The Expression of Anti-Müllerian Hormone Type II Receptor (AMHRII) in Non-Gynecological Solid Tumors Offers Potential for Broad Therapeutic Intervention in Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
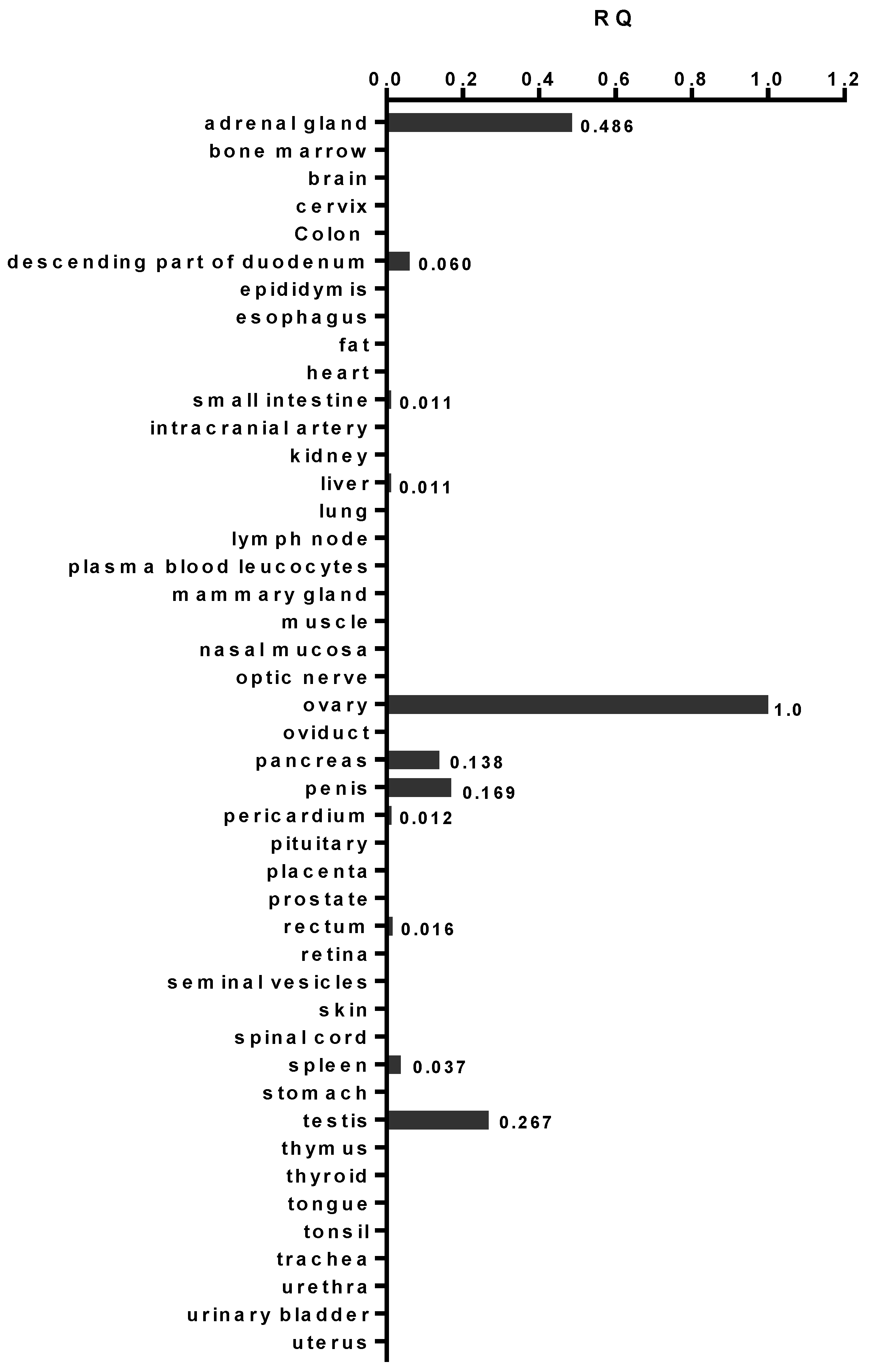
2.1. AMHRII Transcription Is Limited to Very Few Normal Tissue Types
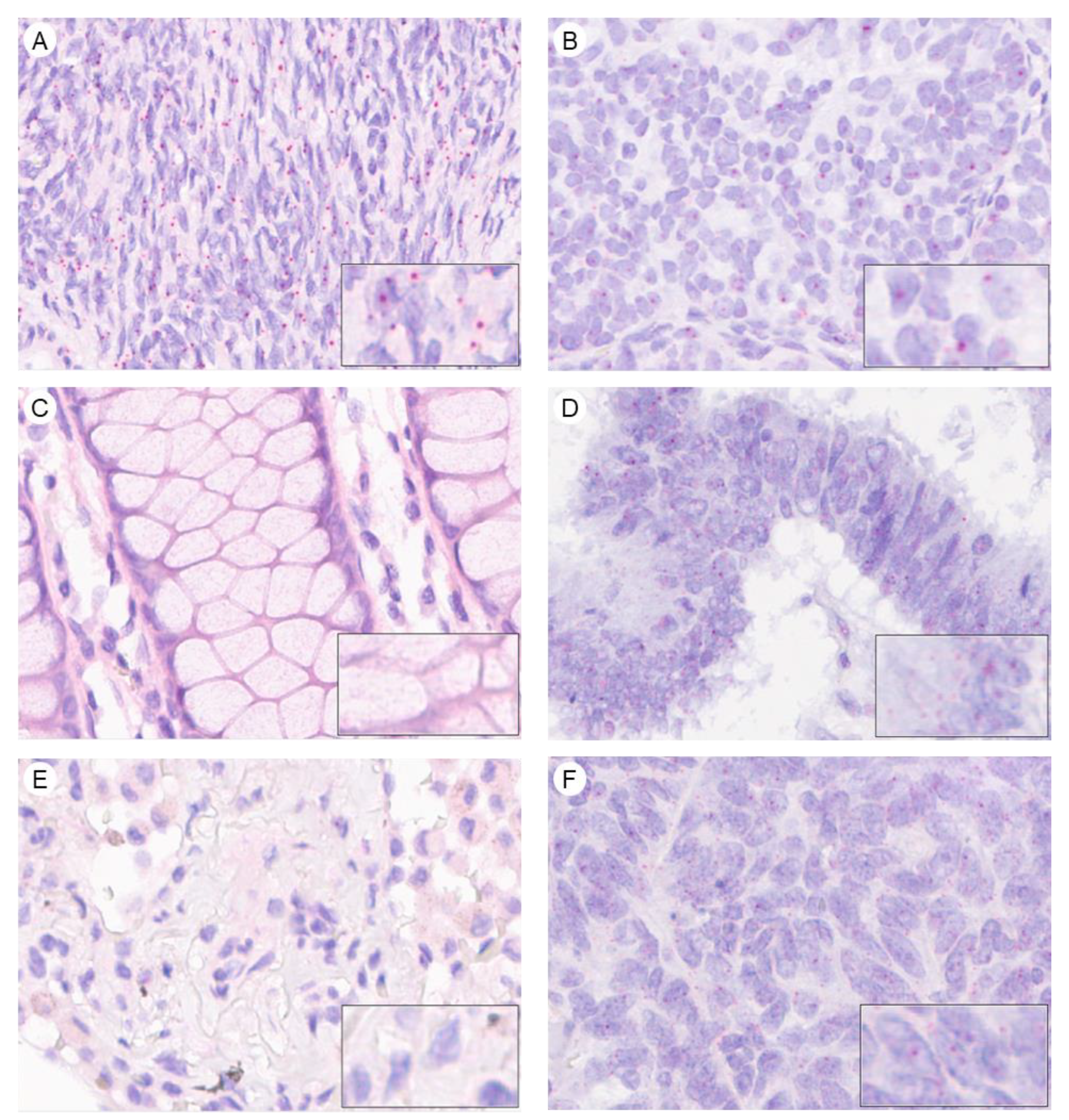
2.2. AMHRII Transcription Is Detected in Gynecological and Non-Gynecological Cancer Tissues
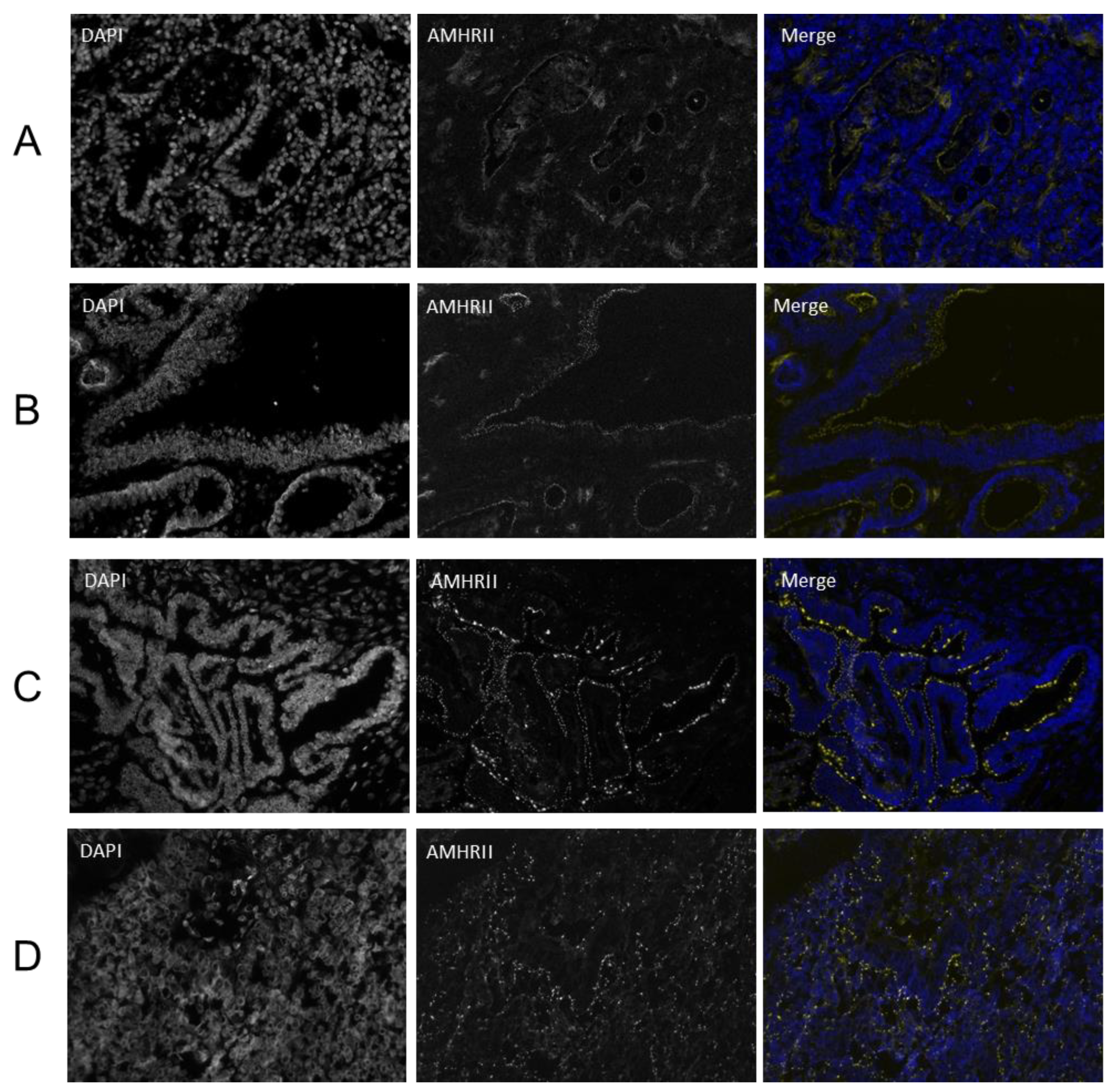
2.3. AMHRII Protein Expression Is Detected in Gynecological and Non-Gynecological Cancer Tissues
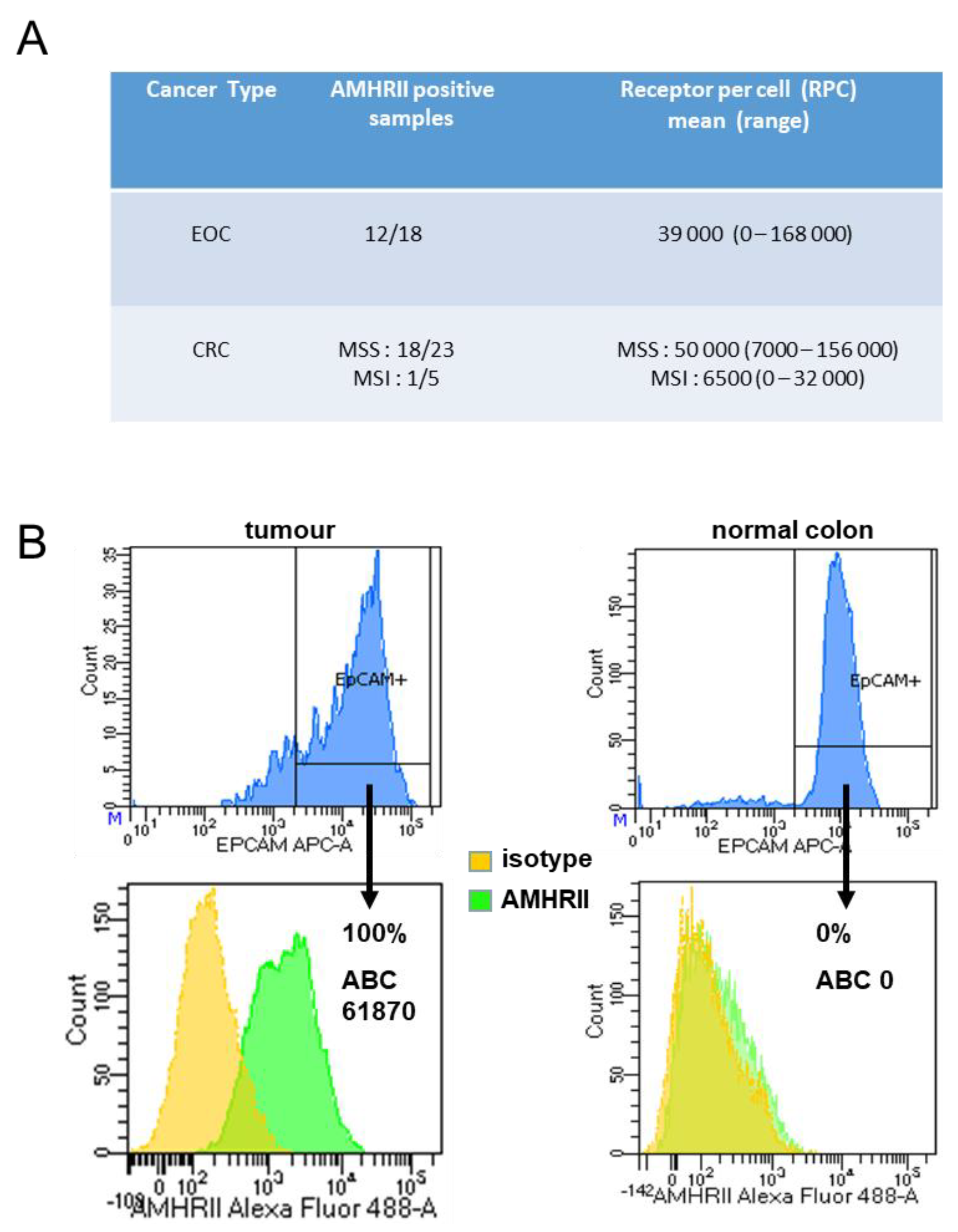
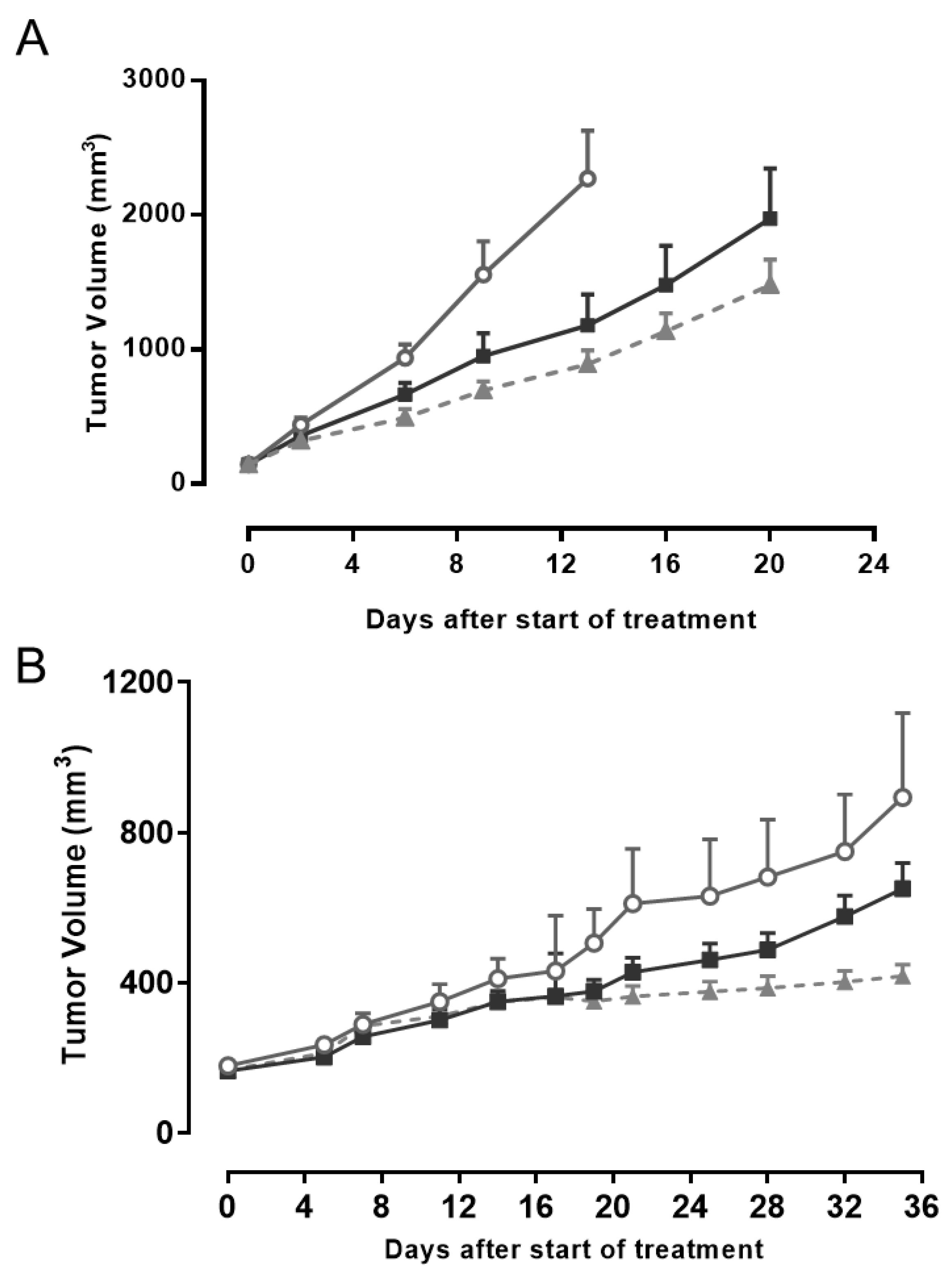
2.4. AMHRII Protein as a Target for Antitumor Activity of Murlentamab, a Low Fucosylated Humanized Anti-AMHRII Antibody
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Patient Specimens
4.3. Reverse Transcriptase Quantitative-PCR
4.4. In Situ Hybridization Using RNAscope
4.5. Immunohistochemistry
4.6. Immunofluorescence
4.7. Flow Cytometry
4.8. Western Blot
4.9. In Vivo Experiments
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baarends, W.M.; van Helmond, M.J.; Post, M.; van der Schoot, P.J.; Hoogerbrugge, J.W.; de Winter, J.P.; Uilenbroek, J.T.; Karels, B.; Wilming, L.G.; Meijers, J.H.; et al. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the Mullerian duct. Development 1994, 120, 189–197. [Google Scholar]
- di Clemente, N.; Wilson, C.; Faure, E.; Boussin, L.; Carmillo, P.; Tizard, R.; Picard, J.Y.; Vigier, B.; Josso, N.; Cate, R. Cloning, expression, and alternative splicing of the receptor for anti-Mullerian hormone. Mol. Endocrinol. 1994, 8, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Belville, C.; Van Vlijmen, H.; Ehrenfels, C.; Pepinsky, B.; Rezaie, A.R.; Picard, J.Y.; Josso, N.; di Clemente, N.; Cate, R.L. Mutations of the anti-Mullerian hormone gene in patients with persistent Mullerian duct syndrome: Biosynthesis, secretion, and processing of the abnormal proteins and analysis using a three-dimensional model. Mol. Endocrinol. 2004, 18, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Jamin, S.P.; Arango, N.A.; Mishina, Y.; Hanks, M.C.; Behringer, R.R. Genetic studies of the AMH/MIS signaling pathway for Mullerian duct regression. Mol. Cell. Endocrinol. 2003, 211, 15–19. [Google Scholar] [CrossRef]
