The Myokine Irisin Promotes Osteogenic Differentiation of Dental Bud-Derived MSCs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dental Bud Stem Cells (DBSCs) Cultures
2.2. Irisin Treatment
2.3. Western Blot
2.4. Real-Time PCR
2.5. Alkaline Phosphatase (ALP)
2.6. Alizarin Red Staining (ARS)
2.7. Statistical Analysis
3. Results
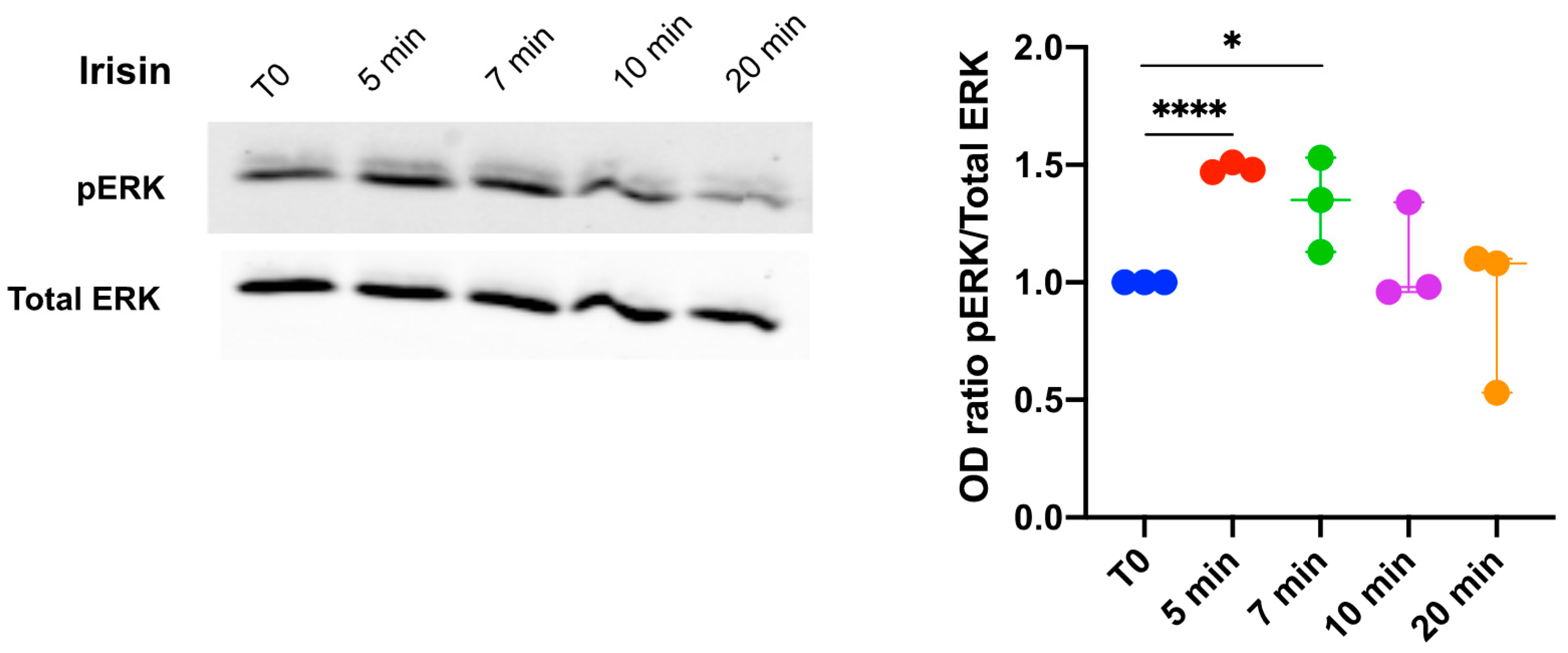
3.1. Extracellular Signal-Regulated Kinase (ERK) Phosphorylation in DBSCs Treated with Irisin
3.2. Irisin Stimulation Increases Osteocalcin (OCN) Expression in DBSCs
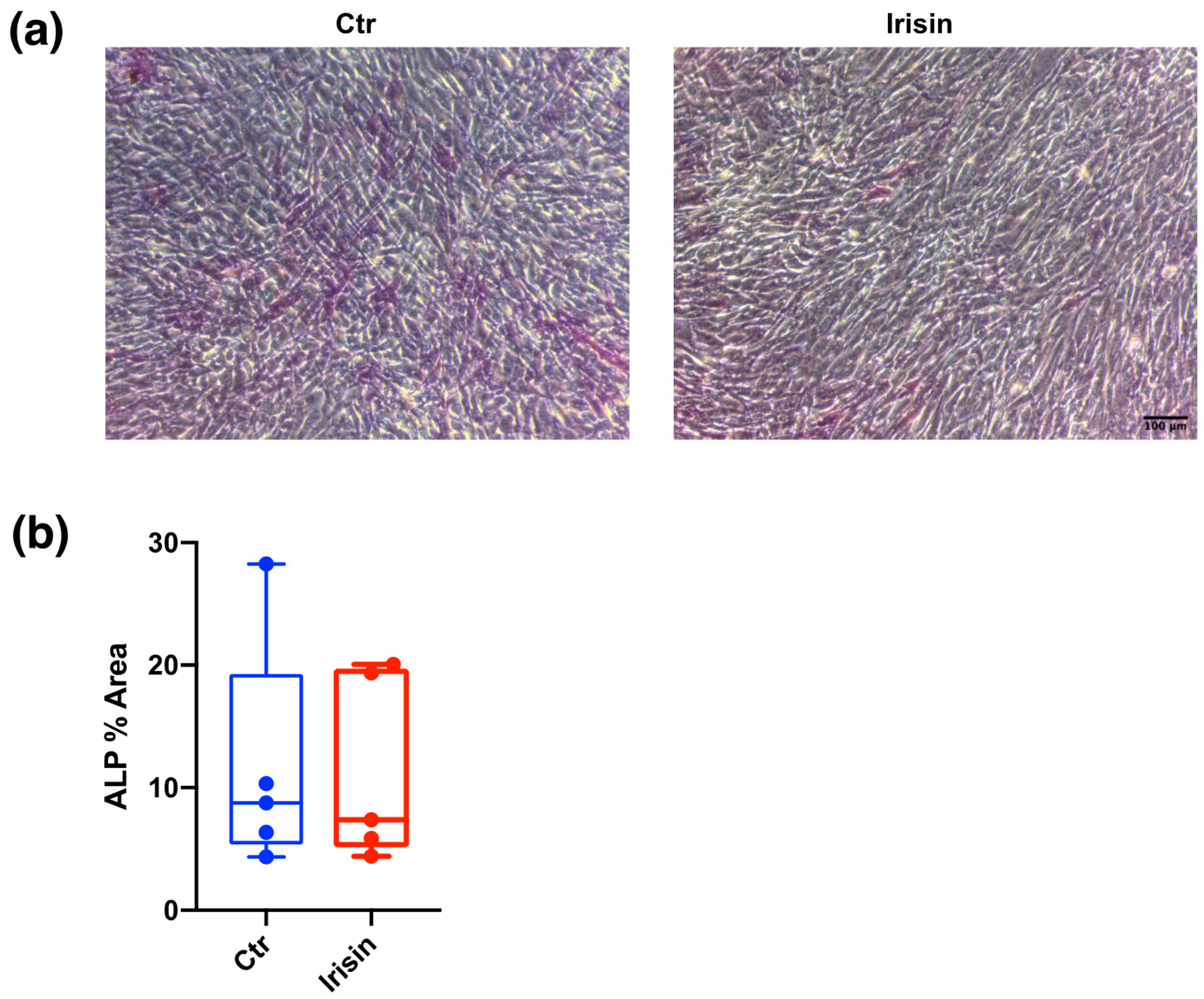
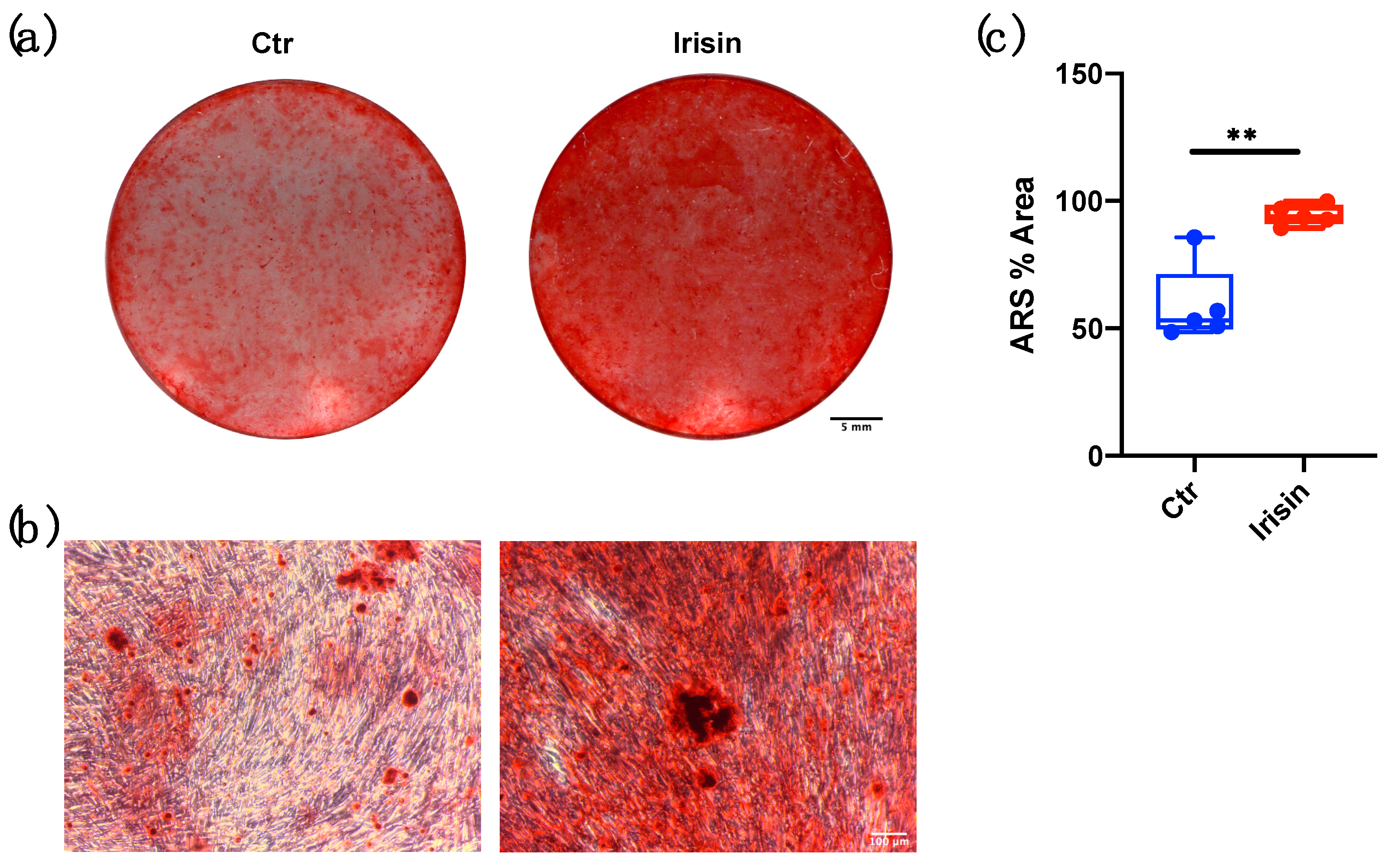
3.3. ALP Positivity and Calcium-Rich Deposit Formation in DBSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodgers, K.; Jadhav, S.S. The application of mesenchymal stem cells to treat thermal and radiation burns. Adv. Drug Deliv. Rev. 2018, 123, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Rodeo, S. Cell therapy in orthopaedics: Where are we in 2019? Bone Jt. J. 2019, 101, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Kebriaei, P.; Isola, L.; Bahceci, E.; Holland, K.; Rowley, S.; McGuirk, J.; Devetten, M.; Jansen, J.; Herzig, R.; Schuster, M. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol. Blood Marrow Transplant. 2009, 15, 804–811. [Google Scholar] [CrossRef]
- Madonna, R.; Geng, Y.-J.; De Caterina, R. Adipose tissue-derived stem cells: Characterization and potential for cardiovascular repair. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1723–1729. [Google Scholar] [CrossRef]
- Řehořová, M.; Vargová, I.; Forostyak, S.; Vacková, I.; Turnovcová, K.; Kupcová Skalníková, H.; Vodička, P.; Kubinová, Š.; Syková, E.; Jendelová, P. A Combination of Intrathecal and Intramuscular Application of Human Mesenchymal Stem Cells Partly Reduces the Activation of Necroptosis in the Spinal Cord of SOD1G93A Rats. Stem Cells Transl. Med. 2019, 8, 535–547. