Intrauterine Infusion of TGF-β1 Prior to Insemination, Alike Seminal Plasma, Influences Endometrial Cytokine Responses but Does Not Impact the Timing of the Progression of Pre-Implantation Pig Embryo Development
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Superovulation and the Detection of Estrus
2.4. Insemination of “Donors”
2.5. Preparation of Sperm-Free Seminal Plasma
2.6. Collection and Evaluation of the Embryos
2.7. Endometrial Tissue Collection
2.8. Endometrial Explant Culture
2.9. Cytokine Analysis
2.10. Statistical Analysis
3. Results
3.1. Reproductive Parameters
3.2. Cytokine Production by Endometrial Explants
3.2.1. Effect of SP and TGF-β1 Infusions Prior to AI on Cytokine Production by Endometrial Explants of “Donor” Sows
3.2.2. Effect of SP and TGF-β1 Infusions Prior to AI on Cytokine Production by Endometrial Explants of “Recipient” Sows
3.2.3. Comparison of Cytokine Profiles between the “Donor” and ”Recipient” Sows
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crawford, G.; Ray, A.; Gudi, A.; Shah, A.; Homburg, R. The role of seminal plasma for improved outcomes during in vitro fertilization treatment: Review of the literature and meta-analysis. Hum. Reprod. Update 2015, 21, 275–284. [Google Scholar] [CrossRef]
- Robertson, S.A. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005, 322, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A. Seminal fluid signaling in the female reproductive tract: Lessons from rodents and pigs. J. Anim. Sci. 2007, 85, 36–44. [Google Scholar] [CrossRef]
- Bromfield, J.J. Seminal fluid and reproduction: Much more than previously thought. J. Assist. Reprod. Genet. 2014, 31, 627–636. [Google Scholar] [CrossRef]
- Weitze, K.F.; Rath, D.; Willmen, T.; Waberski, D.; Lotz, J. Advancement of Ovulation in the Sow Related to Seminal Plasma Application before Insemination. Reprod. Domest. Anim. 1990, 25, 61–67. [Google Scholar] [CrossRef]
- Waberski, D.; Claassen, R.; Hahn, T.; Jungblut, P.W.; Parvizi, N.; Kallweit, E.; Weitze, K.F. LH profile and advancement of ovulation after transcervical infusion of seminal plasma at different stages of oestrus in gilts. J. Reprod. Fertil. 1997, 109, 29–34. [Google Scholar] [CrossRef]
