Beta vulgaris L. (Beetroot) Methanolic Extract Prevents Hepatic Steatosis and Liver Damage in T2DM Rats by Hypoglycemic, Insulin-Sensitizing, Antioxidant Effects, and Upregulation of PPARα
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Animal Diets
2.3. Beetroot Extract Preparation
2.4. Induction of T2DM
2.5. Experimental Design
2.6. Collection of Blood and Tissue Samples
2.7. Extraction of Lipids from the Liver
2.8. Biochemical Analysis
2.9. Real-Time PCR
2.10. Histopathological Analysis
2.11. Statistical Analysis
3. Results
3.1. BE Ameliorates the Gain in Body Weight and Reduces Fasting Glucose and Insulin Levels in T2DM Rats
3.2. BE Attenuates T2DM-Induced Dyslipidemia
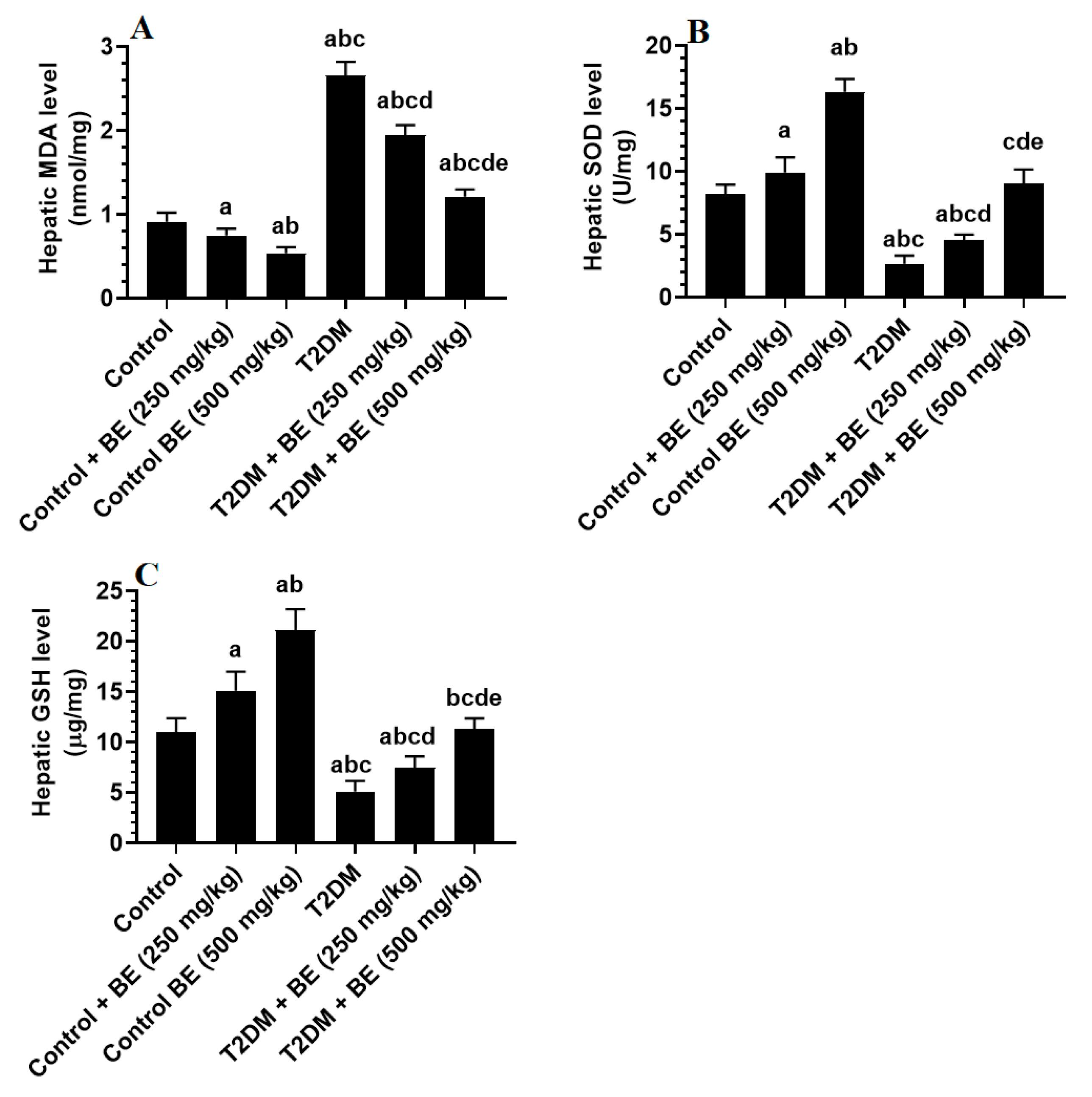
3.3. BE Attenuates Oxidative Stress in the Livers of T2DM Rats
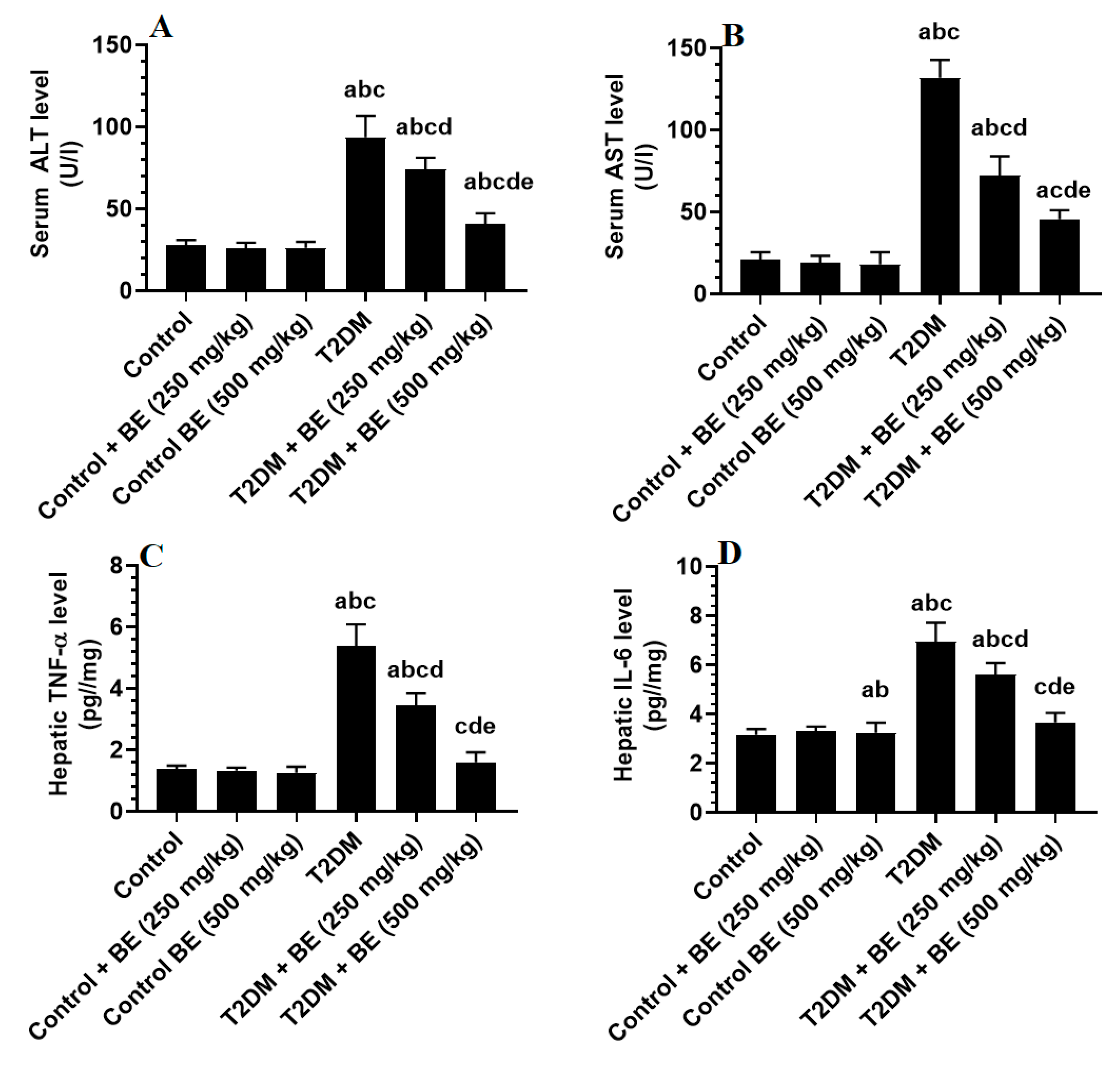
3.4. BE Attenuates Serum Transaminases and Hepatic Inflammatory Markers in T2DM Rats
3.5. BE Downregulates the Transcription SREBP1/2, Stimulates PPARα, and Inhibits Intrinsic Cell Apoptosis in the Livers of T2DM Rats
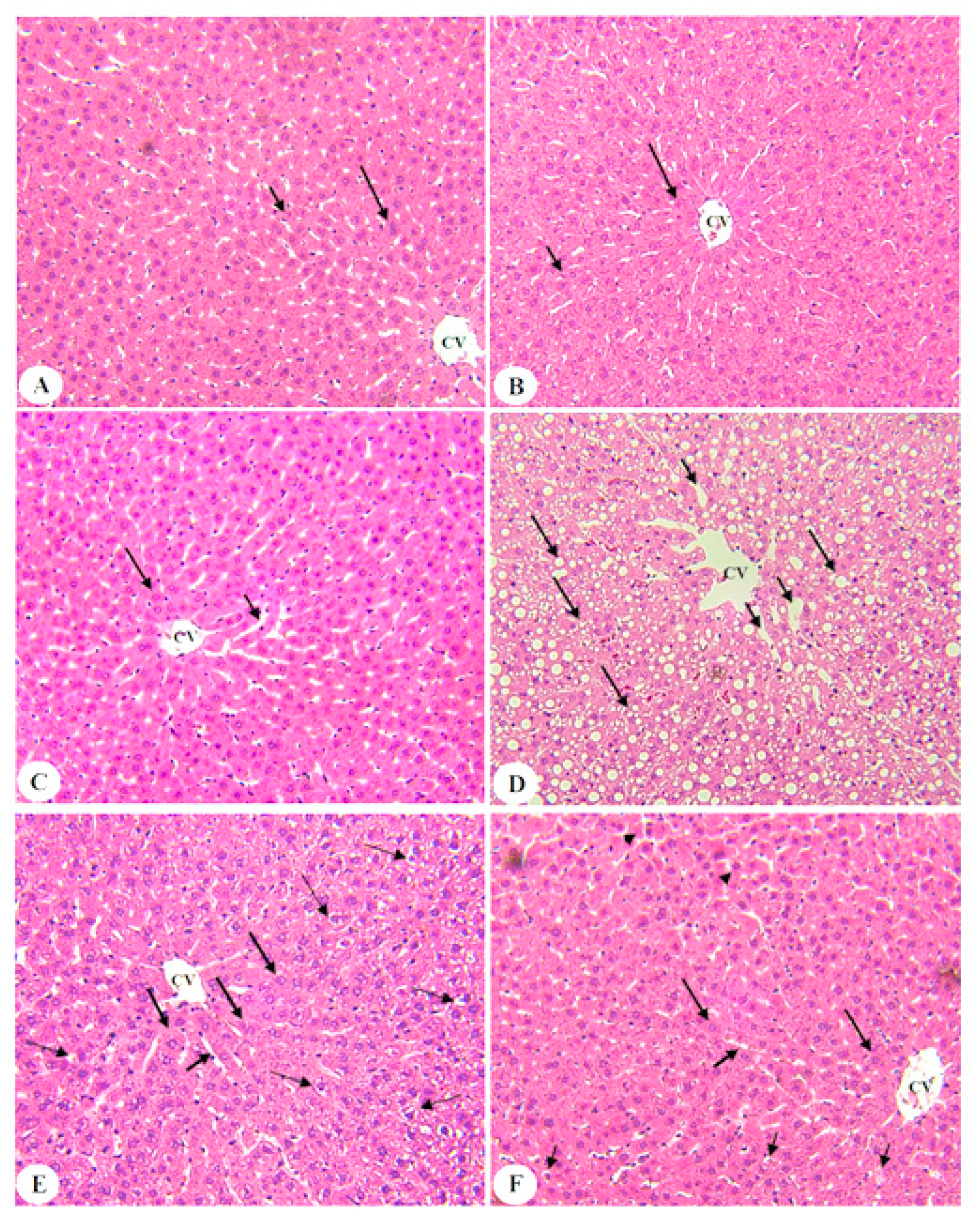
3.6. BE Improved Liver Architectures of T2DM Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Carr, R.M.; Oranu, A.; Khungar, V. Nonalcoholic Fatty Liver Disease: Pathophysiology and Management. Gastroenterol. Clin. N. Am. 2016, 45, 639–652. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metab. Clin. Exp. 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, R.; Xiong, Y.; Du, F.; Zhu, S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017, 16, 203, Erratum in: Lipids Health Dis. 2018;17 (1):33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free. Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Braunersreuther, V.; Viviani, G.L.; Mach, F.; Montecucco, F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J. Gastroenterol. 2012, 18, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Koroglu, E.; Canbakan, B.; Atay, K.; Hatemi, I.; Tuncer, M.; Dobrucali, A.; Sonsuz, A.; Gultepe, I.; Senturk, H. Role of oxidative stress and insulin resistance in disease severity of non-alcoholic fatty liver disease. Turk. J. Gastroenterol. 2016, 27, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Arroyave-Ospina, J.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig. 2008, 118, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, A.; Hamidi-Zad, Z. Role of SREBPs in Liver Diseases: A Mini-review. J. Clin. Transl. Hepatol. 2018, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Souza-Mello, V. Peroxisome proliferator-activated receptors as targets to treat non-alcoholic fatty liver disease. World J. Hepatol. 2015, 7, 1012–1019. [Google Scholar] [CrossRef]