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Huang, G.-J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Mori, G.; Brunetti, G.; Oranger, A.; Carbone, C.; Ballini, A.; Muzio, L.L.; Colucci, S.; Mori, C.; Grassi, F.R.; Grano, M. Dental pulp stem cells: Osteogenic differentiation and gene expression. Ann. N. Y. Acad. Sci. 2011, 1237, 47–52. [Google Scholar] [CrossRef]
- Mori, G.; Centonze, M.; Brunetti, G.; Ballini, A.; Oranger, A.; Mori, C.; Lo, L.M.; Tetè, S.; Ciccolella, F.; Colucci, S. Osteogenic properties of human dental pulp stem cells. J. Biol. Regul. Homeost. Agents 2010, 24, 167–175. [Google Scholar]
- Di Benedetto, A.; Brunetti, G.; Posa, F.; Ballini, A.; Grassi, F.R.; Colaianni, G.; Colucci, S.; Rossi, E.; Cavalcanti-Adam, E.A.; Muzio, L.L. Osteogenic differentiation of mesenchymal stem cells from dental bud: Role of integrins and cadherins. Stem Cell Res. 2015, 15, 618–628. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Posa, F.; De Maria, S.; Ravagnan, G.; Ballini, A.; Porro, C.; Trotta, T.; Grano, M.; Muzio, L.L.; Mori, G. Polydatin, natural precursor of resveratrol, promotes osteogenic differentiation of mesenchymal stem cells. Int. J. Med. Sci. 2018, 15, 944. [Google Scholar] [CrossRef] [PubMed]
- Posa, F.; Di Benedetto, A.; Cavalcanti-Adam, E.A.; Colaianni, G.; Porro, C.; Trotta, T.; Brunetti, G.; Lo Muzio, L.; Grano, M.; Mori, G. Vitamin D promotes MSC osteogenic differentiation stimulating cell adhesion and αVβ3 expression. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef]
- Posa, F.; Di Benedetto, A.; Colaianni, G.; Cavalcanti-Adam, E.A.; Brunetti, G.; Porro, C.; Trotta, T.; Grano, M.; Mori, G. Vitamin D effects on osteoblastic differentiation of mesenchymal stem cells from dental tissues. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, A.; Posa, F.; Marazzi, M.; Kalemaj, Z.; Grassi, R.; Lo Muzio, L.; Comite, M.D.; Cavalcanti-Adam, E.A.; Grassi, F.R.; Mori, G. Osteogenic and Chondrogenic Potential of the Supramolecular Aggregate T-LysYal®. Front. Endocrinol. 