Unravelling the Extent of Diversity within the Iberian Medicinal Leeches (Hirudinea: Hirudo) Using Molecules and Morphology
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
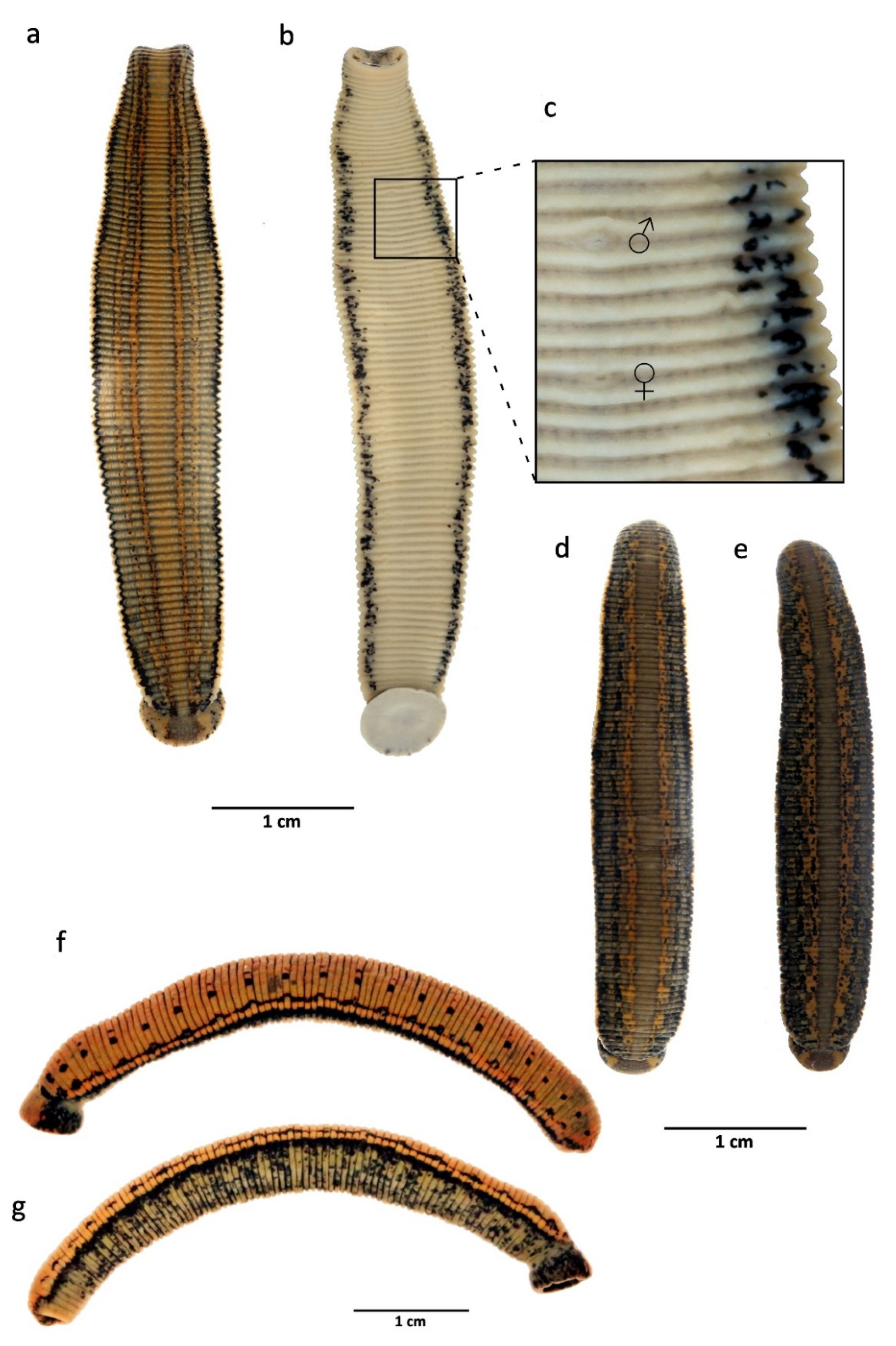
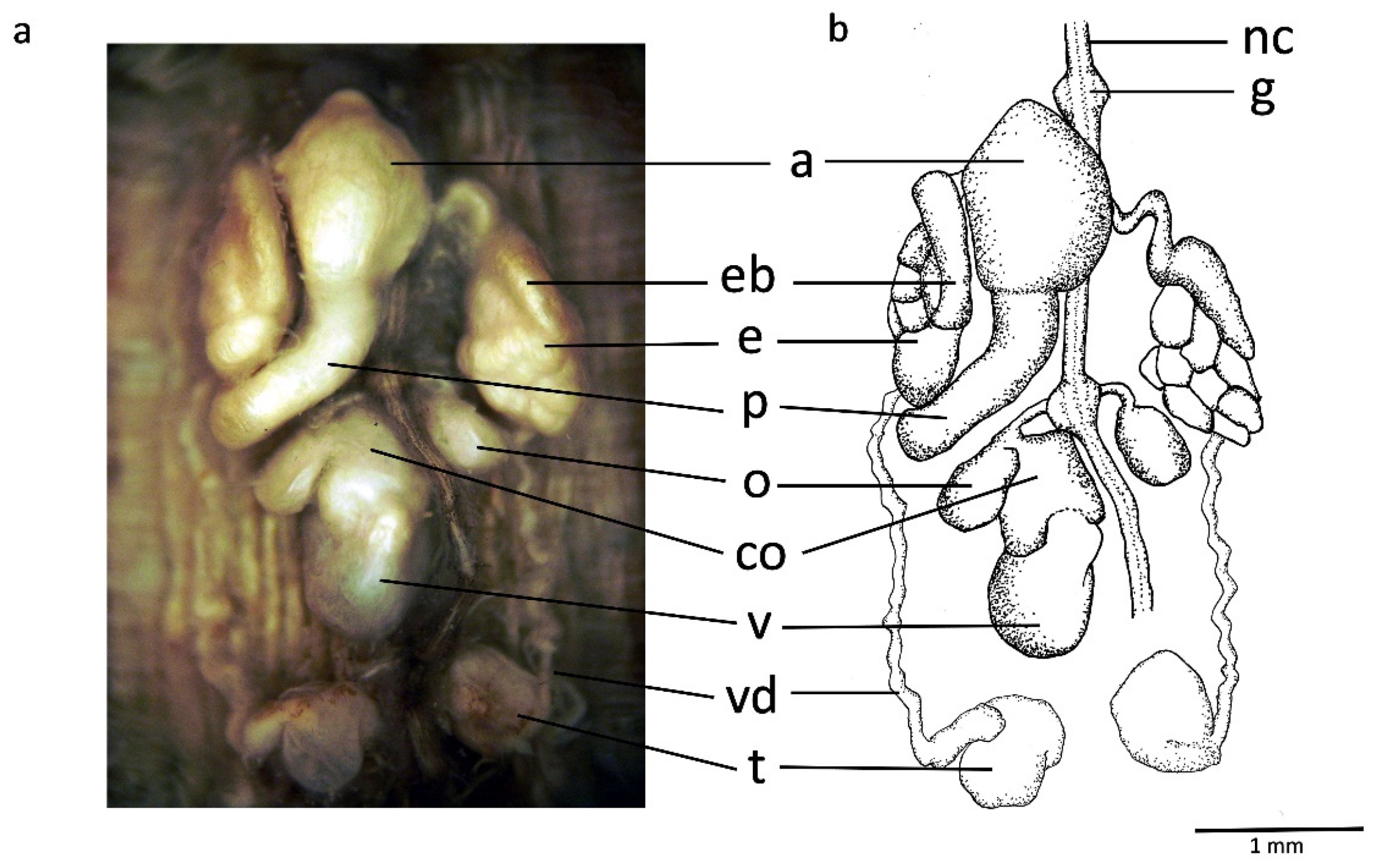
2.1. Species Sampling and Morphological Examination
2.2. Molecular Methods
3. Results
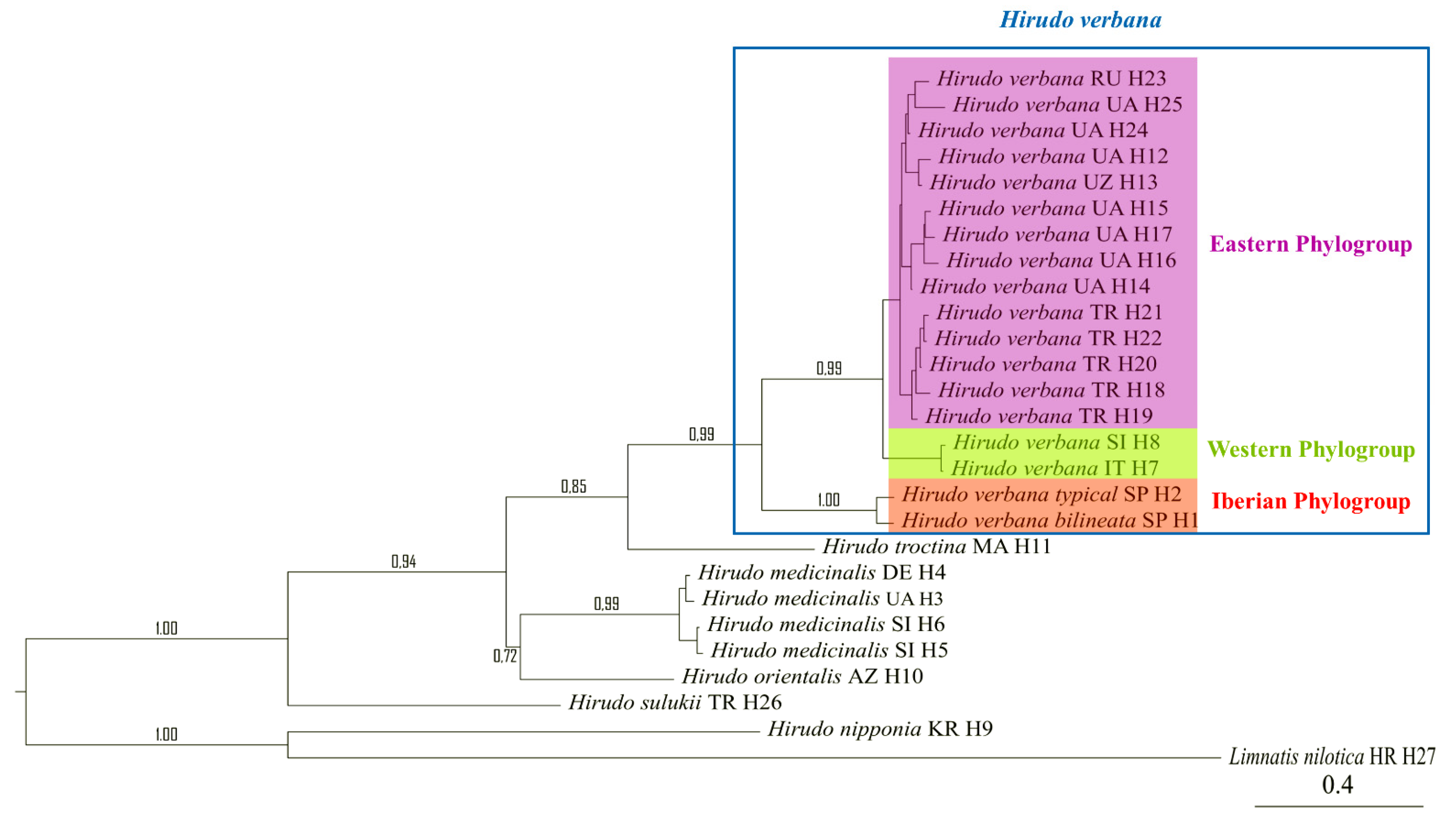
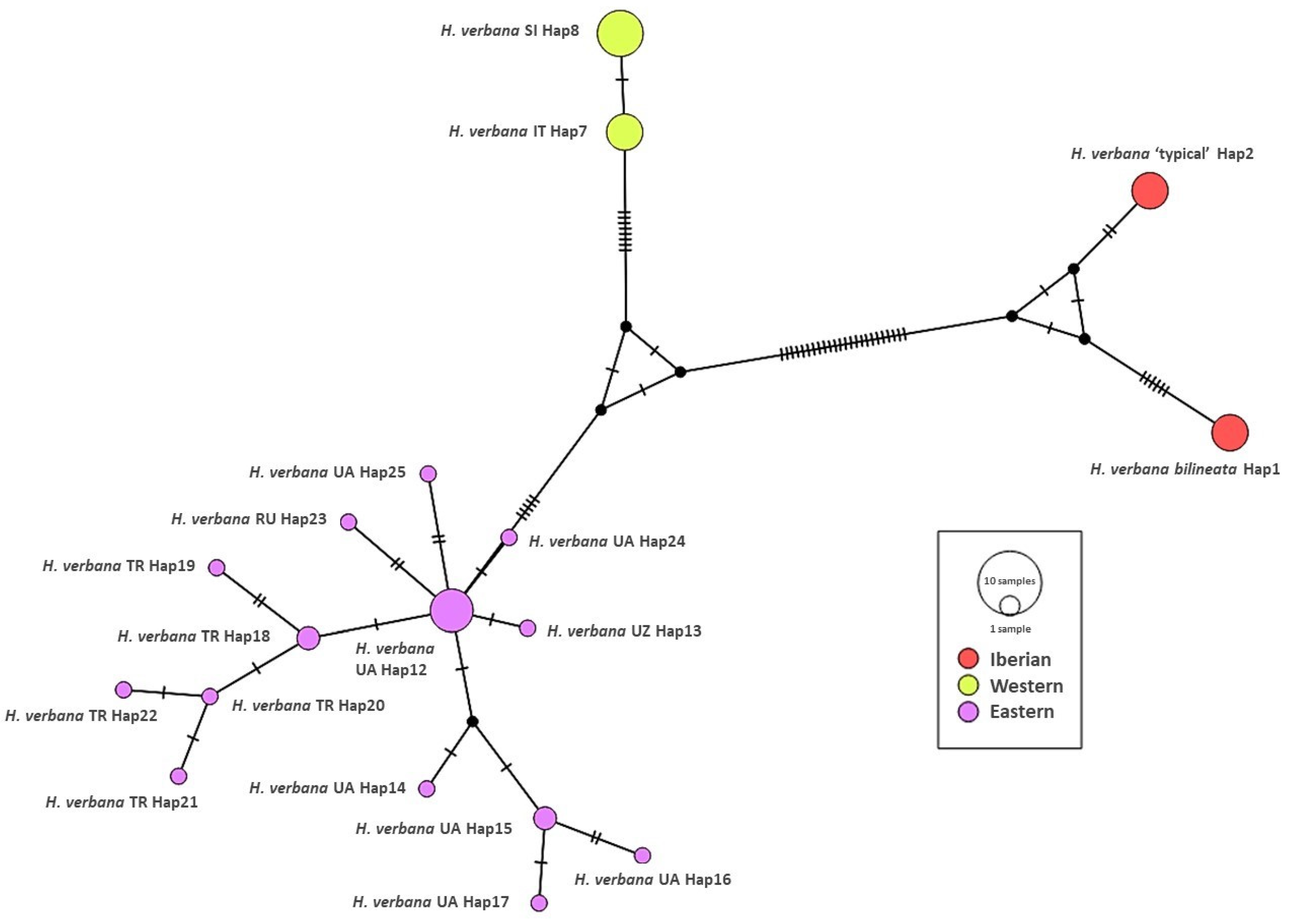
3.1. Phylogenetic Reconstruction
3.2. Taxonomic Account
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species/Provenance | GenBank Acc. No COI | GenBank Acc. No 12S | Haplotype | Reference |
|---|---|---|---|---|
| Limnatis cf. nilotica | AY763152 | AY763161 | H27 | (Trontelj & Utevsky [1]) |
| Hirudo medicinalis Linnaeus, 1758 | ||||
| Gorelaya Dolina, Kharkiv Region, UA | AY763148 | AY763156 | H3 | (Trontelj & Utevsky [1]) |
| Saale River, Rotherburg, Halle, DE | AY763148 | AY763157 | H4 | (Trontelj & Utevsky [1]) |
| Podvinci pond, Ptuj, SI | AY763149 | AY763158 | H5 | (Trontelj & Utevsky [1]) |
| Petajnci gravel pit; Murska Sobota, SI | AY763149 | AY763159 | H6 | (Trontelj & Utevsky [1]) |
| Hirudo verbana Carena, 1820 | ||||
| Lecce, IT | AY763150 | AY763160 | H7 | (Trontelj & Utevsky [1])) |
| Lake Ohrid, Ohrid, MK | AY763150 | AY763160 | H7 | (Trontelj & Utevsky [1]) |
| Pond Globobaj near Povir, Seqana, SI | AY763151 | AY763160 | H8 | (Trontelj & Utevsky [1]) |
| PA55, Island Pag, HR | EF446690 | AY763160 | H8 | (Siddall et al. [5]) |
| PA54, Island Pag, HR | EF446691 | AY763160 | H8 | (Siddall et al. [5]) |
| OH33, Lake Ohrid, MK | EF446693 | AY763160 | H7 | (Siddall et al. [5]) |
| OH31, Lake Ohrid, MK | EF446694 | AY763160 | H7 | (Siddall et al. [5]) |
| KR41, Gabrovica, SW, Sl | EF446695 | AY763160 | H8 | (Siddall et al. [5]) |
| KR40, Gabrovica, SW, Sl | EF446696 | AY763160 | H8 | (Siddall et al. [5]) |
| X1, Lecce, S IT | EF446698 | AY763160 | H7 | (Siddall et al. [5]) |
| KA49 Povir, SW SI | EF446699 | AY763160 | H8 | (Siddall et al. [5]) |
| KA47 Povir, SW SI | EF446700 | AY763160 | H8 | (Siddall et al. [5]) |
| KA46 Povir, SW SI | EF446701 | AY763160 | H8 | (Siddall et al. [5]) |
| Kharkiv Reg., Horila Dolyna, NE, UA | JN083793 | JN104650 | H12 | (Siddall et al. [5]) |
