A Shorter Equilibration Period Improves Post-Warming Outcomes after Vitrification and in Straw Dilution of In Vitro-Produced Bovine Embryos
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Suppliers
2.2. In Vitro Production of Bovine Blastocysts
2.3. Embryo Vitrification and Warming
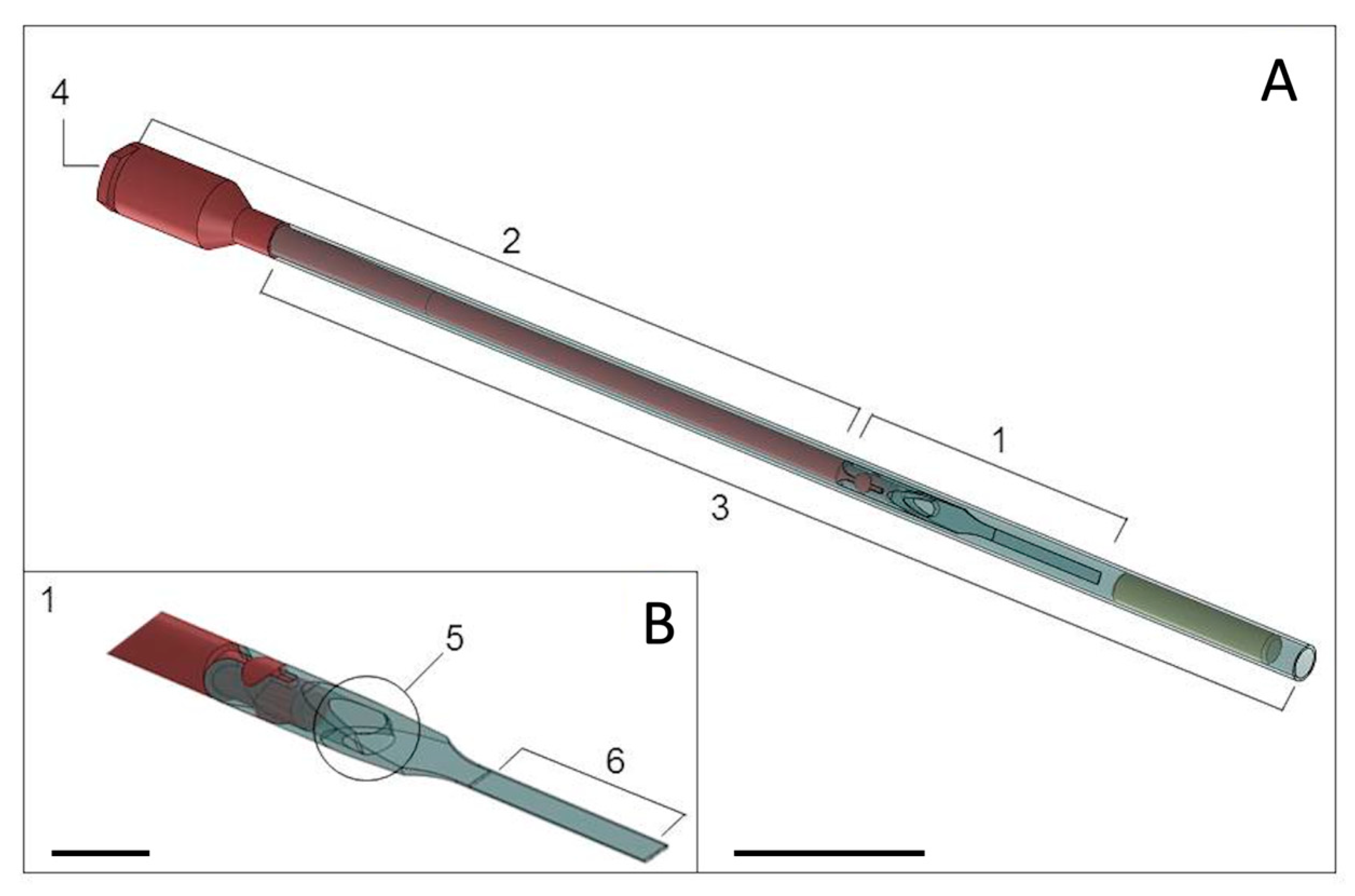
2.3.1. Vitrification Protocol
2.3.2. Warming Protocol
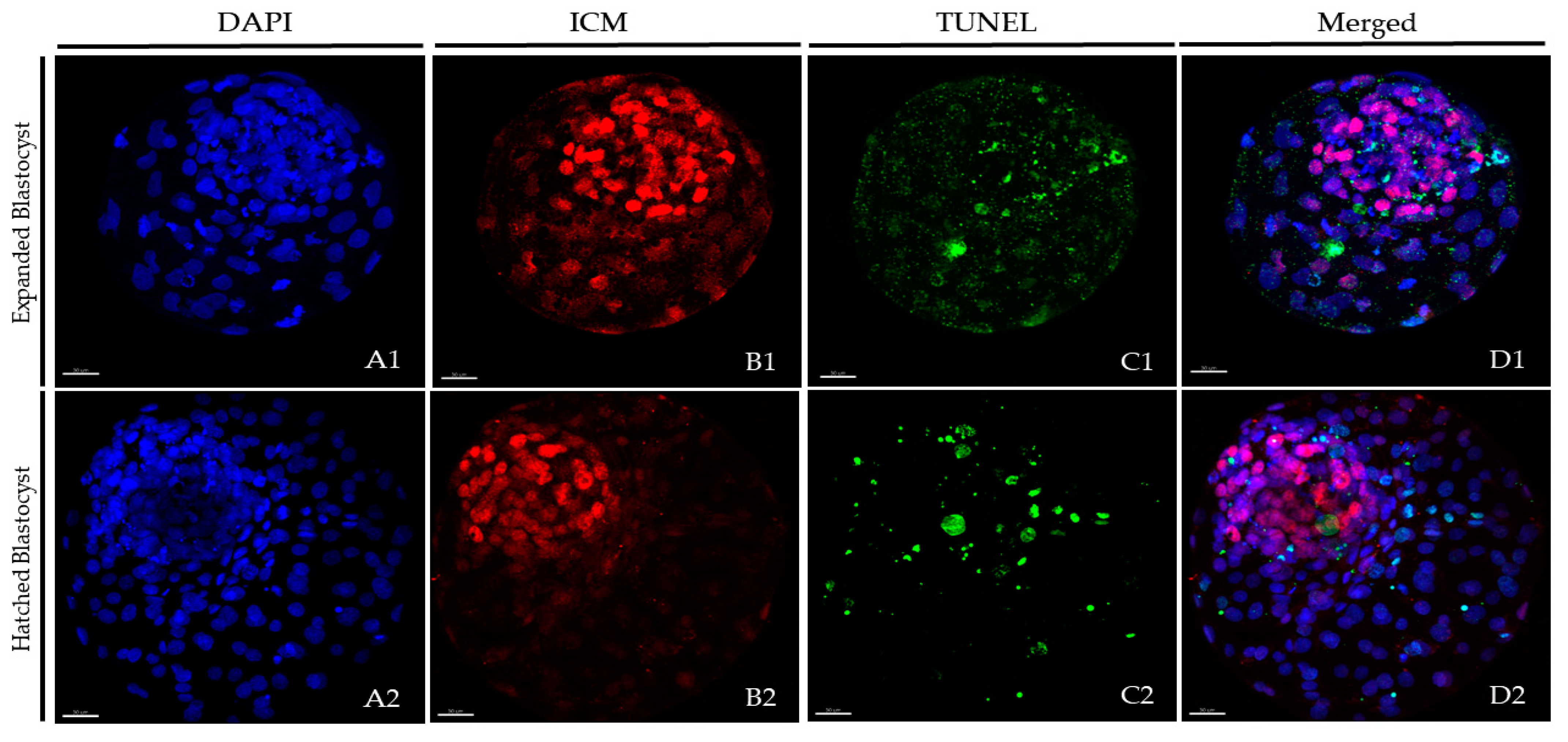
2.4. Differential Staining and TUNEL
2.5. RNA Extraction and Relative Quantification of mRNA by Real-Time Reverse Transcription PCR
2.6. Statistical Analysis
3. Results
3.1. A Shorter Time of Exposure of Embryos to the Equilibrium Solution Leads to Improved Embryo Development (Experiment 1)
3.2. Different Exposure Times to the Equilibration Solution Modify TCN, ICM Cell Numbers, TE Cell Numbers and Apoptosis Rates at 24 h Post-Warming (Experiment 2)
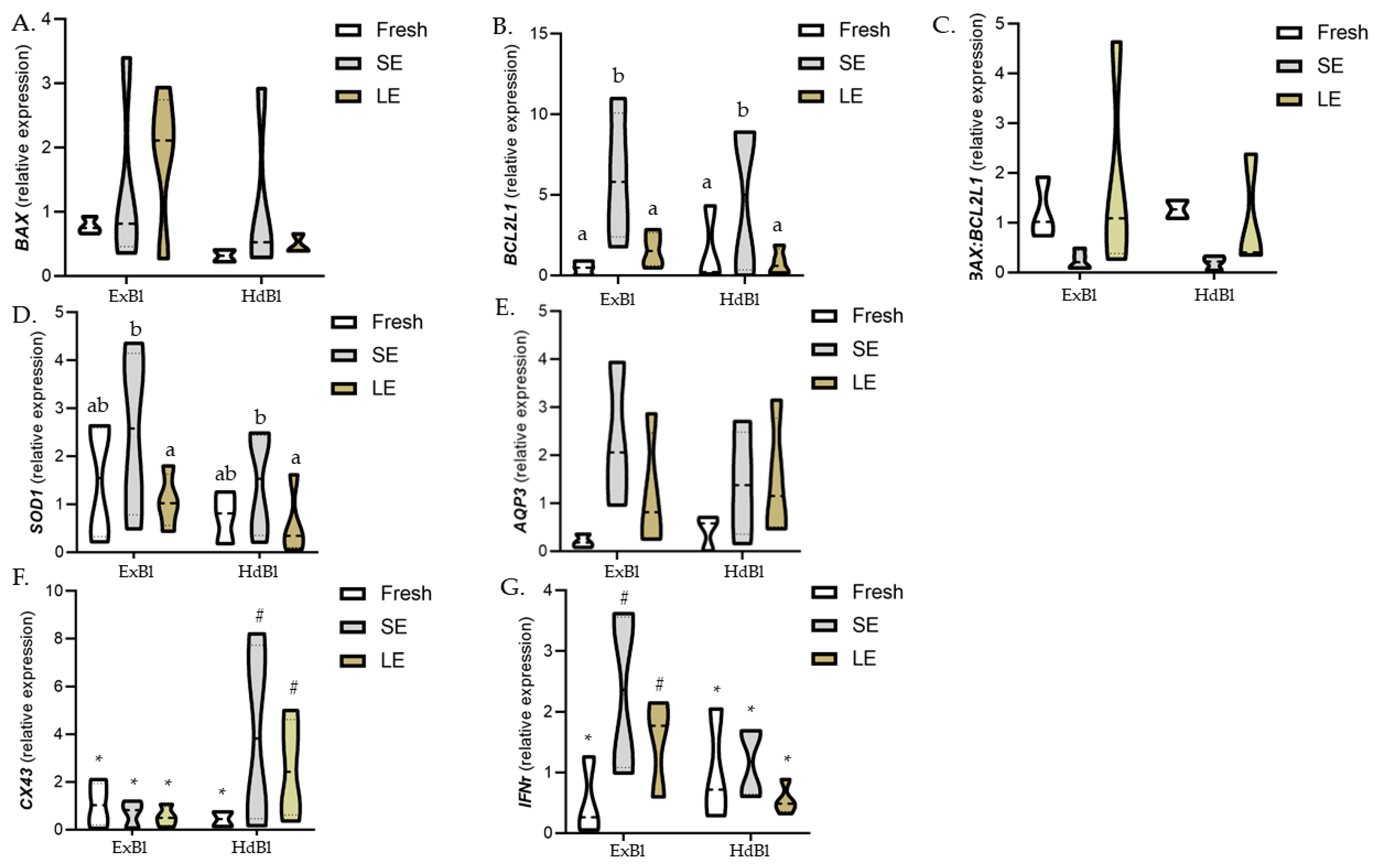
3.3. Different Times of Exposure to the Equilibration Solution Modify Gene Expression Patterns in Warmed Expanded Blastocysts Vitrified Using the VitTrans as the Cryodevice (Experiment 3)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ealy, A.D.; Wooldridge, L.K.; McCoski, S.R. Post-transfer consequences of in vitro-produced embryos in cattle. J. Anim. Sci. 2019, 97, 2555–2568. [Google Scholar] [CrossRef] [PubMed]
- Rizos, D.; Ward, F.; Duffy, P.A.T.; Boland, M.P.; Lonergan, P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 2002, 61, 234–248. [Google Scholar] [CrossRef]
- Heo, Y.T.; Lim, J.K.; Xu, Y.N.; Jang, W.I.; Jeon, S.H.; Kim, N.H. Development of a method of vitrification, thawing, and transfer of mammalian blastocysts using a single closed cryo-straw. Cryo Lett. 2014, 35, 108–113. [Google Scholar] [CrossRef]
- Taniguchi, M.; Ikeda, A.; Arikawa, E.; Wongsrikeao, P.; Agung, B.; Naoi, H.; Nagai, T.; Otoi, T. Effect of cryoprotectant composition on in vitro viability of in vitro fertilized and cloned bovine embryos following vitrification and in-straw dilution. J. Reprod. Dev. 2007, 53, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, X.; Chen, L.; Feng, C.; Bi, J.; Mo, X.; Cheng, K.; Zhang, R.; Li, S.; Zhu, S. A simple and efficient vitrification method for in-straw dilution and direct transfer of bovine embryos. Cryo Lett. 2015, 36, 392–398. [Google Scholar]