- Visser, J.A. AMH signaling: From receptor to target gene. Mol. Cell. Endocrinol. 2003, 211, 65–73. [Google Scholar] [CrossRef]
- Sriraman, V.; Niu, E.; Matias, J.R.; Donahoe, P.K.; MacLaughlin, D.T.; Hardy, M.P.; Lee, M.M. Mullerian inhibiting substance inhibits testosterone synthesis in adult rats. J. Androl. 2001, 22, 750–758. [Google Scholar]
- Mishina, Y.; Rey, R.; Finegold, M.J.; Matzuk, M.M.; Josso, N.; Cate, R.L.; Behringer, R.R. Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 1996, 10, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Y.; Protheroe, A.; Clarkson, A.N.; Imhoff, F.; Koishi, K.; McLennan, I.S. Mullerian inhibiting substance contributes to sex-linked biases in the brain and behavior. Proc. Natl. Acad. Sci. USA 2009, 106, 7203–7208. [Google Scholar] [CrossRef]
- Cimino, I.; Casoni, F.; Liu, X.; Messina, A.; Parkash, J.; Jamin, S.P.; Catteau-Jonard, S.; Collier, F.; Baroncini, M.; Dewailly, D.; et al. Novel role for anti-Mullerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat. Commun. 2016, 7, 10055. [Google Scholar] [CrossRef]
- Behringer, R.R.; Finegold, M.J.; Cate, R.L. Mullerian-Inhibiting substance function during mammalian sexual development. Cell 1994, 79, 415–425. [Google Scholar] [CrossRef]
- Scully, R.E. Recent progress in ovarian cancer. Hum. Pathol. 1970, 1, 73–98. [Google Scholar] [CrossRef]
- Donahoe, P.K.; Swann, D.A.; Hayashi, A.; Sullivan, M.D. Mullerian duct regression in the embryo correlated with cytotoxic activity against human ovarian cancer. Science 1979, 205, 913–915. [Google Scholar] [CrossRef]
- Bakkum-Gamez, J.N.; Aletti, G.; Lewis, K.A.; Keeney, G.L.; Thomas, B.M.; Navarro-Teulon, I.; Cliby, W.A. Mullerian inhibiting substance type II receptor (MISIIR): A novel, tissue-specific target expressed by gynecologic cancers. Gynecol. Oncol. 2008, 108, 141–148. [Google Scholar] [CrossRef]