| Berezivka, SW, UA | JN083793 | JN104650 | H12 | (Siddall et al. [5]) |
| Karateri, UZ | JN083793 | JN104652 | H13 | (Siddall et al. [5]) |
| Kokhany, S, UA | JN083793 | JN104655 | H12 | (Siddall et al. [5]) |
| Krasnodar, RU | JN083793 | JN104649 | H12 | (Trontelj & Utevsky [4]) |
| Severynivka, SW, UA | JN083793 | JN104650 | H12 | (Trontelj & Utevsky [4]) |
| Stavropol, RU | JN083793 | JN104650 | H12 | (Trontelj & Utevsky [4]) |
| Vilkove, SW, UA | JN083793 | JN104649 | H12 | (Trontelj & Utevsky [4]) |
| CRIMEEA. UA | JN083794 | JN104654 | H14 | (Trontelj & Utevsky [4]) |
| Berezivka, SW, UA | JN083795 | JN104650 | H15 | (Trontelj & Utevsky [4]) |
| Shaby, SW, UA | JN083795 | JN104649 | H15 | (Trontelj & Utevsky [4]) |
| Mayaky, Odessa Region, SW, UA | JN083796 | JN104653 | H16 | (Trontelj & Utevsky [4]) |
| Severynivka, SW, UA | JN083798 | JN104651 | H17 | (Trontelj & Utevsky [4]) |
| Izmir, TR | JN083800 | JN104649 | H18 | (Trontelj & Utevsky [4]) |
| North_TR | JN083800 | JN104649 | H18 | (Trontelj & Utevsky [4]) |
| Izmir, TR | JN083801 | JN104649 | H19 | (Trontelj & Utevsky [4]) |
| HV11, Izmir, TR | JN083802 | JN104649 | H20 | (Trontelj & Utevsky [4]) |
| HV12, Izmir, TR | JN083803 | JN104649 | H21 | (Trontelj & Utevsky [4]) |
| HV13, Izmir, TR | JN083804 | JN104649 | H22 | (Trontelj & Utevsky [4]) |
| HV14, Stavropol, RU | JN104641 | JN104650 | H23 | (Trontelj & Utevsky [4]) |
| HV15, Berezivka, SW, UA | JN104642 | JN104650 | H24 | (Trontelj & Utevsky [4]) |
| HV17, MalaK, S, UA | JN104644 | JN104649 | H25 | (Trontelj & Utevsky [4]) |
| ANN003, Bikuña pond, Bikuña, Cuadrilla de Salvatierra, Álava, Basque Country, SP | MT797288 | MT796846 | H1 | Present study |
| ANN004, Bikuña pond, Bikuña, Cuadrilla de Salvatierra, Álava, Basque Country, SP | MT797288 | MT796846 | H1 | Present study |
| ANN005, Bikuña pond, Bikuña, Cuadrilla de Salvatierra, Álava, Basque Country, SP | MT797288 | MT796846 | H1 | Present study |
| ANN006, Bikuña pond, Bikuña, Cuadrilla de Salvatierra, Álava, Basque Country, SP | MT797288 | MT796846 | H1 | Present study |
| ANN007, León, Cueza, Castilla y León, SP | MT797289 | MT796847 | H1 | Present study |
| ANN008, Galicia BUD 3, Gándaras de Budiño pond Pontevedra, Galicia, SP | MT797290 | MT796848 | H2 | Present study |
