.; Condò, R.; Arcuri, C.; Cerroni, L. Autologous blood preparations rich in platelets, fibrin and growth factors. ORAL Implantol. 2015, 8, 96–113. [Google Scholar]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.-Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant. Dent. 2016, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, A.H.; Belal, S.M. Clinical and Radiographic Evaluation of Socket Preservation Using Autologous Concentrated Growth Factors Enriched Bone Graft Matrix (Sticky Bone): A Case Report. EC Dent. Sci. 2016, 5, 1128–1135. [Google Scholar]
- Shyu, S.-S.; Fu, E.; Shen, E.-C. Clinical and Microcomputed Topography Evaluation of the Concentrated Growth Factors as a Sole Material in a Cystic Bony Defect in Alveolar Bone Followed by Dental Implantation. Implant. Dent. 2016, 25, 707–714. [Google Scholar] [CrossRef]
- Kim, J.-M.; Sohn, D.-S.; Bae, M.-S.; Moon, J.-W.; Lee, J.-H.; Park, I.-S. Flapless Transcrestal Sinus Augmentation Using Hydrodynamic Piezoelectric Internal Sinus Elevation with Autologous Concentrated Growth Factors Alone. Implant. Dent. 2014, 23, 168–174. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, Z.; Zheng, D.; Lin, P.; Cai, Y.; Hong, S.; Lai, Y.; Wu, D. Inlay osteotome sinus floor elevation with concentrated growth factor application and simultaneous short implant placement in severely atrophic maxilla. Sci. Rep. 2016, 6, 27348. [Google Scholar] [CrossRef]
- Tadic, A.; Puskar, T.; Petronijevic, B. Application of fibrin rich blocks with concentrated growth factors in pre-implant augmentation procedures. Med. Pregl. 2014, 67, 177–180. [Google Scholar] [CrossRef]
- Gheno, E.; Palermo, A.; Rodella, L.F.; Buffoli, B. The effectiveness of the use of xenogeneic bone blocks mixed with autologous Concentrated Growth Factors (CGF) in bone regeneration techniques: A case series. J. Osseointegration 2014, 6, 37–42. [Google Scholar] [CrossRef]
- Akcan, S.K.; Ünsal, B. Gingival recession treatment with concentrated growth factor membrane: A comparative clinical trial. J. Appl. Oral. Sci. 2020, 28, e20190236. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, T.S. Concentrated Growth Factor Membrane—A Novel Barrier for Accelerated Repair of Gingival Defect Along with Sliding Flap Technique. Int. J. Curr. Res. Rev. 2016, 8, 1–5. [Google Scholar]
- Li, X.; Yang, H.; Zhang, Z.; Yan, Z.; Lv, H.; Zhang, Y.; Wu, B. Concentrated growth factor exudate enhances the proliferation of human periodontal ligament cells in the presence of TNF α. Mol. Med. Rep. 2018, 19, 943–950. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Yu, L.; Zhou, J.; Zheng, D.; Zhang, B. Effect of Concentrated Growth Factor (CGF) on the Promotion of Osteogenesis in Bone Marrow Stromal Cells (BMSC) in vivo. Sci. Rep. 2018, 8, 5876. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; Wang, Z. A comparative study of the effect of Bio-Oss® in combination with concentrated growth factors or bone marrow-derived mesenchymal stem cells in canine sinus grafting. J. Oral Pathol. Med. 2016, 46, 528–536. [Google Scholar] [CrossRef]
- Sohn, D.-S.; Huang, B.; Kim, J.; Park, W.E.; Park, C.C. Utilization of Autologous Concentrated Growth Factors (CGF) Enriched Bone Graft Matrix (Sticky Bone) and CGF-Enriched Fibrin Membrane in Implant Dentistry. J. Implant Adv. Clin. Dent. 2015, 7, 17–29. [Google Scholar]
- Koyuncu, B.Ö.; Çelik, K.I.; Yüce, M.Ö.; Günbay, T.; Çömlekoğlu, M.E. The role of concentrated growth factor on implant stability: A preliminary study. J. Stomatol. Oral. Maxillofac. Surg. 2020, 121, 363–367. [Google Scholar] [CrossRef]
- Isler, S.C.; Soysal, F.; Ceyhanlı, T.; Bakırarar, B.; Unsal, B. Regenerative surgical treatment of peri-implantitis using either a collagen membrane or concentrated growth factor: A 12-month randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2018, 20, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Samadi, P.; Sheykhhasan, M.; Khoshinani, H.M. The Use of Platelet-Rich Plasma in Aesthetic and Regenerative Medicine: A Comprehensive Review. Aesthetic Plast. Surg. 2019, 43, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Mayes, A.E.; Murray, P.G.; A Gunn, D.; Tomlin, C.C.; Catt, S.D.; Wen, Y.B.; Zhou, L.P.; Wang, H.Q.; Catt, M.; Granger, S.P. Ageing appearance in China: Biophysical profile of facial skin and its relationship to perceived age. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, F.; Liborio, F.; Barone, S.; Antonelli, A.; Buffone, C.; Fortunato, L.; Giudice, A. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: A pilot study. Clin. Oral Investig. 2021, 1–9. [Google Scholar] [CrossRef]
- Trink, A.; Sorbellini, E.; Bezzola, P.; Rodella, L.F.; Ramot, Y.; Rezzani, R.; Rinaldi, F. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br. J. Dermatol. 2013, 169, 690–694. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).