- Anttonen, M.; Farkkila, A.; Tauriala, H.; Kauppinen, M.; Maclaughlin, D.T.; Unkila-Kallio, L.; Butzow, R.; Heikinheimo, M. Anti-Mullerian hormone inhibits growth of AMH type II receptor-positive human ovarian granulosa cell tumor cells by activating apoptosis. Lab. Investig. 2011, 91, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Jo, H.H.; Kim, M.R.; Lew, Y.O.; Ryu, K.S.; Cha, J.H.; Kang, C.S.; Donahoe, P.K.; MacLaughlin, D.T.; Kim, J.H. Expression of Mullerian inhibiting substance type II receptor and antiproliferative effects of MIS on human cervical cancer. Int. J. Oncol. 2012, 40, 2013–2021. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kersual, N.; Garambois, V.; Chardes, T.; Pouget, J.P.; Salhi, I.; Bascoul-Mollevi, C.; Bibeau, F.; Busson, M.; Vie, H.; Clemenceau, B.; et al. The human Mullerian inhibiting substance type II receptor as immunotherapy target for ovarian cancer. Validation using the mAb 12G4. MAbs 2014, 6, 1314–1326. [Google Scholar] [CrossRef]
- Pepin, D.; Sosulski, A.; Zhang, L.; Wang, D.; Vathipadiekal, V.; Hendren, K.; Coletti, C.M.; Yu, A.; Castro, C.M.; Birrer, M.J.; et al. AAV9 delivering a modified human Mullerian inhibiting substance as a gene therapy in patient-derived xenografts of ovarian cancer. Proc. Natl. Acad. Sci. USA 2015, 112, E4418–E4427. [Google Scholar] [CrossRef]
- MacLaughlin, D.T.; Donahoe, P.K. Mullerian inhibiting substance/anti-Mullerian hormone: A potential therapeutic agent for human ovarian and other cancers. Future Oncol. 2010, 6, 391–405. [Google Scholar] [CrossRef]
- Kim, J.H.; MacLaughlin, D.T.; Donahoe, P.K. Mullerian inhibiting substance/anti-Mullerian hormone: A novel treatment for gynecologic tumors. Obstet. Gynecol. Sci. 2014, 57, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Barret, J.-M.; Pelegrin, A.; Decaudin, D.; Romeuf, C.D.; Duclos, M.-E.; Bousseau, A.; Combot-Pletan, C.; Prost, J.-F. Preclinical profile of GM102, a first-in-class antibody targeting AMHRII in ovarian cancers. J. Clin. Oncol. 2016, 34, e23196. [Google Scholar] [CrossRef]
- Estupina, P.; Fontayne, A.; Barret, J.M.; Kersual, N.; Dubreuil, O.; Le Blay, M.; Pichard, A.; Jarlier, M.; Pugniere, M.; Chauvin, M.; et al. The anti-tumor efficacy of 3C23K, a glyco-engineered humanized anti-MISRII antibody, in an ovarian cancer model is mainly mediated by engagement of immune effector cells. Oncotarget 2017, 8, 37061–37079. [Google Scholar] [CrossRef] [PubMed]