2020, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Posa, F.; Di Benedetto, A.; Ravagnan, G.; Cavalcanti-Adam, E.A.; Lo Muzio, L.; Percoco, G.; Mori, G. Bioengineering Bone Tissue with 3D Printed Scaffolds in the Presence of Oligostilbenes. Materials 2020, 13, 4471. [Google Scholar] [CrossRef]
- Pullisaar, H.; Colaianni, G.; Lian, A.-M.; Vandevska-Radunovic, V.; Grano, M.; Reseland, J.E. Irisin promotes growth, migration and matrix formation in human periodontal ligament cells. Arch. Oral Biol. 2020, 111, 104635. [Google Scholar] [CrossRef]
- Son, J.W.; Choi, S.H.; Jang, J.H.; Koh, J.T.; Oh, W.M.; Hwang, Y.C.; Lee, B.N. Irisin promotes odontogenic differentiation and angiogenic potential in human dental pulp cells. Int. Endod. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Nie, Y.; Ma, Y.; Chen, Y.; Cheng, R.; Yin, W.; Hu, Y.; Xu, W.; Xu, L. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci. Rep. 2016, 6, 18732. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Oranger, A.; Mori, G.; Brunetti, G.; Colucci, S.; Cinti, S.; Grano, M. Irisin enhances osteoblast differentiation in vitro. Int. J. Endocrinol. 2014, 2014. [Google Scholar] [CrossRef]
- Zeng, R.; Ma, Y.; Qiao, X.; Zhang, J.; Luo, Y.; Li, S.; Liu, L.; Xu, L. The effect of His-tag and point mutation on the activity of irisin on MC3T3-E1 cells. Biosci. Trends 2018, 12, 580–586. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef]
- Luo, Y.; Qiao, X.; Ma, Y.; Deng, H.; Xu, C.C.; Xu, L. Disordered metabolism in mice lacking irisin. Sci. Rep. 2020, 10, 17368. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, Y.; Qiao, X.; Zeng, R.; Cheng, R.; Nie, Y.; Li, S.; Shen, X.; Yang, M.; Xu, C.C.; et al. Irisin ameliorates bone loss in ovariectomized mice. Climacteric J. Int. Menopause Soc. 2020, 23, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, A.; Carbone, C.; Mori, G. Dental pulp stem cells isolation and osteogenic differentiation: A good promise for tissue engineering. Methods Mol. Biol. 2014, 117–130. [Google Scholar] [CrossRef]