| ANN009, Galicia BUD 4 Gándaras de Budiño pond Pontevedra, Galicia, SP | MT797290 | MT796848 | H2 | Present study |
| ANN010, Galicia DON 3 Doniños Lake, A Coruña, Galicia, SP | MT797291 | MT796849 | H2 | Present study |
| ANN011, Galicia DON 4 Doniños Lake, A Coruña, Galicia, SP | MT797291 | MT796849 | H2 | Present study |
| ANN012, Galicia OCE 2 Orense, Galicia, SP | MT797292 | MT796850 | H2 | Present study |
| Hirudo nipponia Whitman, 1886 | ||||
| Leech farm Hans Biomed, KR | AY763153 | AY763162 | H9 | (Trontelj & Utevsky [1]) |
| Hirudo orientalis Utevsky & Trontelj, 2005 | ||||
| Unknown locality in Azerbaijan, AZ | AY763154 | AY763163 | H10 | (Trontelj & Utevsky [1]) |
| Agdam District, AZ | AY763154 | AY768704 | H10 | (Trontelj & Utevsky [1]) |
| Hirudo troctina Johnson, 1816 | ||||
| Bazaar in Marrakech, MA | AY763155 | AY763164 | H11 | (Trontelj & Utevsky [1]) |
| Hirudo sulukii Saglam, Saunders, Lang and Shain 2016 | ||||
| HS1, Kara Lake, Adiyaman, TR | KU216239 | KU216262 | H26 | (Saglam et al. [7]) |
References
- Trontelj, P.; Utevsky, S.Y. Celebrity with a neglected taxonomy: Molecular systematics of the medicinal leech (genus Hirudo). Mol. Phylogenet. Evol. 2005, 34, 616–624. [Google Scholar] [CrossRef]
- Nesemann, H.; Neubert, E. Annelida, Clitellata: Branchiobdellida, Acanthobdellea, Hirudinea. In Süßwasserfauna von Mitteleuropa; Schwoerbel, J., Zwick, P., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 1999; pp. 1–62. [Google Scholar]
- Hechtel, F.O.P.; Sawyer, R.T. Toward a taxonomic revision of the medicinal leech Hirudo medicinalis Linnaeus, 1758 (Hirudinea: Hirudinidae): Re-description of Hirudo troctina Johnston, 1816 from North Africa. J. Nat. Hist. 2002, 36, 1269–1289. [Google Scholar] [CrossRef]
- Trontelj, P.; Utevsky, S.Y. Phylogeny and phylogeography of medicinal leeches (genus Hirudo): Fast dispersal and shallow genetic structure. Mol. Phylogenet. Evol. 2012, 63, 475–485. [Google Scholar] [CrossRef]
- Siddall, M.E.; Trontelj, P.; Utevsky, S.Y.; Nkamany, M.; Macdonald, K.S. Diverse molecular data demonstrate that commercially available medicinal leeches are not Hirudo medicinalis. Proc. R. Soc. B 2007, 274, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Utevsky, S.Y.; Zagmajster, M.; Atemasov, A.; Zinenko, O.; Utevska, O.M.; Utevsky, A.Y.; Trontelj, P. Distribution and status of medicinal leeches (genus Hirudo) in the Western Palaearctic: Anthropogenic, ecological, or historical effects? Aquat. Conserv. 2010, 20, 198–210. [Google Scholar] [CrossRef]