- Bougherara, H.; Nemati, F.; Nicolas, A.; Massonnet, G.; Pugniere, M.; Ngo, C.; Le Frere-Belda, M.A.; Leary, A.; Alexandre, J.; Meseure, D.; et al. The humanized anti-human AMHRII mAb 3C23K exerts an anti-tumor activity against human ovarian cancer through tumor-associated macrophages. Oncotarget 2017, 8, 99950–99965. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.N.; Korobeynikov, V.A.; Kudinov, A.E.; Georgopoulos, R.; Solanki, N.R.; Andrews-Hoke, M.; Kistner, T.M.; Pepin, D.; Donahoe, P.K.; Nicolas, E.; et al. Anti-Mullerian hormone signaling regulates epithelial plasticity and chemoresistance in lung cancer. Cell Rep. 2016, 16, 657–671. [Google Scholar] [CrossRef]
- Masiakos, P.T.; MacLaughlin, D.T.; Maheswaran, S.; Teixeira, J.; Fuller, A.F., Jr.; Shah, P.C.; Kehas, D.J.; Kenneally, M.K.; Dombkowski, D.M.; Ha, T.U.; et al. Human ovarian cancer, cell lines, and primary ascites cells express the human Mullerian inhibiting substance (MIS) type II receptor, bind, and are responsive to MIS. Clin. Cancer Res. 1999, 5, 3488–3499. [Google Scholar] [PubMed]
- Song, J.Y.; Chen, K.Y.; Kim, S.Y.; Kim, M.R.; Ryu, K.S.; Cha, J.H.; Kang, C.S.; MacLaughlin, D.T.; Kim, J.H. The expression of Mullerian inhibiting substance/anti-Mullerian hormone type II receptor protein and mRNA in benign, borderline and malignant ovarian neoplasia. Int. J. Oncol. 2009, 34, 1583–1591. [Google Scholar] [CrossRef][Green Version]
- Alexander, P. Foetal “antigens” in cancer. Nature 1972, 235, 137–140. [Google Scholar] [CrossRef]
- Castro, J.E.; Lance, E.M.; Medawar, P.B.; Zanelli, J.; Hunt, R. Foetal antigens and cancer. Nature 1973, 243, 225–226. [Google Scholar] [CrossRef]