- Schuberth, H.J.; Taylor, U.; Zerbe, H.; Waberski, D.; Hunter, R.; Rath, D. Immunological responses to semen in the female genital tract. Theriogenology 2008, 70, 1174–1181. [Google Scholar] [CrossRef]
- O’Leary, S.; Jasper, M.J.; Robertson, S.A.; Armstrong, D.T. Seminal plasma regulates ovarian progesterone production, leukocyte recruitment and follicular cell responses in the pig. Reproduction 2006, 132, 147–158. [Google Scholar] [CrossRef]
- Murray, F.A.; Grifo, A.P.J.; Parker, C.F. Increased litter size in gilts by intrauterine infusion of seminal and sperm antigens before breeding. J. Anim. Sci. 1983, 56, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Mah, J.; Tilton, J.E.; Williams, G.L.; Johnson, J.N.; Marchello, M.J. The effect of repeated mating at short intervals on reproductive performance of gilts. J. Anim. Sci. 1985, 60, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Flowers, W.L.; Esbenshade, K.L. Optimizing management of natural and artificial matings in swine. J. Reprod. Fertil. Suppl. 1993, 48, 217–228. [Google Scholar]
- Rozeboom, K.J.; Troedsson, M.H.; Hodson, H.H.; Shurson, G.C.; Crabo, B.G. The importance of seminal plasma on the fertility of subsequent artificial inseminations in swine. J. Anim. Sci. 2000, 78, 443–448. [Google Scholar] [CrossRef]
- Peitz, B.; Olds-Clarke, P. Effects of seminal vesicle removal on fertility and uterine sperm motility in the house mouse. Biol. Reprod. 1986, 35, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.F.; Chow, P.H.; Wong, T.M. The role of the seminal vesicles, coagulating glands and prostate glands on the fertility and fecundity of mice. J. Reprod. Fertil. 1979, 56, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Queen, K.; Dhabuwala, C.B.; Pierrepoint, C.G. The effect of the removal of the various accessory sex glands on the fertility of male rats. J. Reprod. Fertil. 1981, 62, 423–426. [Google Scholar] [CrossRef]
- Coulam, C.B.; Stern, J.J. Effect of seminal plasma on implantation rates. Early Pregnancy Biol. Med. Off. J. Soc. Investig. Early Pregnancy 1995, 1, 33–36. [Google Scholar]