- Sağlam, N.; Saunders, R.; Lang, S.A.; Shain, D.H. A new species of Hirudo (Annelida: Hirudinidae): Historical biogeography of Eurasian medicinal leeches. BMC Zool. 2016, 1, 1–12. [Google Scholar] [CrossRef]
- García, M.A. Atlas Climático Ibérico; Agencia Estatal de Meteorología—Instituto de Meteorologia de Portugal—Closas-Orcoyen SL: Madrid, Spain, 2011; pp. 1–8. [Google Scholar]
- Taberlet, P.; Fumagalli, L.; Wust-Saucy, A.G.; Cosson, J.F. Comparative phylogeography and postglacial colonization routes in Europe. Mol. Ecol. 1998, 7, 453–464. [Google Scholar] [CrossRef]
- Hewitt, G.M. Post-glacial re-colonization of European biota. Biol. J. Linn. Soc. 1999, 68, 87–112. [Google Scholar] [CrossRef]
- Gómez, A.; Lunt, D.H. Refugia within refugia: Patterns of phylogeographic concordance in the Iberian Peninsula. In Phylogeography of Southern European Refugia. Evolutionary Perspectives on the Origins and Conservation of European Biodiversity; Weiss, S., Ferrand, N., Eds.; Springer: Dordrecht, Germany, 2007; pp. 155–188. [Google Scholar] [CrossRef]
- Benovics, M.; Desdevises, Y.; Šanda, R.; Vukić, J.; Scheifler, M.; Doadrio, I.; Sousa-Santos, C.; Šimková, A. High diversity of fish ectoparasitic monogeneans (Dactylogyrus) in the Iberian Peninsula: A case of adaptive radiation? Parasitology 2020, 147, 418–430. [Google Scholar] [CrossRef]
- Pastor y López, P. Apuntes Sobre la Fauna Asturiana Bajo su Aspecto Científico e Industrial; Imp. y Lit. de D. Benito González: Oviedo, Spain, 1859; pp. 1–44. [Google Scholar]
- Johansson, L. Hirudineen aus dem nördlichen und ostlichen Spanien, gesammelt von Dr. F. Haas in den Jahren 1914–1919. Abh. Senckenb. Naturforsch. Ges. 1926, 39, 211–217. [Google Scholar]
- García-Más, I.; Muñoz, B. Hirudo medicinalis Linnaeus, 1758. In Los Invertebrados no Insectos de la “Directiva Hábitat” en España; Ramos, M.A., Bragado, D., Fernández, J., Eds.; Ministerio de Medio Ambiente—Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2001; pp. 125–130. [Google Scholar]