- Hylander, B.; Repasky, E.; Shrikant, P.; Intengan, M.; Beck, A.; Driscoll, D.; Singhal, P.; Lele, S.; Odunsi, K. Expression of Wilms tumor gene (WT1) in epithelial ovarian cancer. Gynecol. Oncol. 2006, 101, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Klattig, J.; Sierig, R.; Kruspe, D.; Besenbeck, B.; Englert, C. Wilms’ tumor protein Wt1 is an activator of the anti-Mullerian hormone receptor gene Amhr2. Mol. Cell. Biol. 2007, 27, 4355–4364. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.L.; Chin, T.W.; Epstein, J.; Hudson, P.L.; Powell, D.M.; Donahoe, P.K. Recombinant human Mullerian inhibiting substance inhibits human ocular melanoma cell lines in vitro and in vivo. Cancer Res. 1992, 52, 1182–1186. [Google Scholar]
- Hoshiya, Y.; Gupta, V.; Segev, D.L.; Hoshiya, M.; Carey, J.L.; Sasur, L.M.; Tran, T.T.; Ha, T.U.; Maheswaran, S. Mullerian Inhibiting Substance induces NFkB signaling in breast and prostate cancer cells. Mol. Cell. Endocrinol. 2003, 211, 43–49. [Google Scholar] [CrossRef]
- Hirschhorn, T.; di Clemente, N.; Amsalem, A.R.; Pepinsky, R.B.; Picard, J.Y.; Smorodinsky, N.I.; Cate, R.L.; Ehrlich, M. Constitutive negative regulation in the processing of the anti-Mullerian hormone receptor II. J. Cell Sci. 2015, 128, 1352–1364. [Google Scholar] [CrossRef]
- Catlin, E.A.; Tonnu, V.C.; Ebb, R.G.; Pacheco, B.A.; Manganaro, T.F.; Ezzell, R.M.; Donahoe, P.K.; Teixeira, J. Mullerian inhibiting substance inhibits branching morphogenesis and induces apoptosis in fetal rat lung. Endocrinology 1997, 138, 790–796. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Segev, D.L.; Ha, T.U.; Tran, T.T.; Kenneally, M.; Harkin, P.; Jung, M.; MacLaughlin, D.T.; Donahoe, P.K.; Maheswaran, S. Mullerian inhibiting substance inhibits breast cancer cell growth through an NFkappa B-mediated pathway. J. Biol. Chem. 2000, 275, 28371–28379. [Google Scholar] [CrossRef]
- Segev, D.L.; Hoshiya, Y.; Hoshiya, M.; Tran, T.T.; Carey, J.L.; Stephen, A.E.; MacLaughlin, D.T.; Donahoe, P.K.; Maheswaran, S. Mullerian-Inhibiting substance regulates NF-kappa B signaling in the prostate in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Clendenen, T.V.; Afanasyeva, Y.; Koenig, K.L.; Agnoli, C.; Brinton, L.A.; Dorgan, J.F.; Eliassen, A.H.; Falk, R.T.; Hallmans, G.; et al. Circulating anti-Mullerian hormone and breast cancer risk: A study in ten prospective cohorts. Int. J. Cancer 2018, 142, 2215–2226. [Google Scholar] [CrossRef]