- Pettinelli, P.; del Pozo, T.; Araya, J.; Rodrigo, R.; Araya, A.V.; Smok, G.; Csendes, A.; Gutierrez, L.; Rojas, J.; Korn, O.; et al. Enhancement in liver SREBP-1c/PPAR-α ratio and steatosis in obese patients: Correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2009, 1792, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-M.; Yuan, R.-S.; Zhuang, W.-Y.; Sun, J.-H.; Wu, J.-Y.; Li, H.; Chen, J.-G. Schisandra polysaccharide inhibits hepatic lipid accumulation by downregulating expression of SREBPs in NAFLD mice. Lipids Health Dis. 2016, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Regnier, M.; Polizzi, A.; Smati, S.; Lukowicz, C.; Fougerat, A.; Lippi, Y.; Fouché, E.; Lasserre, F.; Naylies, C.; Bétoulières, C.; et al. Hepatocyte-specific deletion of Pparα promotes NAFLD in the context of obesity. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Ozturk, Z.A.; Kadayifci, A. Insulin sensitizers for the treatment of non-alcoholic fatty liver disease. World J. Hepatol. 2014, 6, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Utzeri, E.; Usai, P. Role of non-steroidal anti-inflammatory drugs on intestinal permeability and nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3954–3963. [Google Scholar] [CrossRef] [PubMed]
- Gezginci-Oktayoglu, S.; Sacan, O.; Bolkent, S.; Ipci, Y.; Kabasakal, L.; Sener, G.; Yanardag, R. Chard (Beta vulgaris L. var. cicla) extract ameliorates hyperglycemia by increasing GLUT2 through Akt2 and antioxidant defense in the liver of rats. Acta Histochem. 2014, 116, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Oztay, F.; Saçan, O.; Kayalar, O.; Bolkent, S.; Ipci, Y.; Kabasakal, L.; Sener, G.; Yanardag, R. Chard (Beta vulgarisvar.cicla) extract improved hyperglycemia-induced oxidative stress and surfactant-associated protein alterations in rat lungs. Pharm. Biol. 2015, 53, 1639–1646. [Google Scholar] [CrossRef]
- Hashem, A.; Soliman, M.S.; Hamed, M.; Swilam, N.F.; Lindequist, U.; Nawwar, M.A. Beta vulgaris subspecies cicla var. flavescens (Swiss chard): Flavonoids, hepatoprotective and hypolipidemic activities. Die Pharm. 2016, 71, 227–232. [Google Scholar]
- Sarfaraz, S.; Najam, R. Evaluation of anti-inflammatory effect of natural dietary supplement Beta vulgaris (Beetroot) in animal models of inflammation. Rawal Med. J. 2017, 42, 385–389. [Google Scholar]