- Tremellen, K.P.; Valbuena, D.; Landeras, J.; Ballesteros, A.; Martinez, J.; Mendoza, S.; Norman, R.J.; Robertson, S.A.; Simon, C. The effect of intercourse on pregnancy rates during assisted human reproduction. Hum. Reprod. 2000, 15, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H.; Kvist, U.; Ernerudh, J.; Sanz, L.; Calvete, J.J. Seminal plasma proteins: What role do they play? Am. J. Reprod. Immunol. 2011, 66, 11–22. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Robertson, S.A. Seminal fluid and immune adaptation for pregnancy—Comparative biology in mammalian species. Reprod. Domest. Anim. 2014, 49, 27–36. [Google Scholar] [CrossRef]
- Waberski, D.; Schäfer, J.; Bölling, A.; Scheld, M.; Henning, H.; Hambruch, N.; Schuberth, H.J.; Pfarrer, C.; Wrenzycki, C.; Hunter, R.H.F. Seminal plasma modulates the immune-cytokine network in the porcine uterine tissue and pre-ovulatory follicles. PLoS ONE 2018, 13, e0202654. [Google Scholar] [CrossRef]
- Alvarez-Rodriguez, M.; Atikuzzaman, M.; Venhoranta, H.; Wright, D.; Rodriguez-Martinez, H. Expression of immune regulatory genes in the porcine internal genital tract is differentially triggered by spermatozoa and seminal plasma. Int. J. Mol. Sci. 2019, 20, 513. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.A.; Cambra, J.M.; Parrilla, I.; Roca, J.; Ferreira-Dias, G.; Pallares, F.J.; Lucas, X.; Vazquez, J.M.; Martinez, E.A.; Gil, M.A.; et al. Seminal Plasma Modifies the Transcriptional Pattern of the Endometrium and Advances Embryo Development in Pigs. Front. Vet. Sci. 2019, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.A.; Cambra, J.M.; Gil, M.A.; Parrilla, I.; Alvarez-Rodriguez, M.; Rodriguez-Martinez, H.; Cuello, C.; Martinez, E.A. Seminal Plasma Induces Overexpression of Genes Associated with Embryo Development and Implantation in Day-6 Porcine Blastocysts. Int. J. Mol. Sci. 2020, 21, 3662. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, S.; Jasper, M.J.; Warnes, G.M.; Armstrong, D.T.; Robertson, S.A. Seminal plasma regulates endometrial cytokine expression, leukocyte recruitment and embryo development in the pig. Reproduction 2004, 128, 237–247. [Google Scholar] [CrossRef]