- Clendenen, T.V.; Ge, W.; Koenig, K.L.; Afanasyeva, Y.; Agnoli, C.; Brinton, L.A.; Darvishian, F.; Dorgan, J.F.; Eliassen, A.H.; Falk, R.T.; et al. Breast cancer risk prediction in women aged 35–50 years: Impact of including sex hormone concentrations in the Gail model. Breast Cancer Res. 2019, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Pellatt, A.J.; Mullany, L.E.; Herrick, J.S.; Sakoda, L.C.; Wolff, R.K.; Samowitz, W.S.; Slattery, M.L. The TGFβ -signaling pathway and colorectal cancer: Associations between dysregulated genes and miRNAs. J. Transl. Med. 2018, 16, 191. [Google Scholar] [CrossRef]
- Pang, B.; Xu, X.; Lu, Y.; Jin, H.; Yang, R.; Jiang, C.; Shao, D.; Liu, Y.; Shi, J. Prediction of new targets and mechanisms for quercetin in the treatment of pancreatic cancer, colon cancer, and rectal cancer. Food Funct. 2019, 10, 5339–5349. [Google Scholar] [CrossRef]
- Meirelles, K.; Benedict, L.A.; Dombkowski, D.; Pepin, D.; Preffer, F.I.; Teixeira, J.; Tanwar, P.S.; Young, R.H.; MacLaughlin, D.T.; Donahoe, P.K.; et al. Human ovarian cancer stem/progenitor cells are stimulated by doxorubicin but inhibited by Mullerian inhibiting substance. Proc. Natl. Acad. Sci. USA 2012, 109, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Vollmer, M.; De Geyter, M.; Litzistorf, Y.; Ladewig, A.; Durrenberger, M.; Guggenheim, R.; Miny, P.; Holzgreve, W.; De Geyter, C. Characterization of an immortalized human granulosa cell line (COV434). Mol. Hum. Reprod. 2000, 6, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.T.; Ma, X.J.; Luo, Y. RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 2012, 14, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Rodina, A.V.; Gukasova, N.V.; Makarov, V.A.; Kondrasheva, I.G.; Khomyakova, A.V.; Posypanova, G.A.; Popova, O.N.; Moskaleva, E.Y.; Severin, S.E. Localization of Mullerian inhibiting substance receptors in various human cancer cell lines. Biochemistry (Moscow) 2008, 73, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Nemati, F.; Daniel, C.; Arvelo, F.; Legrier, M.E.; Froget, B.; Livartowski, A.; Assayag, F.; Bourgeois, Y.; Poupon, M.F.; Decaudin, D. Clinical relevance of human cancer xenografts as a tool for preclinical assessment: Example of in-vivo evaluation of topotecan-based chemotherapy in a panel of human small-cell lung cancer xenografts. Anticancer Drugs 2010, 21, 25–32. [Google Scholar] [CrossRef]