- Ayres, C.; Comensaña-Iglesias, J. Leech presence on Iberian Brown Frog, Rana iberica (Amphibia: Anura: Ranidae) from north-western Spain. Acta Herpetol. 2008, 3, 155–159. [Google Scholar]
- Muñoz, B.; Soriano, O. Hirudo medicinalis. In Bases Ecológicas Preliminares para la Conservación de las Especies de Interés Comunitario en España: Invertebrados; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2012; pp. 1–51. [Google Scholar]
- Blanchard, R. Sanguijuelas de la Península Ibérica. An. Soc. Española Hist. Nat. 1893, 22, 234–258. [Google Scholar]
- Rivas-Mateos, M. El Hirudo troctina Johnson, de Extremadura. Boletín Real Soc. Española Hist. Nat. Sección Biológica 1901, 1, 175–377. [Google Scholar]
- García-Más, I.; Jiménez, J.M. Introducción al estudio de las comunidades macrobentónicas de los ríos Asturianos: Hirudineos. Limnetica 1984, 1, 179–186. [Google Scholar]
- Utevsky, S.Y.; Zinenko, A.I.; Atemasov, A.A.; Huseynov, M.A.; Utevska, O.M.; Utevsky, A.Y. New information on the distribution of the medicinal leech (genus Hirudo) in the Iberian Peninsula, the Caucasus and Central Asia. Lauterbornia 2008, 65, 119–130. [Google Scholar]
- Kovalenko, M.V.; Utevsky, S.Y. Transitional morphology in hybrids of Hirudo verbana and H. orientalis (Clitellata, Hirudinida). Vestn. Zool. 2013, 47, 32–36. [Google Scholar] [CrossRef]
- Tessler, M.; de Carle, D.; Voiklis, M.L.; Gresham, O.A.; Neumann, J.S.; Cios, S.; Siddall, M.E. Worms that suck: Phylogenetic analysis of Hirudinea solidifies the position of Acanthobdellida and necessitates the dissolution of Rhynchobdellida. Mol. Phylogenet. Evol. 2018, 127, 129–134. [Google Scholar] [CrossRef]
- Phillips, A.J.; Siddall, M.E. Poly-paraphyly of Hirudinidae: Many lineages of medicinal leeches. BMC Evol. Biol. 2009, 9, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Pennak, R.W. Freshwater Invertebrates of United States; The Ronald Press Company: New York, NY, USA, 1953; pp. 1–769. [Google Scholar] [CrossRef]
- Madoz, P. Diccionario Geográfico-Estadístico-Histórico de España y sus Posesiones de Ultramar; Establecimiento Tipográfico de P. Madoz y L. Sagasti: Madrid, Spain, 1850; Volume I–XVI, pp. 1–625. [Google Scholar]