- Ha, A.N.; Lee, S.R.; Jeon, J.S.; Park, H.S.; Lee, S.H.; Jin, J.I.; Sessions, B.R.; Wang, Z.; White, K.L.; Kong, I.K. Development of a modified straw method for vitrification of in vitro-produced bovine blastocysts and various genes expression in between the methods. Cryobiology 2014, 68, 57–64. [Google Scholar] [CrossRef]
- Tajimi, H.; Yamazaki, T.; Oike, S.; Yoshida, T.; Okada, K.; Kuwayama, M.; Ushijima, H. Vitrification for bovine embryos with low-quality grade. Anim. Sci. J. 2018, 89, 1194–1200. [Google Scholar] [CrossRef]
- Rodriguez-Villamil, P.; Ongaratto, F.L.; Fernandez Taranco, M.; Bo, G.A. Solid-surface vitrification and in-straw dilution after warming of in vitro-produced bovine embryos. Reprod. Domest. Anim. 2014, 49, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Caamaño, J.N.; Gómez, E.; Trigal, B.; Muñoz, M.; Carrocera, S.; Martín, D.; Díez, C. Survival of vitrified invitro-produced bovine embryos after a one-step warming in-straw cryoprotectant dilution procedure. Theriogenology 2015, 83, 881–890. [Google Scholar] [CrossRef]
- Inaba, Y.; Aikawa, Y.; Hirai, T.; Hashiyada, Y.; Yamanouchi, T.; Misumi, K.; Ohtake, M.; Somfai, T.; Kobayashi, S.; Saito, N. In-straw cryoprotectant dilution for bovine embryos vitrified using Cryotop. J. Reprod. Dev. 2011, 57, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Pugh, P.A.; Tervit, H.R.; Niemann, H. Effects of vitrification medium composition on the survival of bovine in vitro produced embryos, following in straw-dilution, in vitro and in vivo following transfer. Anim. Reprod. Sci. 2000, 58, 9–22. [Google Scholar] [CrossRef]
- Vieira, A.D.; Forell, F.; Feltrin, C.; Rodrigues, J.L. In-straw cryoprotectant dilution of IVP bovine blastocysts vitrified in hand-pulled glass micropipettes. Anim. Reprod. Sci. 2007, 99, 377–383. [Google Scholar] [CrossRef]
- Saha, S.; Rajamahendran, R.; Boediono, A.; Sumantri, C.; Suzuki, T. Viability of bovine blastocysts obtained after 7, 8 or 9 days of culture in vitro following vitrification and one-step rehydration. Theriogenology 1996, 46, 331–343. [Google Scholar] [CrossRef]
- Vajta, G.; Murphy, C.N.; Machaty, Z.; Prather, R.S.; Greve, T.; Callesen, H. In-straw dilution of bovine blastocysts after vitrification with the open-pulled straw method. Vet. Rec. 1999, 7, 180–181. [Google Scholar] [CrossRef]