| Cancer Type | Subtype | Number of Samples | % of Samples with GHs ≥ 1.5 |
|---|---|---|---|
| Adrenocortical cancer | 4 | 75 | |
| Renal cell carcinoma | 34 | 71 | |
| Clear cell carcino. | 33 | 73 | |
| Papillary carcino. | 1 | 0 | |
| Ovarian cancer | 76 | 66 | |
| Serous adeno. | 56 | 73 | |
| Mucinous adeno. | 6 | 50 | |
| Endometrioid adeno. | 5 | 40 | |
| Clear cell adeno. | 5 | 80 | |
| Carcinosarcoma | 2 | 50 | |
| Metastatic adeno. | 1 | 0 | |
| Undifferentiated | 1 | 0 | |
| Colorectal cancer | 30 | 53 | |
| Adeno. | 27 | 56 | |
| Mucinous adeno. | 2 | 50 | |
| Carcino. | 1 | 0 | |
| Liver cancer | Hepatocarcinoma | 62 | 53 |
| Non-small cell lung cancer | 18 | 44 | |
| Large cell carcino. | 4 | 50 | |
| Sarcomatoid carcino. | 1 | 100 | |
| Adeno. | 9 | 56 | |
| Squamous cell carcino. | 4 | 0 | |
| Cervical cancer | 45 | 42 | |
| Adeno. | 5 | 0 | |
| Adenosquam. carcino. | 2 | 100 | |
| Squamous cell carcino. | 35 | 43 | |
| Undifferentiated carcino. | 3 | 66 | |
| Testicular cancer | 48 | 27 | |
| Spermatocytoma | 42 | 31 | |
| Spermatocytic seminoma | 3 | 33 | |
| Anaplastic spermacytoma | 3 | 0 | |
| Breast cancer | 102 | 26 | |
| Inv. ductal carcino. Her2+ | 38 | 32 | |
| Inv. ductal carcino. lum. A | 9 | 11 | |
| Inv. ductal carcino. lum. B Her2− | 3 | 0 | |
| Inv. ductal carcino. lum. B Her2+ | 5 | 0 | |
| Inv. ductal carcino. lum. TN | 42 | 26 | |
| Medullary carcino. TN | 5 | 60 | |
| Thyroid cancer | 19 | 21 | |
| Papillary | 11 | 9 | |
| Follicular | 2 | 50 | |
| Medullary | 2 | 0 | |
| Anaplastic | 3 | 33 | |
| Not specified | 1 | 0 | |
| Gastric cancer | Adeno. | 40 | 20 |
| Endometrial cancer | 185 | 18 | |
| Endometrioid adeno. | 130 | 22 | |
| Adenosquam. carcino. | 9 | 1 | |
| Chorionic carcino. | 4 | 0 | |
| Clear cell carcino. | 2 | 0 | |
| Polypoidal endometrial carcino. | 1 | 0 | |
| Squam. cell carcino. | 4 | 0 | |
| Stromal sarcoma | 6 | 0 | |
| Non specified | 29 | 14 | |
| Bladder | 19 | 11 | |