- Ballini, A.; Di Benedetto, A.; De Vito, D.; Scarano, A.; Scacco, S.; Perillo, L.; Posa, F.; Dipalma, G.; Paduano, F.; Contaldo, M.; et al. Stemness genes expression in naive vs. osteodifferentiated human dental-derived stem cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2916–2923. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, G.; Di Benedetto, A.; Posa, F.; Colaianni, G.; Faienza, M.F.; Ballini, A.; Colucci, S.; Passeri, G.; Lo Muzio, L.; Grano, M.; et al. High expression of TRAIL by osteoblastic differentiated dental pulp stem cells affects myeloma cell viability. Oncol. Rep. 2018, 39, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J. Irisin mediates effects on bone and fat via αV integrin receptors. Cell 2018, 175, 1756–1768.e1717. [Google Scholar] [CrossRef]
- Chen, Q.; Kinch, M.S.; Lin, T.H.; Burridge, K.; Juliano, R. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J. Biol. Chem. 1994, 269, 26602–26605. [Google Scholar] [CrossRef]
- Hanna, H.; Mir, L.M.; Andre, F.M. In vitro osteoblastic differentiation of mesenchymal stem cells generates cell layers with distinct properties. Stem Cell Res. Ther. 2018, 9, 203. [Google Scholar] [CrossRef]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Otsuka, A.; Poser, J.W.; Kristaponis, J.; Raman, N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc. Natl. Acad. Sci. USA 1976, 73, 1447–1451. [Google Scholar] [CrossRef]
- Van de Loo, P.; Soute, B.; Van Haarlem, L.; Vermeer, C. The effect of Gla-containing proteins on the precipitation of insoluble salts. Biochem. Biophys. Res. Commun. 1987, 142, 113–119. [Google Scholar] [CrossRef]