- Oliveira, C.S.; da Silva Feuchard, V.L.; de Freitas, C.; da Silva Rosa, P.M.; dos Camargo, A.J.R.; Saraiva, N.Z. In-straw warming protocol improves survival of vitrified embryos and allows direct transfer in cattle. Cryobiology 2020, 97, 222–225. [Google Scholar] [CrossRef]
- Morató, R.; Mogas, T. New device for the vitrification and in-straw warming of in vitro produced bovine embryos. Cryobiology 2014, 68, 288–293. [Google Scholar] [CrossRef]
- Szurek, E.A.; Eroglu, A. Comparison and avoidance of toxicity of penetrating cryoprotectants. PLoS ONE 2011, 6, e27604. [Google Scholar] [CrossRef]
- Paynter, S.J.; Cooper, A.; Gregory, L.; Fuller, B.J.; Shaw, R.W. Permeability characteristics of human oocytes in the presence of the cryoprotectant dimethylsulphoxide. Hum. Reprod. 1999, 14, 2338–2342. [Google Scholar] [CrossRef]
- Sanches, B.V.; Lunardelli, P.A.; Tannura, J.H.; Cardoso, B.L.; Colombo Pereira, M.H.; Gaitkoski, D.; Basso, A.C.; Arnold, D.R.; Seneda, M.M. A new direct transfer protocol for cryopreserved IVF embryos. Theriogenology 2016, 85, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Rios, G.L.; Mucci, N.C.; Kaiser, G.G.; Alberio, R.H. Effect of container, vitrification volume and warming solution on cryosurvival of in vitro-produced bovine embryos. Anim. Reprod. Sci. 2010, 118, 19–24. [Google Scholar] [CrossRef]
- Gómez, E.; Carrocera, S.; Martín, D.; Pérez-Jánez, J.J.; Prendes, J.; Prendes, J.M.; Vázquez, A.; Murillo, A.; Gimeno, I.; Muñoz, M. Efficient one-step direct transfer to recipients of thawed bovine embryos cultured in vitro and frozen in chemically defined medium. Theriogenology 2020, 146, 39–47. [Google Scholar] [CrossRef]
- Morató, R.; Izquierdo, D.; Paramio, M.T.; Mogas, T. Survival and apoptosis rates after vitrification in cryotop devices of in vitro-produced calf and cow blastocysts at different developmental stages. Reprod. Fertil. Dev. 2010, 22, 1141–1147. [Google Scholar] [CrossRef]
- Arcarons, N.; Morató, R.; Spricigo, J.F.W.; Ferraz, M.A.; Mogas, T. Spindle configuration and developmental competence of in vitro-matured bovine oocytes exposed to NaCl or sucrose prior to Cryotop vitrification. Reprod. Fertil. Dev. 2016, 28, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Vendrell-Flotats, M.; García-Martínez, T.; Martínez-Rodero, I.; Lopez-Bejar, M.; LaMarre, J.; Yeste, M.; Mogas, T. In Vitro Maturation with Leukemia Inhibitory Factor Prior to the Vitrification of Bovine Oocytes Improves Their Embryo Developmental Potential and Gene Expression in Oocytes and Embryos. Int. J. Mol. Sci. 2020, 21, 7067. [Google Scholar] [CrossRef]