- Sarfaraz, S.; Ikram, R. Evaluation of nephroprotective effect of Beta vulgaris at different doses. Pak. J. Pharmacol. 2019, 36, 9–13. [Google Scholar]
- Sarfaraz, S.; Rahela, I.; Rabia, M.; Muhammad, O.; Sabiha, G.; Muhammad, S. Rising trend of Nutraceuticals: Evaluation of lyophilized beetroot powder at different doses for its hypolipidemic effects. Pak. J. Pharm. Sci. 2021, 4, 1315–1322. [Google Scholar]
- Kumar, S.; Kumari, S.; Dubey, N.K.; Usha, D. Anti-Diabetic and Haematinic Effects of Beetroot Juice (Beta vulgaris L.) in Alloxan Induced Type-1 Diabetic Albino Rats. J. Diabetes Res. Ther. 2020, 6, 1–3. [Google Scholar]
- Al-Dosari, M.; Alqasoumi, S.; Ahmed, M.; Al-Yahya, M.; Ansari, M.N.; Rafatullah, S. Effect of Beta vulgaris L. on cholesterol rich diet-induced hypercholesterolemia in rats. Farmacia 2011, 59, 669–678. [Google Scholar]
- Afzali, N.; Ebadi, S.S.; Afzali, H.; Sharif, M.R.; Vazirian, M.; Shahkarami, V.; Rahimi, H. Effect of Beta vulgaris Extract on Liver Enzymes in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Zahedan J. Res. Med Sci. 2020, 20, 102125. [Google Scholar] [CrossRef]
- Babarykin, D.; Smirnova, G.; Markovs, J.; Vasiljeva, S.; Basova, N.; Simanis, R.; Viksna, L. Therapeutic Effect of Fractionated by Ultrafiltration Red Beetroot (Beta vulgaris L.) Juice in Rats with Food-Induced Fatty Liver. Eur. J. Biol. Res. 2019, 9, 1–9. [Google Scholar]
- Van Herck, M.A.; Vonghia, L.; Francque, S.M. Animal Models of Nonalcoholic Fatty Liver Disease-A Starter’s Guide. Nutrients 2017, 9, 1072. [Google Scholar] [CrossRef]
- Albasher, G.; Almeer, R.; Al-Otibi, F.O.; Al-Kubaisi, N.; Mahmoud, A.M. Ameliorative Effect of Beta vulgaris Root Extract on Chlorpyrifos-Induced Oxidative Stress, Inflammation and Liver Injury in Rats. Biomolecules 2019, 9, 261. [Google Scholar] [CrossRef]
- Dwivedi, D.; Jena, G.B. NLRP3 inhibitor glibenclamide attenuates high-fat diet and streptozotocin-induced non-alcoholic fatty liver disease in rat: Studies on oxidative stress, inflammation, DNA damage and insulin signalling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-C.; Song, J.-M.; Kim, C.-J.; Yoon, S.-Y.; Kim, I.-R.; Park, B.-S.; Shin, S.-H. Combined effect of bisphosphonate and recombinant human bone morphogenetic protein 2 on bone healing of rat calvarial defects. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 16. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Salgado, A.L.; Carvalho, L.D.; Oliveira, A.C.; Santos, V.N.; Vieira, J.G.; Parise, E.R. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq. Gastroenterol. 