- Price, P.A.; Williamson, M.K.; Haba, T.; Dell, R.B.; Jee, W. Excessive mineralization with growth plate closure in rats on chronic warfarin treatment. Proc. Natl. Acad. Sci. USA 1982, 79, 7734–7738. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C. Increased bone formation in osteocalcin-deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef]
- Colaianni, G.; Faienza, M.F.; Sanesi, L.; Brunetti, G.; Pignataro, P.; Lippo, L.; Bortolotti, S.; Storlino, G.; Piacente, L.; D’Amato, G. Irisin serum levels are positively correlated with bone mineral status in a population of healthy children. Pediatric Res. 2019, 85, 484–488. [Google Scholar] [CrossRef]
- Colaianni, G.; Errede, M.; Sanesi, L.; Notarnicola, A.; Celi, M.; Zerlotin, R.; Storlino, G.; Pignataro, P.; Oranger, A.; Pesce, V. Irisin Correlates Positively with BMD in a Cohort of Older Adult Patients and Downregulates the Senescent Marker p21 in Osteoblasts. J. Bone Miner. Res. 2020. [Google Scholar] [CrossRef]
- Ritter, N.M.; Farach-Carson, M.C.; Butler, W.T. Evidence for the formation of a complex between osteopontin and osteocalcin. J. Bone Miner. Res. 1992, 7, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Hoang, Q.Q.; Sicheri, F.; Howard, A.J.; Yang, D.S. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature 2003, 425, 977–980. [Google Scholar] [CrossRef]
- Hauschka, P.V.; Carr, S.A. Calcium-dependent alpha-helical structure in osteocalcin. Biochemistry 1982, 21, 2538–2547. [Google Scholar] [CrossRef] [PubMed]
- Poundarik, A.A.; Diab, T.; Sroga, G.E.; Ural, A.; Boskey, A.L.; Gundberg, C.M.; Vashishth, D. Dilatational band formation in bone. Proc. Natl. Acad. Sci. USA 2012, 109, 19178–19183. [Google Scholar] [CrossRef] [PubMed]
- Nikel, O.; Laurencin, D.; McCallum, S.A.; Gundberg, C.M.; Vashishth, D. NMR investigation of the role of osteocalcin and osteopontin at the organic–inorganic interface in bone. Langmuir 2013, 29, 13873–13882. [Google Scholar] [CrossRef]