- Elliott, J.M.; Mann, K.H. A key to British Freshwater Leeches, with Notes on Their Life Cycles and Ecology; Freshwater Biological Association: Ambleside, UK, 1979; pp. 1–72. [Google Scholar]
- Zurita, A.; García-Sánchez, A.M.; Cutillas, C. Ctenophthalmus baeticus boisseauorum (Beaucournu, 1968) and Ctenophthalmus apertus allani (Smit, 1955) (Siphonaptera: Ctenophthalmidae) as synonymous taxa: Morphometric, phylogenetic, and molecular characterization. Bull. Entomol. Res. 2020, 110, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Goyanes, G. Parque Nacional de la Montaña de Covadonga; SNPPC: Madrid, Spain, 1976; pp. 1–226. [Google Scholar]
- Laborda, A.J. Fauna: Naturaleza Leonesa III; Edilesa: León, Spain, 1994; pp. 1–180. [Google Scholar]
- Ocharan, F.J. Anélidos y otros grupos afines. In Zoología: Invertebrados; Anadón, E., Ed.; Júcar: Oviedo, Spain, 1981; pp. 107–109. [Google Scholar]
- Sawyer, R.T. The Portuguese Leech Trade in the 19th Century: The First TransAtlantic Commerce in Medicinal Leeches. O Negócio Português de Sanguessugas no Século XIX: O Primeiro Comércio Transatlântico de Sanguessugas Medicinais. Anu. Cent. Estud. Hist. Atl. 2015, 7, 283–322. [Google Scholar]
- Álvarez, D. Depredación de Mesotriton alpestris por Hirudo medicinalis en los Picos de Europa. Bol. Asoc. Herpetol. Esp. 2010, 21, 25–26. [Google Scholar]
- Fernández-Bernaldo-de-Quirós, C. Contribución al estudio de las sanguijuelas (Hirudinea) de las aguas dulces de Asturias (N. de España). Bol. Cien. Natur. 1982, 30, 107–125. [Google Scholar]
- García-Más, F.; Martínez, F.; Pujante, A. Sanguijuelas y moluscos de las aguas de La Mancha (España). Cuad. Estud. Manch. 1990, 21, 123–148. [Google Scholar]
- Sawyer, R.T. Leech Biology and Behaviour; Oxford University Press: Oxford, UK, 1986; pp. 1–1065. [Google Scholar]
- Folmer, O.; Black, M.; Hoen, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-Del Barrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Datasets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Med. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. Popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Lanfear, R.; Brett, C.; Simon, Y.W.H.; Stephane, G. PartitionFinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.4.2. 2014. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 17 June 2020).