- Arcarons, N.; Vendrell-Flotats, M.; Yeste, M.; Mercade, E.; López-Béjar, M.; Mogas, T. Cryoprotectant role of exopolysaccharide of Pseudomonas sp. ID1 in the vitrification of IVM cow oocytes. Reprod. Fertil. Dev. 2019, 31, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Liu, J.X.; Gao, Y.; Liu, W.W.; Wu, L.H.; Han, W.; Zhang, X.D.; Han, S.B.; Liu, D.Y.; Huang, G.N. Shortened equilibration time can compromise clinical outcomes in human embryo vitrification. Hum. Fertil. 2016, 19, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Herrid, M.; Billah, M.; Malo, C.; Skidmore, J.A. Optimization of a vitrification protocol for hatched blastocysts from the dromedary camel (Camelus dromedarius). Theriogenology 2016, 85, 585–590. [Google Scholar] [CrossRef]
- Do, V.H.; Walton, S.; Catt, S.; Taylor-Robinson, A.W. A comparative analysis of the efficacy of three cryopreservation protocols on the survival of in vitro-derived cattle embryos at pronuclear and blastocyst stages. Cryobiology 2017, 77, 58–63. [Google Scholar] [CrossRef]
- Kader, A.; Choi, A.; Sharma, R.K.; Falcone, T.; Agarwal, A. Effect of varying equilibration time in a two-step vitrification method on the post-warming DNA integrity of mouse blastocysts. Fertil. Steril. 2010, 93, 2640–2645. [Google Scholar] [CrossRef]
- Bagis, H.; Mercan, H.O.; Cetin, S.; Sekmen, S. The effect of equilibration time on survival and development rates of mouse pronuclear-stage embryos vitrified in solid surface (SSV) and convential straws: In vitro and In vivo evaluations. Mol. Reprod. Dev. Inc. Gamete Res. 2005, 72, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudzadeh, A.R.; Van Soom, A.; Bols, P.; Ysebaert, M.T.; De Knuif, A. Optimization of a simple vitrification procedure for bovine embryos produced in vitro: Effect of developmental stage, two-step addition of cryoprotectant and sucrose dilution on embryonic survival. J. Reprod. Fertil. 1995, 103, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Dinnyés, A.; Lonergan, P.; Fair, T.; Boland, M.P.; Yang, X. Timing of the first cleavage post-insemination affects cryosurvival of in vitro–produced bovine blastocysts. Mol. Reprod. Dev. 1999, 53, 318–324. [Google Scholar] [CrossRef]