- Berezovska, O.; Yildirim, G.; Budell, W.; Yagerman, S.; Pidhaynyy, B.; Bastien, C.; van der Meulen, M.; Dowd, T. Osteocalcin affects bone mineral and mechanical properties in female mice. Bone 2019, 128, 115031. [Google Scholar] [CrossRef] [PubMed]


| Gene | Sense (5′–3′) | Antisense (5′–3′) |
|---|---|---|
| RANK-L | ACAGCACATCAGAGCAGAG | AGGACAGACTCACTTTATGGG |
| OPG | CAAAGGCAGGCGATACTTCC | ATGGAGATGTCCAGAAACACGA |
| OCN | CTCACACTCCTCGCCCTATTG | GCTTGGACACAAAGGCTGCAC |
| Coll I (COL1A1) | TGAAGGGACACAGAGGTTTCAG | GTAGCACCATCATTTCCACGA |
| RUNX-2 | CGCCTCACAAACAACCACAG | ACTGCTTGCAGCCTTAAATGAC |
| BSP | CAATCTGTGCCACTCACTGC | TTTGGTGATTGCTTCCTCTGG |
| β-Actin (ACTB) | AATCGTGCGTGACATTAAG | GAAGGAAGGCTGGAAGAG |
| β2 Microglobulin (B2M) | ATGAGTATGCCTGCCGTGTGA | GGCATCTTCAAACCTCCATG |
| GAPDH | GGAGTCAACGGATTTGGT | GTGATGGGATTTCCATTGAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posa, F.; Colaianni, G.; Di Cosola, M.; Dicarlo, M.; Gaccione, F.; Colucci, S.; Grano, M.; Mori, G. The Myokine Irisin Promotes Osteogenic Differentiation of Dental Bud-Derived MSCs. Biology 2021, 10, 295. https://doi.org/10.3390/biology10040295
Posa F, Colaianni G, Di Cosola M, Dicarlo M, Gaccione F, Colucci S, Grano M, Mori G. The Myokine Irisin Promotes Osteogenic Differentiation of Dental Bud-Derived MSCs. Biology. 2021; 10(4):295. https://doi.org/10.3390/biology10040295
Chicago/Turabian StylePosa, Francesca, Graziana Colaianni, Michele Di Cosola, Manuela Dicarlo, Francesco Gaccione, Silvia Colucci, Maria Grano, and Giorgio Mori. 2021. "The Myokine Irisin Promotes Osteogenic Differentiation of Dental Bud-Derived MSCs" Biology 10, no. 4: 295. https://doi.org/10.3390/biology10040295
APA StylePosa, F., Colaianni, G., Di Cosola, M., Dicarlo, M., Gaccione, F., Colucci, S., Grano, M., & Mori, G. (2021). The Myokine Irisin Promotes Osteogenic Differentiation of Dental Bud-Derived MSCs. Biology, 10(4), 295. https://doi.org/10.3390/biology10040295