| Transitional cell carcino. | 15 | 13 | |
| Squam. cell carcino. | 2 | 0 | |
| Neuroendo. Small cell carcino. | 2 | 0 | |
| Pancreatic cancer | 78 | 9 | |
| Ductal carcino. | 69 | 9 | |
| Adenosquam. carcino. | 9 | 11 | |
| Prostate cancer | 30 | 7 | |
| Acinar adeno. | 4 | 0 | |
| Other adeno. | 26 | 8 | |
| Head & Neck cancer | Epidermoid carcino. | 52 | 2 |
| Small cell lung cancer | Neuroendo. carcino. | 76 | 1 |
| Non-Hodgkin’s lymphoma | 18 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barret, J.-M.; Nicolas, A.; Jarry, A.; Dubreuil, O.; Meseure, D.; Passat, T.; Perrial, E.; Deleine, C.; Champenois, G.; Gaillard, S.; et al. The Expression of Anti-Müllerian Hormone Type II Receptor (AMHRII) in Non-Gynecological Solid Tumors Offers Potential for Broad Therapeutic Intervention in Cancer. Biology 2021, 10, 305. https://doi.org/10.3390/biology10040305
Barret J-M, Nicolas A, Jarry A, Dubreuil O, Meseure D, Passat T, Perrial E, Deleine C, Champenois G, Gaillard S, et al. The Expression of Anti-Müllerian Hormone Type II Receptor (AMHRII) in Non-Gynecological Solid Tumors Offers Potential for Broad Therapeutic Intervention in Cancer. Biology. 2021; 10(4):305. https://doi.org/10.3390/biology10040305
Chicago/Turabian StyleBarret, Jean-Marc, André Nicolas, Anne Jarry, Olivier Dubreuil, Didier Meseure, Tilda Passat, Emeline Perrial, Cécile Deleine, Gabriel Champenois, Solenne Gaillard, and et al. 2021. "The Expression of Anti-Müllerian Hormone Type II Receptor (AMHRII) in Non-Gynecological Solid Tumors Offers Potential for Broad Therapeutic Intervention in Cancer" Biology 10, no. 4: 305. https://doi.org/10.3390/biology10040305
APA StyleBarret, J.-M., Nicolas, A., Jarry, A., Dubreuil, O., Meseure, D., Passat, T., Perrial, E., Deleine, C., Champenois, G., Gaillard, S., Duchalais, E., Ray-Coquard, I., Lahmar, M., Dumontet, C., Bennouna, J., Bossard, C., Roman-Roman, S., & Prost, J.-F. (2021). The Expression of Anti-Müllerian Hormone Type II Receptor (AMHRII) in Non-Gynecological Solid Tumors Offers Potential for Broad Therapeutic Intervention in Cancer. Biology, 10(4), 305. https://doi.org/10.3390/biology10040305