- Tremellen, K.P.; Seamark, R.F.; Robertson, S.A. Seminal transforming growth factor beta1 stimulates granulocyte-macrophage colony-stimulating factor production and inflammatory cell recruitment in the murine uterus. Biol. Reprod. 1998, 58, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, D.J.; Tremellen, K.P.; Jasper, M.J.; Gemzell-Danielsson, K.; Robertson, S.A. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J. Immunol. 2012, 188, 2445–2454. [Google Scholar] [CrossRef]
- Robertson, S.A.; Sjoblom, C.; Jasper, M.J.; Norman, R.J.; Seamark, R.F. Granulocyte-macrophage colony-stimulating factor promotes glucose transport and blastomere viability in murine preimplantation embryos. Biol. Reprod. 2001, 64, 1206–1215. [Google Scholar] [CrossRef]
- de Moraes, A.A.; Hansen, P.J. Granulocyte-macrophage colony-stimulating factor promotes development of in vitro produced bovine embryos. Biol. Reprod. 1997, 57, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Sjoblom, C.; Wikland, M.; Robertson, S.A. Granulocyte-macrophage colony-stimulating factor promotes human blastocyst development in vitro. Hum. Reprod. 1999, 14, 3069–3076. [Google Scholar] [CrossRef] [PubMed]
- Sjoblom, C.; Wikland, M.; Robertson, S.A. Granulocyte-macrophage colony-stimulating factor (GM-CSF) acts independently of the beta common subunit of the GM-CSF receptor to prevent inner cell mass apoptosis in human embryos. Biol. Reprod. 2002, 67, 1817–1823. [Google Scholar] [CrossRef][Green Version]
- Robertson, S.A. GM-CSF regulation of embryo development and pregnancy. Cytokine Growth Factor Rev. 2007, 18, 287–298. [Google Scholar] [CrossRef]
- O’Leary, S.; Armstrong, D.T.; Robertson, S.A. Transforming growth factor-β (TGFβ) in porcine seminal plasma. Reprod. Fertil. Dev. 2011, 23, 748–758. [Google Scholar] [CrossRef]
- Robertson, S.A.; Chin, P.-Y.; Femia, J.G.; Brown, H.M. Embryotoxic cytokines-Potential roles in embryo loss and fetal programming. J. Reprod. Immunol. 2018, 125, 80–88. [Google Scholar] [CrossRef]
- Robertson, S.A.; Moldenhauer, L.M. Immunological determinants of implantation success. Int. J. Dev. Biol. 2014, 58, 205–217. [Google Scholar] [CrossRef]