2010, 47, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Dharmalingam, M.; Yamasandhi, P.G. Nonalcoholic fatty liver disease and Type 2 diabetes mellitus. Indian J. Endocrinol. Metab. 2018, 22, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Lo, L.; McLennan, S.V.; Williams, P.F.; Bonner, J.; Chowdhury, S.; Mccaughan, G.; Gorrell, M.; Yue, D.K.; Twigg, S.M. Diabetes is a progression factor for hepatic fibrosis in a high fat fed mouse obesity model of non-alcoholic steatohepatitis. J. Hepatol. 2011, 55, 435–444. [Google Scholar] [CrossRef]
- Fujii, M.; Shibazaki, Y.; Wakamatsu, K.; Honda, Y.; Kawauchi, Y.; Suzuki, K.; Arumugam, S.; Watanabe, K.; Ichida, T.; Asakura, H.; et al. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med Mol. Morphol. 2013, 46, 141–152. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, S.R.; Jung, U.J. Myricitrin Ameliorates Hyperglycemia, Glucose Intolerance, Hepatic Steatosis, and Inflammation in High-Fat Diet/Streptozotocin-Induced Diabetic Mice. Int. J. Mol. Sci. 2020, 21, 1870. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.; Nazratun Nafizah, A.H.; Zariyantey, A.H.; Budin, S.B. Mechanisms of Diabetes-Induced Liver Damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132–e141. [Google Scholar] [CrossRef] [PubMed]
- Lasker, S.; Rahman, M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S.B.; Subhan, N.; Ahsan, G.U.; et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019, 9, 20026. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Tang, K.; Chen, R.; Nie, H.; Liang, S.; Zhang, J.; Zhang, Y.; Yang, Q. Berberine attenuates hepatic oxidative stress in rats with non-alcoholic fatty liver disease via the Nrf2/ARE signalling pathway. Exp. Ther. Med. 2019, 17, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liu, Y.; Fang, N.; Guo, Y. Hepatoprotective effects of Cassia semen ethanol extract on non-alcoholic fatty liver disease in experimental rat. Pharm. Biol. 2019, 57, 98–104. [Google Scholar] [CrossRef]
- Xia, H.; Wang, J.; Xie, X.; Xu, L.; Tang, S. Green tea polyphenols attenuate hepatic steatosis, and reduce insulin resistance and inflammation in high-fat diet-induced rats. Int. J. Mol. Med. 2019, 44, 1523–1530. [Google Scholar] [CrossRef]
- Yin, K.; Zhou, X.; Jiang, W.; Wang, L.; Dai, Z.; Tang, B. Jiangzhi Ligan Decoction Inhibits GSDMD-Mediated Canonical/Noncanonical Pyroptosis Pathways and Alleviates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease. Dis. Markers 2021, 2021, 9963534. [Google Scholar] [CrossRef] [PubMed]