- Iwasaki, S.; Yoshiba, N.; Ushijima, H.; Watanabe, S.; Nakahara, T. Morphology and proportion of inner cell mass of bovine blastocysts fertilized in vitro and in vivo. Reproduction 1990, 90, 279–284. [Google Scholar] [CrossRef]
- Loureiro, B.; Bonilla, L.; Block, J.; Fear, J.M.; Bonilla, A.Q.S.; Hansen, P.J. Colony-stimulating factor 2 (CSF-2) improves development and posttransfer survival of bovine embryos produced in vitro. Endocrinology 2009, 150, 5046–5054. [Google Scholar] [CrossRef]
- Van Soom, A.; Boerjan, M.; Ysebaert, M.; de Kruif, A. Cell allocation to the inner cell mass and the trophectoderm in bovine embryos cultured in two different media. Mol. Reprod. 1996, 45, 171–182. [Google Scholar] [CrossRef]
- Leese, H.J.; Donnay, I.; Thompson, J.G. Human assisted conception: A cautionary tale. Lessons from domestic animals. Hum. Reprod. 1998, 13, 184–202. [Google Scholar] [CrossRef]
- Inaba, Y.; Miyashita, S.; Somfai, T.; Geshi, M.; Matoba, S.; Dochi, O.; Nagai, T. Cryopreservation method affects DNA fragmentation in trophectoderm and the speed of re-expansion in bovine blastocysts. Cryobiology 2016, 72, 86–92. [Google Scholar] [CrossRef]
- Kaidi, S.; Bernard, S.; Lambert, P.; Massip, A.; Dessy, F.; Donnay, I. Effect of conventional controlled-rate freezing and vitrification on morphology and metabolism of bovine blastocysts produced in vitro. Biol. Reprod. 2001, 65, 1127–1134. [Google Scholar] [CrossRef]
- Mucci, N.; Aller, J.; Kaiser, G.G.; Hozbor, F.; Cabodevila, J.; Alberio, R.H. Effect of estrous cow serum during bovine embryo culture on blastocyst development and cryotolerance after slow freezing or vitrification. Theriogenology 2006, 65, 1551–1562. [Google Scholar] [CrossRef]
- Abe, H.; Yamashita, S.; Satoh, T.; Hoshi, H. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol. Reprod. Dev. 2002, 61, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Gómez, E.; Muñoz, M.; Rodríguez, A.; Caamaño, J.N.; Facal, N.; Díez, C. Vitrification of Bovine blastocysts produced in Vitro inflicts selective damage to the inner cell mass. Reprod. Domest. Anim. 2009, 44, 194–199. [Google Scholar] [CrossRef]
- Mogas, T. Update on the vitrification of bovine oocytes and in vitro-produced embryos. Reprod. Fertil. Dev. 2019, 31, 105–117. [Google Scholar] [CrossRef]
- Marsico, T.V.; de Camargo, J.; Valente, R.S.; Sudano, M.J. Embryo competence and cryosurvival: Molecular and cellular features. Anim. Reprod. 2019, 16, 423–439. [Google Scholar] [CrossRef]