- Syriou, V.; Papanikolaou, D.; Kozyraki, A.; Goulis, D.G. Cytokines and male infertility. Eur. Cytokine Netw. 2018, 29, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Mau, V.J.; Tremellen, K.P.; Seamark, R.F. Role of high molecular weight seminal vesicle proteins in eliciting the uterine inflammatory response to semen in mice. J. Reprod. Fertil. 1996, 107, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, D.J.; Macpherson, A.M.; Tremellen, K.P.; Robertson, S.A. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol. Hum. Reprod. 2007, 13, 491–501. [Google Scholar] [CrossRef]
- Martinez, E.A.; Cuello, C.; Parrilla, I.; Martinez, C.A.; Nohalez, A.; Vazquez, J.L.; Vazquez, J.M.; Roca, J.; Gil, M.A. Recent advances toward the practical application of embryo transfer in pigs. Theriogenology 2016, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.A.; Martinez, C.A.; Cambra, J.M.; Maside, C.; Lucas, X.; Vazquez, J.L.; Vazquez, J.M.; Roca, J.; Rodriguez-Martinez, H.; Gil, M.A.; et al. Achievements and future perspectives of embryo transfer technology in pigs. Reprod. Domest. Anim. 2019, 54 (Suppl. 4), 4–13. [Google Scholar] [CrossRef]
- Nohalez, A.; Martinez, C.A.; Reixach, J.; Diaz, M.; Vila, J.; Colina, I.; Parrilla, I.; Vazquez, J.L.; Roca, J.; Gil, M.A.; et al. Factors of importance when selecting sows as embryo donors. Animal 2017, 11, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Pursel, V.G.; Johnson, L.A. Freezing of boar spermatozoa: Fertilizing capacity with concentrated semen and a new thawing procedure. J. Anim. Sci. 1975, 40, 99–102. [Google Scholar] [CrossRef]
- Martinez, C.A.; Nohalez, A.; Parrilla, I.; Vazquez, J.L.; Roca, J.; Cuello, C.; Rodriguez-Martinez, H.; Martinez, E.A.; Gil, M.A. Surgical embryo collection but not nonsurgical embryo transfer compromises postintervention prolificacy in sows. Theriogenology 2017, 87, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.A.; Martinez, C.A.; Nohalez, A.; Sanchez-Osorio, J.; Vazquez, J.M.; Roca, J.; Parrilla, I.; Gil, M.A.; Cuello, C. Nonsurgical deep uterine transfer of vitrified, in vivo-derived, porcine embryos is as effective as the default surgical approach. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Wright, J.M. Photographic Illustrations of Embryo Developmental Stage and Quality Codes. In Manual of the International Embryo Transfer Society; Stringfellow, D.A., Seidel, S.M., Eds.; International Embryo Transfer Society (IETS): Savoy, IL, USA, 1998. [Google Scholar]