- Panasiuk, A.; Dzięcioł, J.; Panasiuk, B.; Prokopowicz, D. Expression of p53, Bax and Bcl-2 proteins in hepatocytes in non-alcoholic fatty liver disease. World J. Gastroenterol. 2006, 12, 6198–6202. [Google Scholar] [CrossRef] [PubMed]
- Li, C.P.; Li, J.H.; He, S.Y.; Li, P.; Zhong, X.L. Roles of Fas/Fasl, Bcl-2/Bax, and Caspase-8 in rat nonalcoholic fatty liver disease pathogenesis. Genet. Mol. Res. 2014, 13, 3991–3999. [Google Scholar] [CrossRef]
- Mu, W.; Cheng, X.-F.; Liu, Y.; Lv, Q.-Z.; Liu, G.-L.; Zhang, J.-G.; Li, X.-Y. Potential Nexus of Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Insulin Resistance between Hepatic and Peripheral Tissues. Front. Pharmacol. 2019, 9, 1566. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.N.C.; Manchali, S. Anti-diabetic Potentials of Red Beet Pigments and Other Constituents. In Red Beet Biotechnology; Neelwarne, B., Ed.; Springer: Boston, MA, USA, 2013; pp. 155–174. [Google Scholar]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef]
- Khalifi, S.; Rahimipour, A.; Jeddi, S.; Ghanbari, M.; Kazerouni, F.; Ghasemi, A. Dietary nitrate improves glucose tolerance and lipid profile in an animal model of hyperglycemia. Nitric Oxide 2015, 44, 24–30. [Google Scholar] [CrossRef]
- Holy, B.; Isaac, N.N.; Ngoye, B.O. Post-prandial effect of beetroot (beta vulgaris) juice on glucose and lipids levels of apparently healthy subjects. Eur. J. Pharm. Med. Res. 2017, 4, 60–62. [Google Scholar]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Ignatowicz, E.; Murias, M.; Ewertowska, M.; Mikołajczyk, K.; Jodynis-Liebert, J. Protective Effect of Red Beetroot against Carbon Tetrachloride- and N-Nitrosodiethylamine-Induced Oxidative Stress in Rats. J. Agric. Food Chem. 2009, 57, 2570–2575. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.; Prescott, S.L. Stability of the antioxidant capacity of twenty-five commercially available fruit juices subjected to an in vitro digestion. Int. J. Food Sci. Technol. 2010, 45, 1191–1197. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Vulić, J.J.; Ćebović, T.N.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Čanadanović, V.M.; Djilas, S.M.; Tumbas Šaponjac, V.T. In vivo and in vitro antioxidant effects of beetroot pomace extracts. J. Funct. Foods 2014, 6, 168–175. [Google Scholar] [CrossRef]
- Szaefer, H.; Krajka-Kuźniak, V.; Ignatowicz, E.; Adamska, T.; Baer-Dubowska, W. Evaluation of the Effect of Beetroot Juice on DMBA-induced Damage in Liver and Mammary Gland of Female Sprague-Dawley Rats. Phytotherapy Res. 2014, 28, 55–61. [Google Scholar] [CrossRef]