- Rizos, D.; Clemente, M.; Bermejo-Alvarez, P.; De La Fuente, J.; Lonergan, P.; Gutiérrez-Adán, A. Consequences of in vitro culture conditions on embryo development and quality. Reprod. Domest. Anim. 2008, 43, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Neuber, E.; Luetjens, C.M.; Chan, A.W.S.; Schatten, G.P. Analysis of DNA fragmentation of in vitro cultured bovine blastocysts using TUNEL. Theriogenology 2002, 57, 2193–2202. [Google Scholar] [CrossRef]
- Paula-Lopes, F.F.; Hansen, P.J. Apoptosis is an adaptive response in bovine preimplantation embryos that facilitates survival after heat shock. Biochem. Biophys. Res. Commun. 2002, 295, 37–42. [Google Scholar] [CrossRef]
- Kader, A.; Agarwal, A.; Abdelrazik, H.; Sharma, R.K.; Ahmady, A.; Falcone, T. Evaluation of post-thaw DNA integrity of mouse blastocysts after ultrarapid and slow freezing. Fertil. Steril. 2009, 91, 2087–2094. [Google Scholar] [CrossRef]
- Yang, M.Y.; Rajamahendran, R. Expression of Bcl-2 and Bax proteins in relation to quality of bovine oocytes and embryos produced in vitro. Anim. Reprod. Sci. 2002, 70, 159–169. [Google Scholar] [CrossRef]
- Vandaele, L.; Goossens, K.; Peelman, L.; Van Soom, A. mRNA expression of Bcl-2, Bax, caspase-3 and-7 cannot be used as a marker for apoptosis in bovine blastocysts. Anim. Reprod. Sci. 2008, 106, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, Y.; Zhao, Y.; Gao, Q.; Jin, Q.; Yan, C.; Xu, Y. l-carnitine supplementation during in vitro culture regulates oxidative stress in embryos from bovine aged oocytes. Theriogenology 2020, 143, 64–73. [Google Scholar] [CrossRef]
- Rizos, D.; Gutierrez-Adan, A.; Perez-Garnelo, S.; De La Fuente, J.; Boland, M.P.; Lonergan, P. Bovine embryo culture in the presence or absence of serum: Implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol. Reprod. 2003, 68, 236–243. [Google Scholar] [CrossRef]
- Gutiérrez-Adán, A.; Rizos, D.; Fair, T.; Moreira, P.N.; Pintado, B.; De La Fuente, J.; Boland, M.P.; Lonergan, P. Effect of speed of development on mRNA expression pattern in early bovine embryos cultured in vivo or in vitro. Mol. Reprod. Dev. 2004, 68, 441–448. [Google Scholar] [CrossRef]
- Jin, B.; Kawai, Y.; Hara, T.; Takeda, S.; Seki, S.; Nakata, Y.; Matsukawa, K.; Koshimoto, C.; Kasai, M.; Edashige, K. Pathway for the movement of water and cryoprotectants in bovine oocytes and embryos. Biol. Reprod. 2011, 85, 834–847. [Google Scholar] [CrossRef]