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
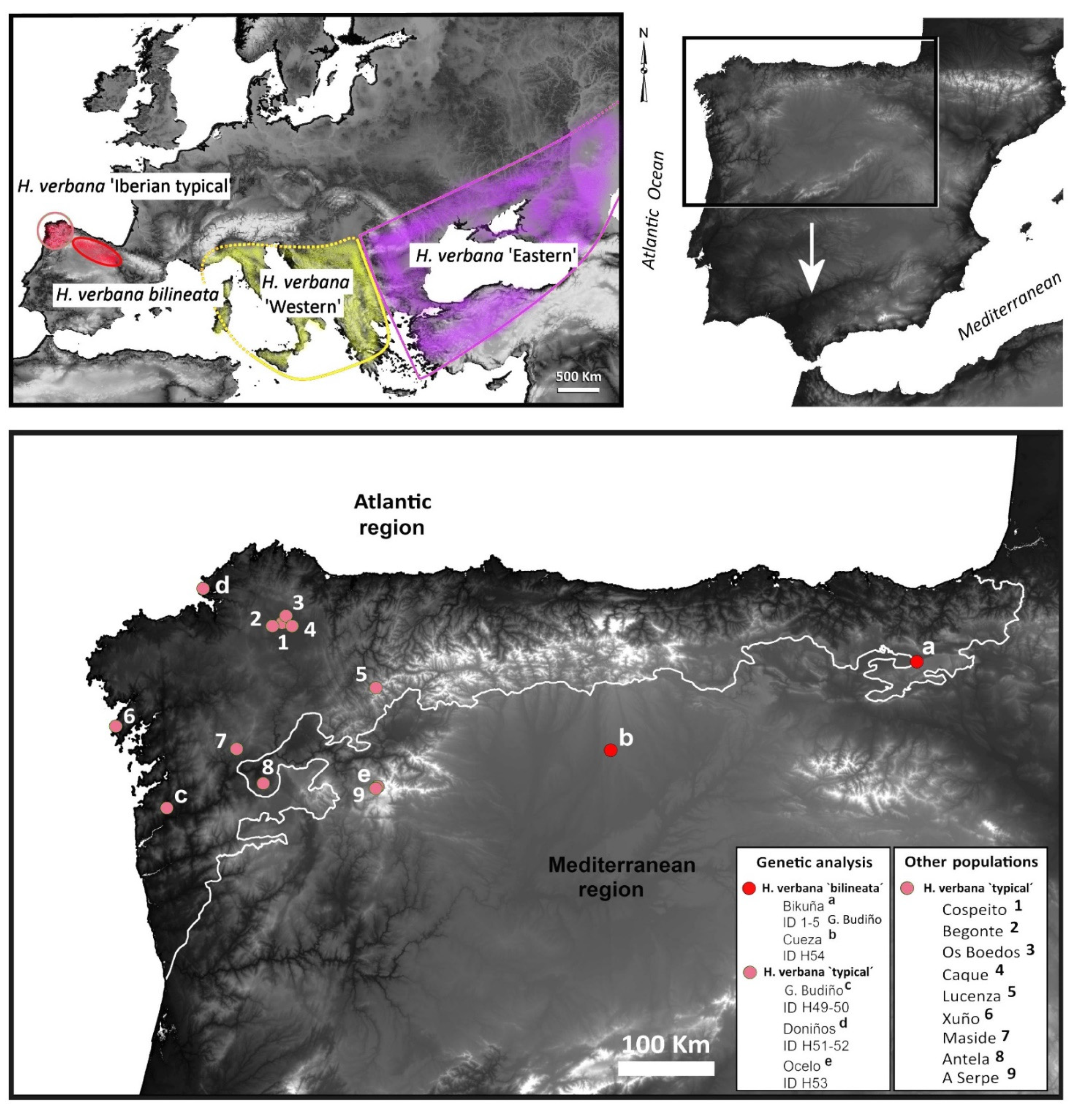



| Sample ID | Species | Morphotype | Locality | Collector /Date |
|---|---|---|---|---|
| ANN003 | Hirudo verbana | “bilineated” | Bikuña pond, 42°50′16″ N, 2°19′24″ W, Álava, Basque Country | A. Arias 26 September 2015 |
| ANN004 | H. verbana | “bilineated” | Bikuña pond, 42°50′16″ N, 2°19′24″ W, Álava, Basque Country | A. Arias 26 September 2015 |
| ANN005 | H. verbana | “bilineated” | Bikuña pond, 42°50′16″ N, 2°19′24″ W, Álava, Basque Country | A. Arias 26 September 2015 |
| ANN006 | H. verbana | “bilineated” | Bikuña pond, 42°50′16″ N, 2°19′24″ W, Álava, Basque Country | A. Arias 26 September 2015 |
| ANN007 | H. verbana. | “bilineated” | Cueza pond, 42°25′35″ N, 4°55′56″ W, León, Castilla y León | R. Carballeira 22 May 2016 |
| ANN008 | H. verbana | “typical” | Gándaras de Budiño, 42°06′43″ N, 8°37′48″ W, Pontevedra, Galicia | R. Carballeira 13 June 2015 |
| ANN009 | H. verbana | “typical” | Gándaras de Budiño, 42°06′43″ N, 8°37′48″ W, Pontevedra, Galicia | R. Carballeira 13 June 2015 |
| ANN010 | H. verbana | “typical” | Doniños Lake, 42°29′31″ N, 8°18′44″ W, A Coruña, Galicia | R. Carballeira 13 June 2015 |
| ANN011 | H. verbana | “typical” | Doniños Lake, 42°29′31″ N, 8°18′44″ W, A Coruña, Galicia Spain | R. Carballeira 13 June 2015 |
| ANN012 | H. verbana | “typical” | Ocelo, 42°13′36″ N, 6°52′22″ W, Orense, Galicia | R. Carballeira 13 June 2015 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 H. medicinalis | 1.3% | 0.9% | 0.9% | 0.9% | 0.9% | 0.9% | 0.9% | 0.9% | 1.4% | |
| 2 H. nipponia | 20% | 1.4% | 1.3% | 1.3% | 1.3% | 1.3% | 1.3% | 1.3% | 1.4% | |
| 3 H. orientalis | 7.3% | 20.8% | 0.9% | 0.9% | 0.9% | 0.9% | 0.9% | 1% | 1.4% | |
| 4 H. troctina | 8% | 18.9% | 8.4% | 0.9% | 0.9% | 0.9% | 0.9% | 1% | 1.4% | |
| 5 H. verbana Eastern Phylogroup | 8.3% | 19.5% | 8.1% | 8.6% | 0.4% | 0.6% | 0.6% | 1% | 1.4% | |
| 6 H. verbana Western Phylogroup | 7.7% | 18.8% | 7.6% | 7.7% | 1.8% | 0.7% | 0.7% | 1% | 1.4% | |
| 7 H. verbana verbana (Iberian) | 7.5% | 18.8% | 7.5% | 7.6% | 3.9% | 4.1% | 0.3% | 1% | 1.4% | |
| 8 H. verbana bilineata | 7.7% | 19.6% | 7.6% | 7.9% | 4.3% | 4.3% | 1% | 1% | 1.4% | |
| 9 H. sulukii | 9.4% | 19.7% | 9.6% | 10.3% | 10% | 9.8% | 9.7% | 10% | 1.4% | |
| 10 Limnatis cf. nilotica | 23.1% | 24.7% | 22.9% | 22.9% | 23.7% | 23.4% | 23.2% | 23.4% | 22.5% |
| Species | No. of Specimens | Habitat | Locality | Coll. Date | Reg. Number |
|---|---|---|---|---|---|