- Arazi, H.; Eghbali, E. Possible Effects of Beetroot Supplementation on Physical Performance through Metabolic, Neuroendocrine, and Antioxidant Mechanisms: A Narrative Review of the Literature. Front. Nutr. 2021, 8, 660150. [Google Scholar] [CrossRef] [PubMed]
- Baião, D.D.S.; da Silva, V.; Paschoalin, M. Beetroot, a Remarkable Vegetable: Its Nitrate and Phytochemical Contents Can be Adjusted in Novel Formulations to Benefit Health and Support Cardiovascular Disease Therapies. Antioxidants 2020, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- El Gamal, A.A.; AlSaid, M.S.; Raish, M.; Al-Sohaibani, M.; Al-Massarani, S.M.; Ahmad, A.; Hefnawy, M.; Al-Yahya, M.; Basoudan, O.A.; Rafatullah, S. Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediat. Inflamm. 2014, 2014, 983952. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Szaefer, H.; Ignatowicz, E.; Adamska, T.; Baer-Dubowska, W. Beetroot juice protects against N-nitrosodiethylamine-induced liver injury in rats. Food Chem. Toxicol. 2012, 50, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Dif, N.; Euthine, V.; Gonnet, E.; Laville, M.; Vidal, H.; Lefai, E. Insulin activates human sterol-regulatory-element-binding protein-1c (SREBP-1c) promoter through SRE motifs. Biochem. J. 2006, 400, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Pacot, C.; Dugail, I.; Lemarchand, P.; Guichard, C.; Le Lièpvre, X.; Berthelier-Lubrano, C.; Spiegelman, B.; Kim, J.B.; Ferré, P.; et al. ADD1/SREBP-1c is required in the activation of hepatic lipogenic gene expression by glucose. Mol. Cell Biol. 1999, 19, 3760–3768. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, M.; Hiraishi, A.; Touyama, M.; Sakamoto, K. Oxidative stress induced lipid accumulation via SREBP1c activation in HepG2 cells. Biochem. Biophys. Res. Commun. 2008, 375, 602–607. [Google Scholar] [CrossRef]
- Xu, X.; So, J.-S.; Park, J.-G.; Lee, A.-H. Transcriptional Control of Hepatic Lipid Metabolism by SREBP and ChREBP. Semin. Liver Dis. 2013, 33, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Wandrer, F.; Liebig, S.; Marhenke, S.; Vogel, A.; John, K.; Manns, M.P.; Teufel, A.; Itzel, T.; Longerich, T.; Maier, O.; et al. TNF-Receptor-1 inhibition reduces liver steatosis, hepatocellular injury and fibrosis in NAFLD mice. Cell Death Dis. 2020, 11, 212. [Google Scholar] [CrossRef]
- Montagner, A.; Polizzi, A.; Fouché, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef]