- Lopera-Vasquez, R.; Hamdi, M.; Maillo, V.; Lloreda, V.; Coy, P.; Gutierrez-Adan, A.; Bermejo-Alvarez, P.; Rizos, D. Effect of bovine oviductal fluid on development and quality of bovine embryos produced in vitro. Reprod. Fertil. Dev. 2017, 29, 621–629. [Google Scholar] [CrossRef]
| Symbol | Primer Sequences (5′-3′) | Amplicon Size (bp) | GenBank Accession No. |
|---|---|---|---|
| BCL2 associated X apoptosis regulator (BAX) | F: ACCAAGAAGCTGAGCGAGTG | 116 | NM_173894.1 |
| R: CGGAAAAAGACCTCTCGGGG | |||
| BCL2-like 1 (BCL2L1) | F: GAGTTCGGAGGGGTCATGTG | 211 | NM_001166486.1 |
| R: TGAGCAGTGCCTTCAGAGAC | |||
| Superoxide dismutase 1 (SOD1) | F: ACACAAGGCTGTACCAGTGC | 102 | NM_174615.2 |
| R: CACATTGCCCAGGTCTCCAA | |||
| Aquaporin 3 (AQP3) | F: GTGGACCCCTACAACAACCC | 222 | NM_001079794.1 |
| R: CAGGAGCGGAGAGACAATGG | |||
| Connexin 43 (CX43) | F: TGGAATGCAAGAGAGGTTGAAAGAGG | 294 | NM_174068.2 |
| R: AACACTCTCCAGAACACATGATCG | |||
| Interferon tau (IFNτ) | F: CTGAAGGTTCACCCAGACCC | 197 | AF238612 |
| R: GAGTCTGTTCATTCGGGCCA | |||
| Peptidylprolyl isomerase A (PPIA) | F: CATACAGGTCCTGGCATCTTGTCC | 108 | NM_178320.2 |
| R: CACGTGCTTGCCATCCAACC | |||
| H3.3 histone A (H3F3A) | F: CATGGCTCGTACAAAGCAGA | 136 | NM_001014389.2 |
| R: ACCAGGCCTGTAACGATGAG |
| Day 7 Blastocysts | Day 8 Blastocysts | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Survival (%) (3 h) | Survival (%) (24 h) | Hatching Rate (%) (24 h) | n | Survival (%) (3 h) | Survival (%) (24 h) | Hatching Rate (%) (24 h) | |
| Control | 86 | 100 a,A | 100 a,A | 35.9 ± 4.0 a,A | 40 | 100 a,A | 100 a,A | 50.0 ± 7.0 a,B |
| SE | 86 | 60.6 ± 1.5 b,A | 78.4 ± 2.0 b,A | 31.4 ± 3.7 a,A | 33 | 48.6 ± 5.3 b,B | 63.0 ± 5.5 b,B | 19.9 ± 2.7 b,B |
| LE | 83 | 57.5 ±4.0 b,A | 63.1 ± 2.6 c,A | 10.1 ± 2.4 b,A | 36 | 39.4 ± 4.7 b,B | 55.3 ± 5.0 c,B | 8.1 ± 2.7 c,A |
| Day 7 Blastocysts | |||||||||
| TCN | ICM Cell Number | TE Cell Number | AR | ||||||
| n | Expanded | Hatched | Expanded | Hatched | Expanded | Hatched | Expanded | Hatched | |
| Control | 30 | 140.3 ± 8.6 a,A | 189.8 ± 4.4 a,B | 24.4 ± 1.6 a,A | 38.6 ± 1.6 a,B | 115.9 ± 7.9 a,A | 151.3 ± 4.1 a,B | 3.7 ± 0.4 a | 4.7 ± 0.7 a |
| SE | 23 | 110.0±2.7 b,A | 195.2 ± 3.5 a,B | 22.0 ± 2,1 a,A | 35.1 ± 1.5 a,B | 87.1 ± 2.3 b,A | 160.1 ± 3.1 a,B | 11.5 ± 1.1 b | 9.1 ± 0.9 b |