| Hirudo troctina Johnson, 1816 | 6 | Niebla stream | Plasencia, Cáceres, Extremadura | May 1944 | MNCN 16.02/85 |
| Hirudo troctina Johnson, 1816 | 1 | Alagón river | Coria, Cáceres, Extremadura | May 1944 | MNCN 16.02/84 |
| Hirudo troctina Johnson, 1816 | 3 | Tiétar river | Casavieja, Ávila, Castilla y León | May 1932 | MNCN 16.02/81 |
| Hirudo troctina Johnson, 1816 | 1 | Jerte river | Plasencia, Cáceres, Extremadura | May 1944 | MNCN 16.02/71 |
| Hirudo troctina Johnson, 1816 | 1 | Arrago river | Moraleja, Cáceres, Extremadura | June 1944 | MNCN 16.02/70 |
| Hirudo troctina Johnson, 1816 | 1 | Panes stream | Panes, Peñamellera Baja, Asturias | August 1992 | BOS Collection |
| Hirudo troctina Johnson, 1816 | 1 | Panes stream | Panes, Peñamellera Baja, Asturias | August 1992 | Utevsky S. Collection |
| Hirudo troctina Johnson, 1816 | 1 | Bedón river | Naves, Llanes, Asturias | 1980s | BOS Collection |
| Hirudo troctina Johnson, 1817 | 1 | Pas river | Vioño de Piélagos, Piélagos, Cantabria | 1980s | BOS Collection |
| Hirudo verbana cf. verbana Carena, 1820 | 1 | Kuartango Valley ponds | Zuazo de Cuartango, Álava, Basque Country | 1980-1990 | Utevsky S. Collection |
| Hirudo verbana cf. verbana Carena, 1820 | 20 | Kuartango Valley ponds | Zuazo de Cuartango, Álava, Basque Country | 1980-1990 | BOS Collection |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias, A.; Surugiu, V.; Carballeira, R.; Popa, O.P.; Popa, L.O.; Utevsky, S. Unravelling the Extent of Diversity within the Iberian Medicinal Leeches (Hirudinea: Hirudo) Using Molecules and Morphology. Biology 2021, 10, 315. https://doi.org/10.3390/biology10040315
Arias A, Surugiu V, Carballeira R, Popa OP, Popa LO, Utevsky S. Unravelling the Extent of Diversity within the Iberian Medicinal Leeches (Hirudinea: Hirudo) Using Molecules and Morphology. Biology. 2021; 10(4):315. https://doi.org/10.3390/biology10040315
Chicago/Turabian StyleArias, Andrés, Victor Surugiu, Rafael Carballeira, Oana Paula Popa, Luis Ovidiu Popa, and Serge Utevsky. 2021. "Unravelling the Extent of Diversity within the Iberian Medicinal Leeches (Hirudinea: Hirudo) Using Molecules and Morphology" Biology 10, no. 4: 315. https://doi.org/10.3390/biology10040315
APA StyleArias, A., Surugiu, V., Carballeira, R., Popa, O. P., Popa, L. O., & Utevsky, S. (2021). Unravelling the Extent of Diversity within the Iberian Medicinal Leeches (Hirudinea: Hirudo) Using Molecules and Morphology. Biology, 10(4), 315. https://doi.org/10.3390/biology10040315