- Rabeh, M.N.; Ibrahim, E.M. Antihypercholesterolemic effects of beet (Beta vulgaris L.) root waste extract on hypercholesterolemic rats and its antioxidant potential properties. Pak. J. Nutr. 2014, 13, 500. [Google Scholar] [CrossRef][Green Version]
- Singh, A.; Verma, S.; Singh, V.; Jappa, C.; Roopa, N.; Raju, P.; Singh, S. Beetroot Juice Supplementation Increases High Density Lipoprotein-Cholesterol and Reduces Oxidative Stress in Physically Active Individuals. J. Pharm. Nutr. Sci. 2015, 5, 179–185. [Google Scholar] [CrossRef]
- Lotfi, M.; Azizi, M.; Tahmasebi, W.; Bashiri, P.; Physiology, M.O.E.; Professor, S. Efficacy of Beetroot Juice Consumption on the Lipid Profile of Female Soccer Players. Med. Lab. J. 2020, 14, 26–30. [Google Scholar] [CrossRef]
- Haswell, C.; Ali, A.; Page, R.; Hurst, R.; Rutherfurd-Markwick, K. Potential of Beetroot and Blackcurrant Compounds to Improve Metabolic Syndrome Risk Factors. Metabolites 2021, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Xu, J.X.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primers (5′→3′) | GenBank Accession # | Product Length |
|---|---|---|---|
| PPARα | F: TGCGGACTACCAGTACTTAGGG R: GCTGGAGAGAGGGTGTCTGT | NM_013196.1 | 116 |
| SREBP-1c | F: GCA AGG CCA TCG ACT ACA TC R: TTT CAT GCC CTC CAT AGA CAC | NM_001276707.1 | 161 |
| Bcl2 | F: TGGGATGCCTTTGTGGAACT R: TCTTCAGAGACTGCCAGGAGAAA | U34964.1 | 73 |
| SREBP-2 | F: CTGACCACAATGCCGGTAAT R: CTTGTGCATCTTGGCATCTG | NM_001033694.1 | 204 |
| Bax | F: ATGGAGCTGCAGAGGATGATT R: TGAAGTTGCCATCAGCAAACA | NM_017059 | 97 |
| Caspase-3 | F: AATTCAAGGGACGGGTCATG R: R-GCTTGTGCGCGTACAGTTTC | U49930 | 67 |
| Β-actin | F: ATC TGG CAC CAC ACC TTC R: AGC CAG GTC CAG ACG CA | NM_031144 | 291 |
| Items | Control | Control + BE (250 mg/kg) | Control + BE (500 mg/kg) | T2DM | T2DM + BE (250 mg/kg) + CC | T2DM + BE (500 mg/kg) |
|---|---|---|---|---|---|---|
| Final body weight (g) | 442 ± 24.3 | 446 ± 17.5 | 454 ± 27.6 | 576 ± 25.4 abc | 536 ± 21.6 abcd | 451 ± 18.9 de |
| Plasma fasting glucose (mg/dL) | 100 ± 8.6 | 96 ± 7.8 | 65 ± 11.3 ab | 253 ± 18.5 abc | 189 ± 15.1 abcd | 121 ± 10.1 abcde |
| Plasma fasting insulin | 4.8 ± 0.71 | 4.6 ± 0.73 | 4.7 ± 61 | 9.3 ± 0.81 abc | 6.9 ± 0.41 abcd | 5.0 ± 0.56 de |
| (ng/mL) | ||||||
| HOMA-IR | 1.19 ± 0.23 | 1.11 ± 0.19 | 0.75 ± 0.11 ab | 5.8 ± 0.82 abc | 3.2 ± 0.33 abcd | 1.5 ± 0.24 abcde |
| Control | Control + BE (250 mg/kg) | Control + BE (500 mg/kg) | T2DM | T2DM + BE (250 mg/kg) + CC | T2DM + BE (500 mg/kg) | |
|---|---|---|---|---|---|---|
| Serum | ||||||