| LE | 21 | 113.2 ± 4.5 b,A | 170.6 ± 1.4 b,B | 23.3 ± 1.3 a,A | 33.2 ± 3.3 a,B | 89.9 ± 4.3 b,A | 135.4 ± 2.8 b,B | 15.2 ± 0.3 c | 13.6 ± 1.2 c |
| Day 8 Blastocysts | |||||||||
| TCN | ICM Cell Number | TE Cell Number | AR | ||||||
| n | Expanded | Hatched | Expanded | Hatched | Expanded | Hatched | Expanded | Hatched | |
| Control | 40 | 125.3 ± 5.4 a,A | 206.8 ± 12 a,B | 29.2 ± 1.9 a,A | 43.1 ± 1.2 a,B | 96.0 ± 4.5 a,A | 163.6 ± 5.6 a,B | 5.6 ± 0.4 a | 4.6 ± 0.7 a |
| SE | 21 | 128.1 ± 2.8 a,A | 169.7 ± 5.6 b,B | 22.2 ± 1.0 b,A | 32.0 ± 1.2 b,B | 105.9 ± 2.8 a,A | 137.8 ± 4.0 b,B | 15.1±0.6 b | 12.7 ± 0.8 b |
| LE | 20 | 108.4 ± 1.3 b,A | 154.6 ± 2.1 b,B | 19.7 ± 0.8 b,A | 29.0 ± 2.0 b,B | 88.7 ± 1.3 b,A | 125.0 ± 2.7 b,B | 25.1 ± 1.5 c | 23.2 ± 2.1 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Rodero, I.; García-Martínez, T.; Ordóñez-León, E.A.; Vendrell-Flotats, M.; Olegario Hidalgo, C.; Esmoris, J.; Mendibil, X.; Azcarate, S.; López-Béjar, M.; Yeste, M.; et al. A Shorter Equilibration Period Improves Post-Warming Outcomes after Vitrification and in Straw Dilution of In Vitro-Produced Bovine Embryos. Biology 2021, 10, 142. https://doi.org/10.3390/biology10020142
Martínez-Rodero I, García-Martínez T, Ordóñez-León EA, Vendrell-Flotats M, Olegario Hidalgo C, Esmoris J, Mendibil X, Azcarate S, López-Béjar M, Yeste M, et al. A Shorter Equilibration Period Improves Post-Warming Outcomes after Vitrification and in Straw Dilution of In Vitro-Produced Bovine Embryos. Biology. 2021; 10(2):142. https://doi.org/10.3390/biology10020142
Chicago/Turabian StyleMartínez-Rodero, Iris, Tania García-Martínez, Erika Alina Ordóñez-León, Meritxell Vendrell-Flotats, Carlos Olegario Hidalgo, Joseba Esmoris, Xabier Mendibil, Sabino Azcarate, Manel López-Béjar, Marc Yeste, and et al. 2021. "A Shorter Equilibration Period Improves Post-Warming Outcomes after Vitrification and in Straw Dilution of In Vitro-Produced Bovine Embryos" Biology 10, no. 2: 142. https://doi.org/10.3390/biology10020142
APA StyleMartínez-Rodero, I., García-Martínez, T., Ordóñez-León, E. A., Vendrell-Flotats, M., Olegario Hidalgo, C., Esmoris, J., Mendibil, X., Azcarate, S., López-Béjar, M., Yeste, M., & Mogas, T. (2021). A Shorter Equilibration Period Improves Post-Warming Outcomes after Vitrification and in Straw Dilution of In Vitro-Produced Bovine Embryos. Biology, 10(2), 142. https://doi.org/10.3390/biology10020142