- Martinez, C.A.; Cambra, J.M.; Nohalez, A.; Parrilla, I.; Roca, J.; Vazquez, J.L.; Rodriguez-Martinez, H.; Gil, M.A.; Martinez, E.A.; Cuello, C. Prevention of hatching of porcine morulae and blastocysts by liquid storage at 20 degrees C. Sci. Rep. 2019, 9, 6219. [Google Scholar] [CrossRef] [PubMed]
- Petters, R.M.; Wells, K.D. Culture of pig embryos. J. Reprod. Fertil. Suppl. 1993, 48, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Maeda, T.; Fukunaga, E.; Shiba, R.; Okano, S.; Kinutani, M.; Horiuchi, T. Selection of high-quality and viable blastocysts based on timing of morula compaction and blastocyst formation. Reprod. Med. Biol. 2020, 19, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Ruber, M.; Perez-Patino, C.; Atikuzzaman, M.; Martinez, E.A.; Roca, J.; Rodriguez-Martinez, H. The Seminal Plasma of the Boar is Rich in Cytokines, with Significant Individual and Intra-Ejaculate Variation. Am. J. Reprod. Immunol. 2015, 74, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Padilla, L.; Perez-Patino, C.; Vazquez, J.M.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J.; Parrilla, I. Seminal Plasma Cytokines Are Predictive of the Outcome of Boar Sperm Preservation. Front. Vet. Sci. 2019, 6, 436. [Google Scholar] [CrossRef]
- Rhodes, M.; Brendemuhl, J.H.; Hansen, P.J. Litter characteristics of gilts artificially inseminated with transforming growth factor-β. Am. J. Reprod. Immunol. 2006, 56, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Hyytiäinen, M.; Penttinen, C.; Keski-Oja, J. Latent TGF-beta binding proteins: Extracellular matrix association and roles in TGF-beta activation. Crit. Rev. Clin. Lab. Sci. 2004, 41, 233–264. [Google Scholar] [CrossRef] [PubMed]
- Taylor, U.; Schuberth, H.J.; Rath, D.; Michelmann, H.W.; Sauter-Louis, C.; Zerbe, H. Influence of inseminate components on porcine leucocyte migration in vitro and in vivo after pre- and post-ovulatory insemination. Reprod. Domest. Anim. 2009, 44, 180–188. [Google Scholar] [CrossRef]