| TGs (mg/dl) | 51.1 ± 4.8 | 40.3 ± 2.8 a | 30.5 ± 2.9 ab | 120 ± 8.3 abc | 85.4 ± 4.2 abcd | 55.8 ± 3.6 bcde |
| CHOL (mg/dl) | 79.8 ± 6.1 | 68.8± 3.9 a | 55.1 ± 4.3 ab | 162 ± 10.9 abc | 136 ± 10.1 abcd | 95.2 ± 5.5 abcde |
| LDL-c (mg/dl) | 42.4 ± 4.1 | 32.1 ± 5.4 a | 27.8 ± 4.2 ab | 96.1 ± 5.6 abc | 77.1 ± 4.6 abcd | 54.6 ± 3.7 abcde |
| FFAs (µM/l) | 199 ± 13.1 | 167 ± 10.3 a | 135 ± 7.5 ab | 756 ± 114 abc | 462 ± 79 abcd | 244 ± 32 abcde |
| Liver | ||||||
| TGs (mg/g) | 0.41 ± 0.06 | 0.33 ± 0.04 a | 0.24 ± 0.03 ab | 0.88 ± 0.12 abc | 0.7 ± 0.06 abcd | 0.43 ± 0.05 bcde |
| CHOL (mg/g) | 2.8 ± 0.22 | 2.11 ± 0.17 a | 1.7 ± 0.09 ab | 6.3 ± 0.51 abc | 4.9 ± 0.36 abcd | 3.0 ± 0.26 bcde |
| FFAs (µM/g) | 59.6 ± 7.9 | 49.1 ± 4.2 a | 32.9 ± 3.1 ab | 340 ± 27.1 abc | 236± 26 abcd | 110 ± 16.2 abcde |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Harbi, L.N.; Alshammari, G.M.; Al-Dossari, A.M.; Subash-Babu, P.; Binobead, M.A.; Alhussain, M.H.; AlSedairy, S.A.; Al-Nouri, D.M.; Shamlan, G. Beta vulgaris L. (Beetroot) Methanolic Extract Prevents Hepatic Steatosis and Liver Damage in T2DM Rats by Hypoglycemic, Insulin-Sensitizing, Antioxidant Effects, and Upregulation of PPARα. Biology 2021, 10, 1306. https://doi.org/10.3390/biology10121306
Al-Harbi LN, Alshammari GM, Al-Dossari AM, Subash-Babu P, Binobead MA, Alhussain MH, AlSedairy SA, Al-Nouri DM, Shamlan G. Beta vulgaris L. (Beetroot) Methanolic Extract Prevents Hepatic Steatosis and Liver Damage in T2DM Rats by Hypoglycemic, Insulin-Sensitizing, Antioxidant Effects, and Upregulation of PPARα. Biology. 2021; 10(12):1306. https://doi.org/10.3390/biology10121306
Chicago/Turabian StyleAl-Harbi, Laila Naif, Ghedeir M. Alshammari, Alhanouf Mohammed Al-Dossari, Pandurangan Subash-Babu, Manal Abdulaziz Binobead, Maha H. Alhussain, Sahar Abdulaziz AlSedairy, Doha M. Al-Nouri, and Ghalia Shamlan. 2021. "Beta vulgaris L. (Beetroot) Methanolic Extract Prevents Hepatic Steatosis and Liver Damage in T2DM Rats by Hypoglycemic, Insulin-Sensitizing, Antioxidant Effects, and Upregulation of PPARα" Biology 10, no. 12: 1306. https://doi.org/10.3390/biology10121306
APA StyleAl-Harbi, L. N., Alshammari, G. M., Al-Dossari, A. M., Subash-Babu, P., Binobead, M. A., Alhussain, M. H., AlSedairy, S. A., Al-Nouri, D. M., & Shamlan, G. (2021). Beta vulgaris L. (Beetroot) Methanolic Extract Prevents Hepatic Steatosis and Liver Damage in T2DM Rats by Hypoglycemic, Insulin-Sensitizing, Antioxidant Effects, and Upregulation of PPARα. Biology, 10(12), 1306. https://doi.org/10.3390/biology10121306