- Jiwakanon, J.; Persson, E.; Berg, M.; Dalin, A.M. Influence of seminal plasma, spermatozoa and semen extender on cytokine expression in the porcine endometrium after insemination. Anim. Reprod. Sci. 2011, 123, 210–220. [Google Scholar] [CrossRef]
- Jalali, B.M.; Kitewska, A.; Wasielak, M.; Bodek, G.; Bogacki, M. Effects of seminal plasma and the presence of a conceptus on regulation of lymphocyte-cytokine network in porcine endometrium. Mol. Reprod. Dev. 2014, 81, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Bidarimath, M.; Tayade, C. Pregnancy and spontaneous fetal loss: A pig perspective. Mol. Reprod. Dev. 2017, 84, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; O’Leary, S.; Armstrong, D.T. Influence of semen on inflammatory modulators of embryo implantation. Soc. Reprod. Fertil. Suppl. 2006, 62, 231–245. [Google Scholar] [PubMed]
- Jones, R.L.; Stoikos, C.; Findlay, J.K.; Salamonsen, L.A. TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction 2006, 132, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, I.; Martinez, C.A.; Cambra, J.M.; Lucas, X.; Ferreira-Dias, G.; Rodriguez-Martinez, H.; Cuello, C.; Gil, M.A.; Martinez, E.A. Blastocyst-Bearing Sows Display a Dominant Anti-Inflammatory Cytokine Profile Compared to Cyclic Sows at Day 6 of the Cycle. Animals 2020, 10, 2028. [Google Scholar] [CrossRef]
- Fujiwara, H.; Araki, Y.; Toshimori, K. Is the zona pellucida an intrinsic source of signals activating maternal recognition of the developing mammalian embryo? J. Reprod. Immunol. 2009, 81, 1–8. [Google Scholar] [CrossRef]
- Almiñana, C.; Heath, P.R.; Wilkinson, S.; Sanchez-Osorio, J.; Cuello, C.; Parrilla, I.; Gil, M.A.; Vazquez, J.L.; Vazquez, J.M.; Roca, J.; et al. Early developing pig embryos mediate their own environment in the maternal tract. PLoS ONE 2012, 7, e33625. [Google Scholar] [CrossRef] [PubMed]
- Talukder, A.K.; Yousef, M.S.; Rashid, M.B.; Awai, K.; Acosta, T.J.; Shimizu, T.; Okuda, K.; Shimada, M.; Imakawa, K.; Miyamoto, A. Bovine embryo induces an anti-inflammatory response in uterine epithelial cells and immune cells in vitro: Possible involvement of interferon tau as an intermediator. J. Reprod. Dev. 2017, 63, 425–434. [Google Scholar] [CrossRef]
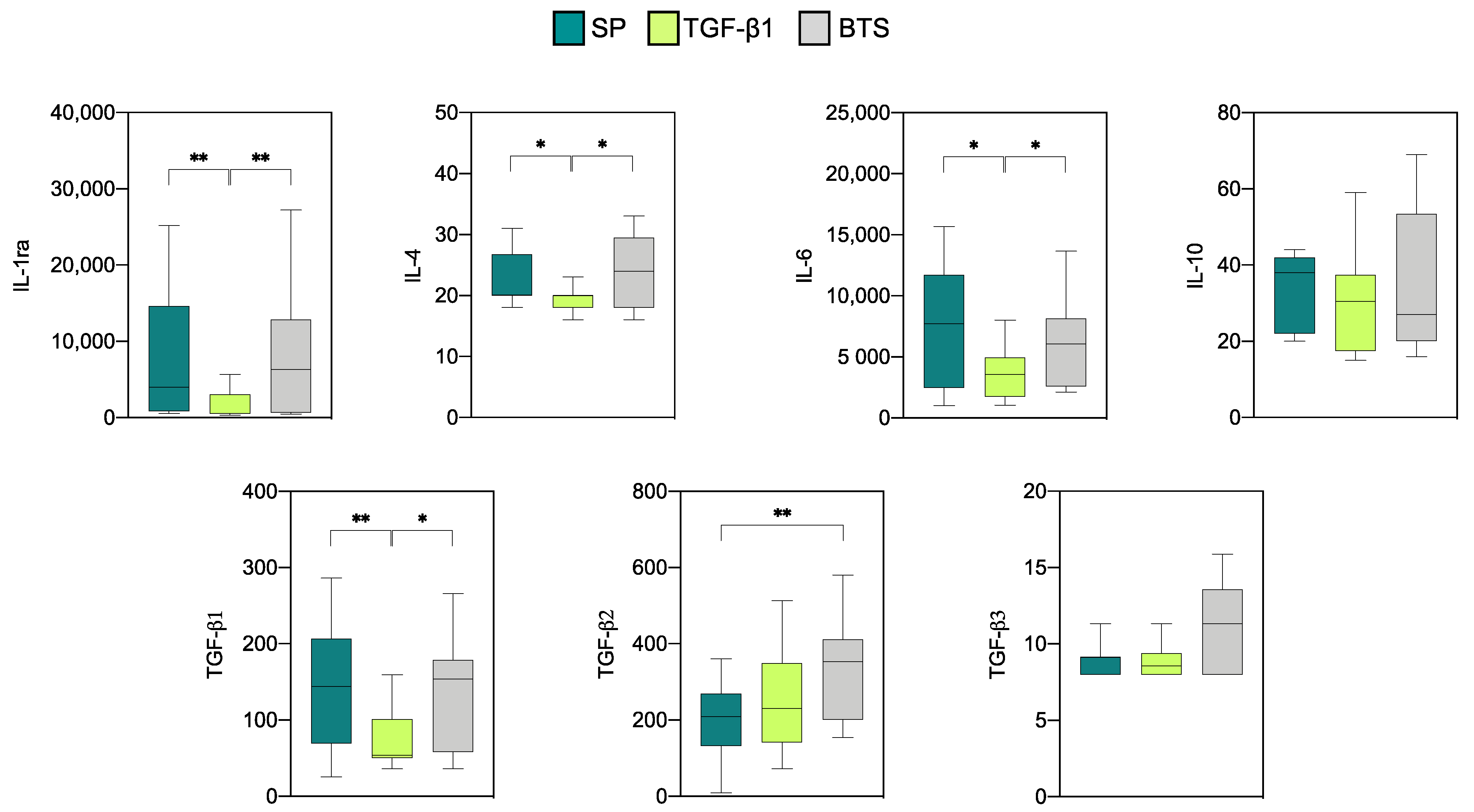

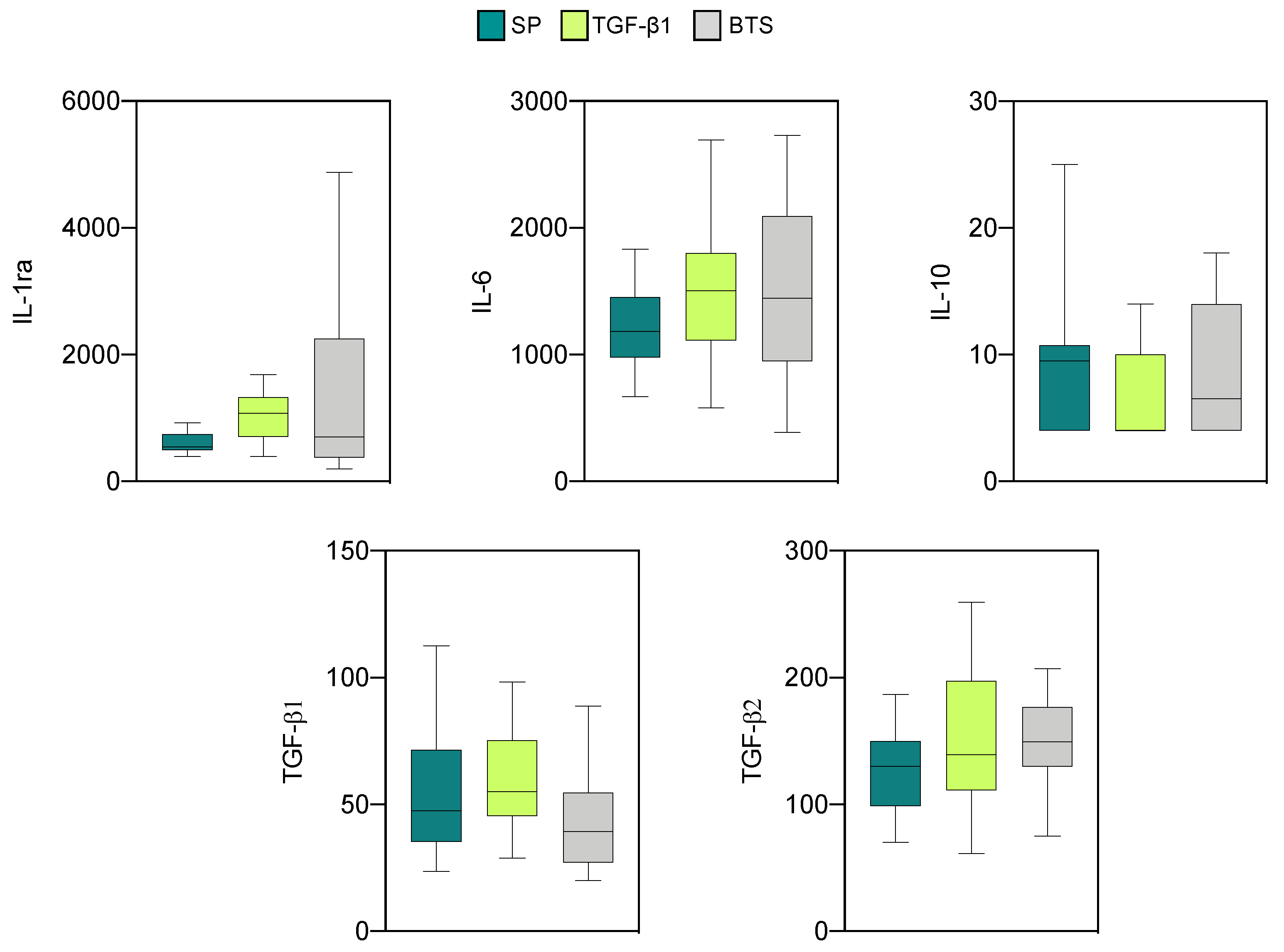
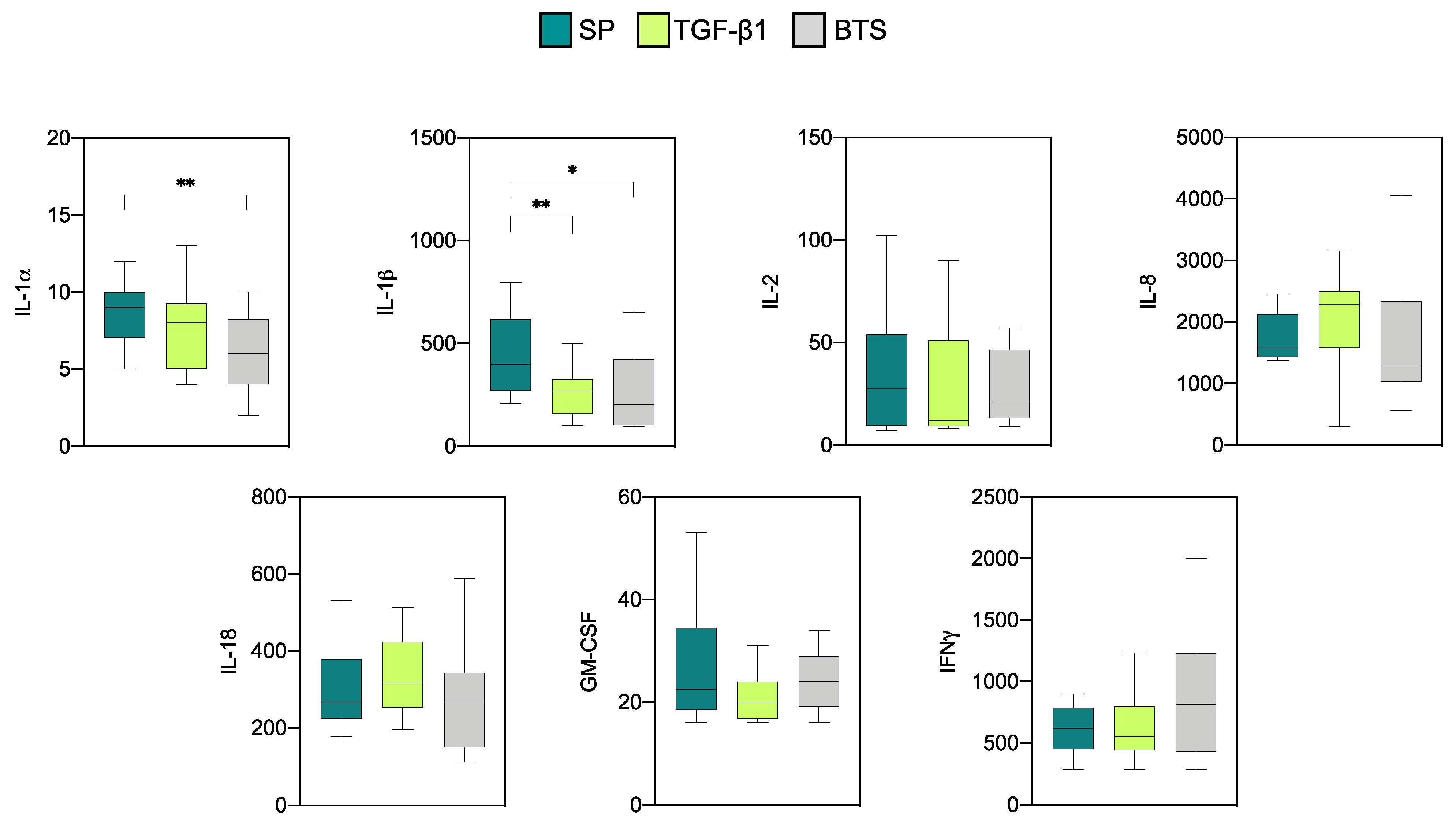

| Treatment | Sows (n) | Corpora Lutea | Recovery Rate (%) | Viable Embryos | Fertilization Rate (%) | Embryonic Stage 1 |
|---|---|---|---|---|---|---|
| SP | 18 | 24.1 ± 8.4 | 93.3 ± 11.0 | 20.1 ± 7.4 | 89.1 ± 19.3 | 2.3 ± 0.7 a |
| TGF-β1 | 14 | 23.7 ± 5.3 | 92.9 ± 12.1 | 19.9 ± 6.0 | 90.4 ± 14.2 | 1.8 ± 0.6 b |
| BTS | 15 | 23.5 ± 6.1 | 93.2 ± 11.5 | 21.3 ± 6.4 | 91.5 ± 14.8 | 1.9 ± 0.7 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, C.A.; Cambra, J.M.; Lucas, X.; Ferreira-Dias, G.; Rodriguez-Martinez, H.; Gil, M.A.; Martinez, E.A.; Cuello, C.; Parrilla, I. Intrauterine Infusion of TGF-β1 Prior to Insemination, Alike Seminal Plasma, Influences Endometrial Cytokine Responses but Does Not Impact the Timing of the Progression of Pre-Implantation Pig Embryo Development. Biology 2021, 10, 159. https://doi.org/10.3390/biology10020159
Martinez CA, Cambra JM, Lucas X, Ferreira-Dias G, Rodriguez-Martinez H, Gil MA, Martinez EA, Cuello C, Parrilla I. Intrauterine Infusion of TGF-β1 Prior to Insemination, Alike Seminal Plasma, Influences Endometrial Cytokine Responses but Does Not Impact the Timing of the Progression of Pre-Implantation Pig Embryo Development. Biology. 2021; 10(2):159. https://doi.org/10.3390/biology10020159
Chicago/Turabian StyleMartinez, Cristina A., Josep M. Cambra, Xiomara Lucas, Graça Ferreira-Dias, Heriberto Rodriguez-Martinez, Maria A. Gil, Emilio A. Martinez, Cristina Cuello, and Inmaculada Parrilla. 2021. "Intrauterine Infusion of TGF-β1 Prior to Insemination, Alike Seminal Plasma, Influences Endometrial Cytokine Responses but Does Not Impact the Timing of the Progression of Pre-Implantation Pig Embryo Development" Biology 10, no. 2: 159. https://doi.org/10.3390/biology10020159
APA StyleMartinez, C. A., Cambra, J. M., Lucas, X., Ferreira-Dias, G., Rodriguez-Martinez, H., Gil, M. A., Martinez, E. A., Cuello, C., & Parrilla, I. (2021). Intrauterine Infusion of TGF-β1 Prior to Insemination, Alike Seminal Plasma, Influences Endometrial Cytokine Responses but Does Not Impact the Timing of the Progression of Pre-Implantation Pig Embryo Development. Biology, 10(2), 159. https://doi.org/10.3390/biology10020159










