Accumulation and Release of Mercury in the Lichen Evernia prunastri (L.) Ach
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Experimental Design
2.3. Chemical Analysis
2.4. Physiological Parameters
2.5. Statistical Analysis
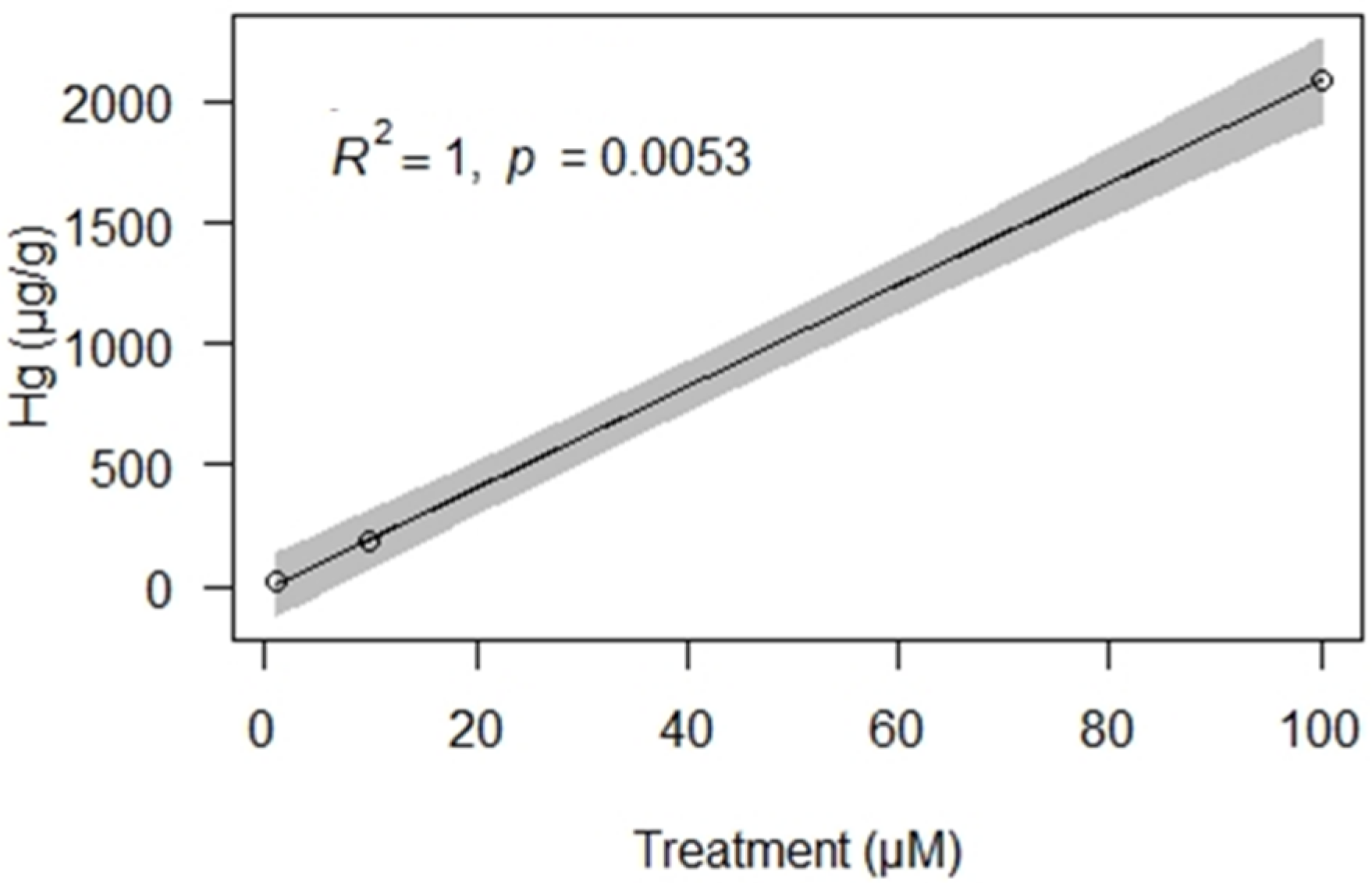
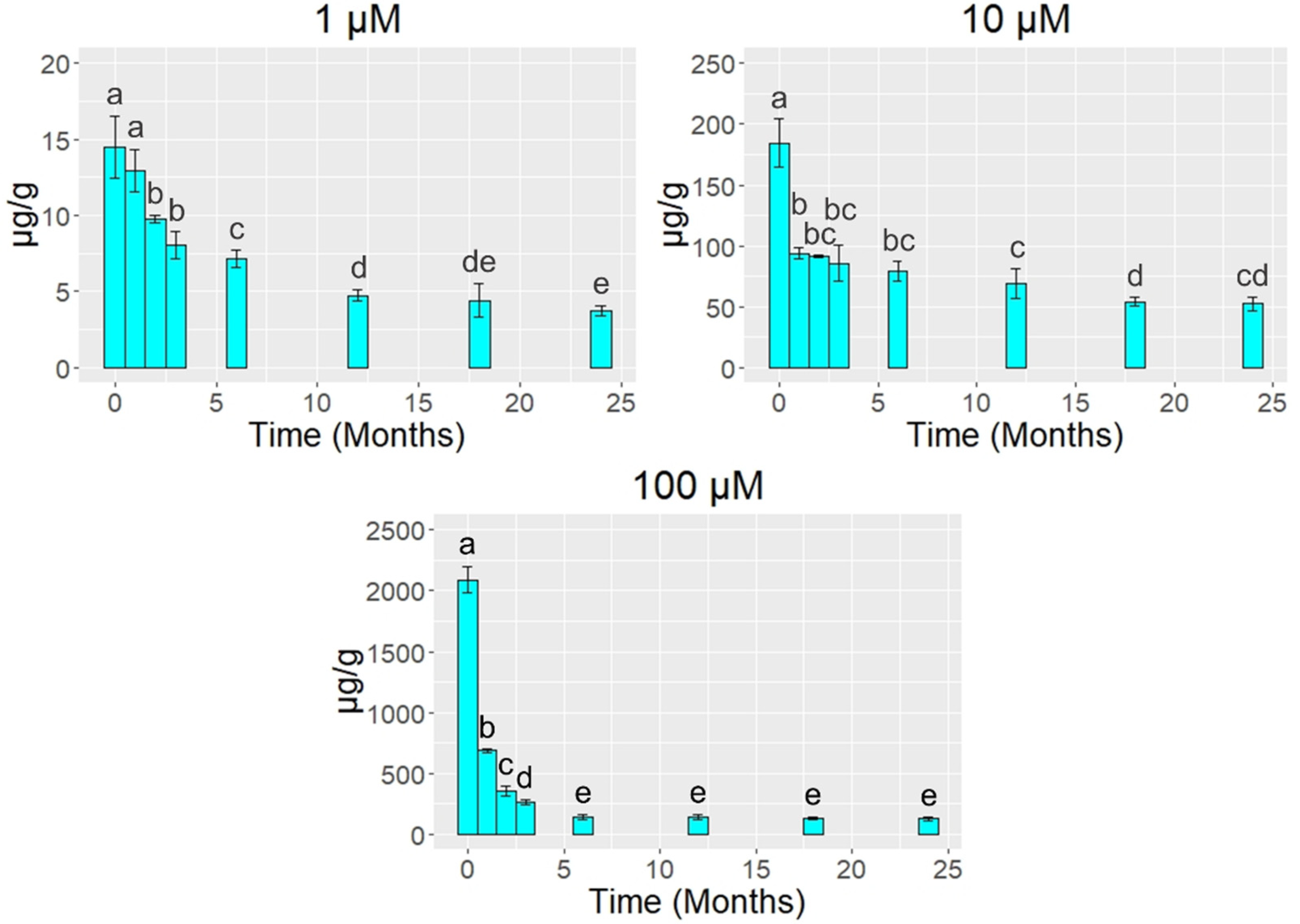
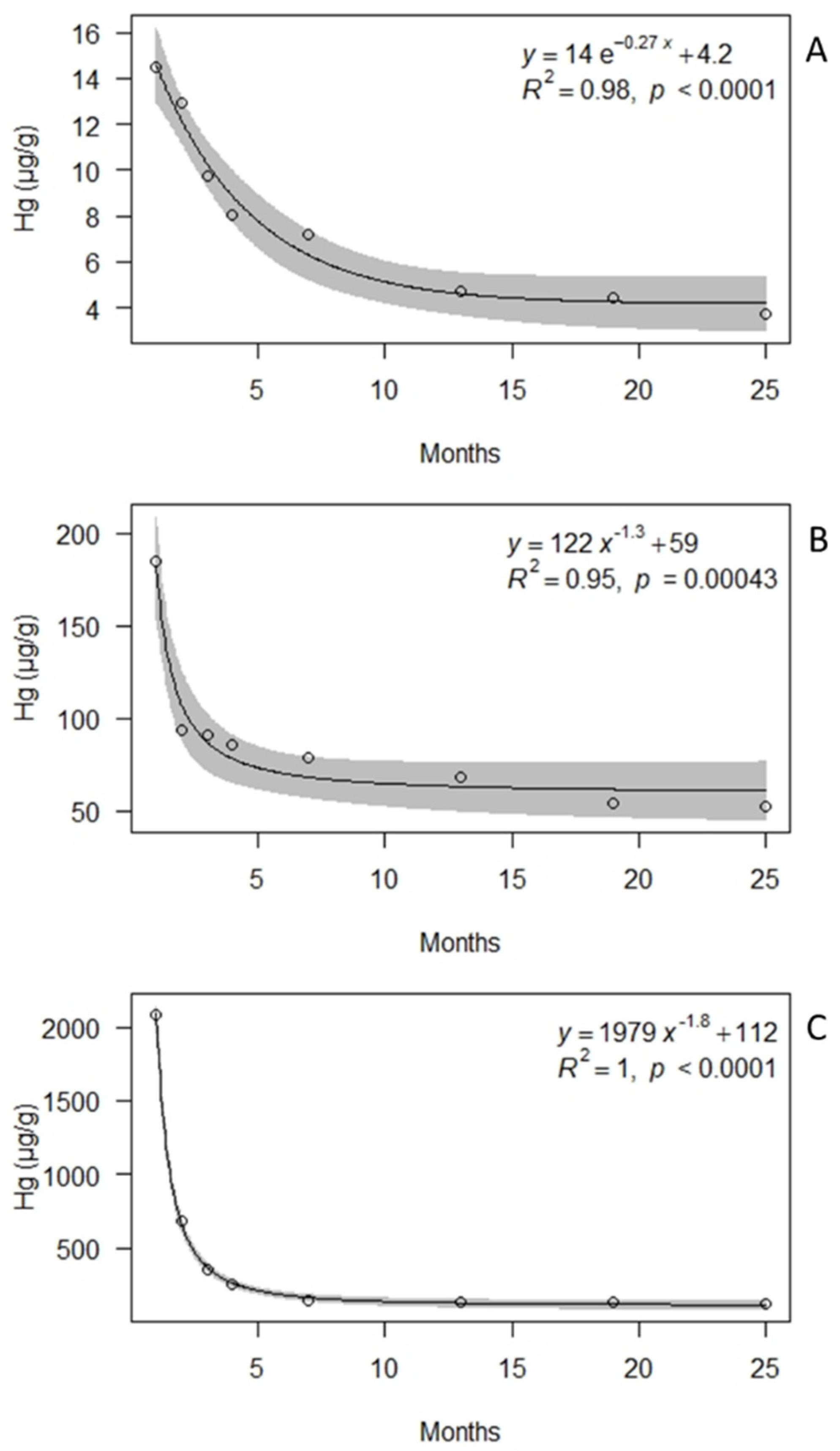
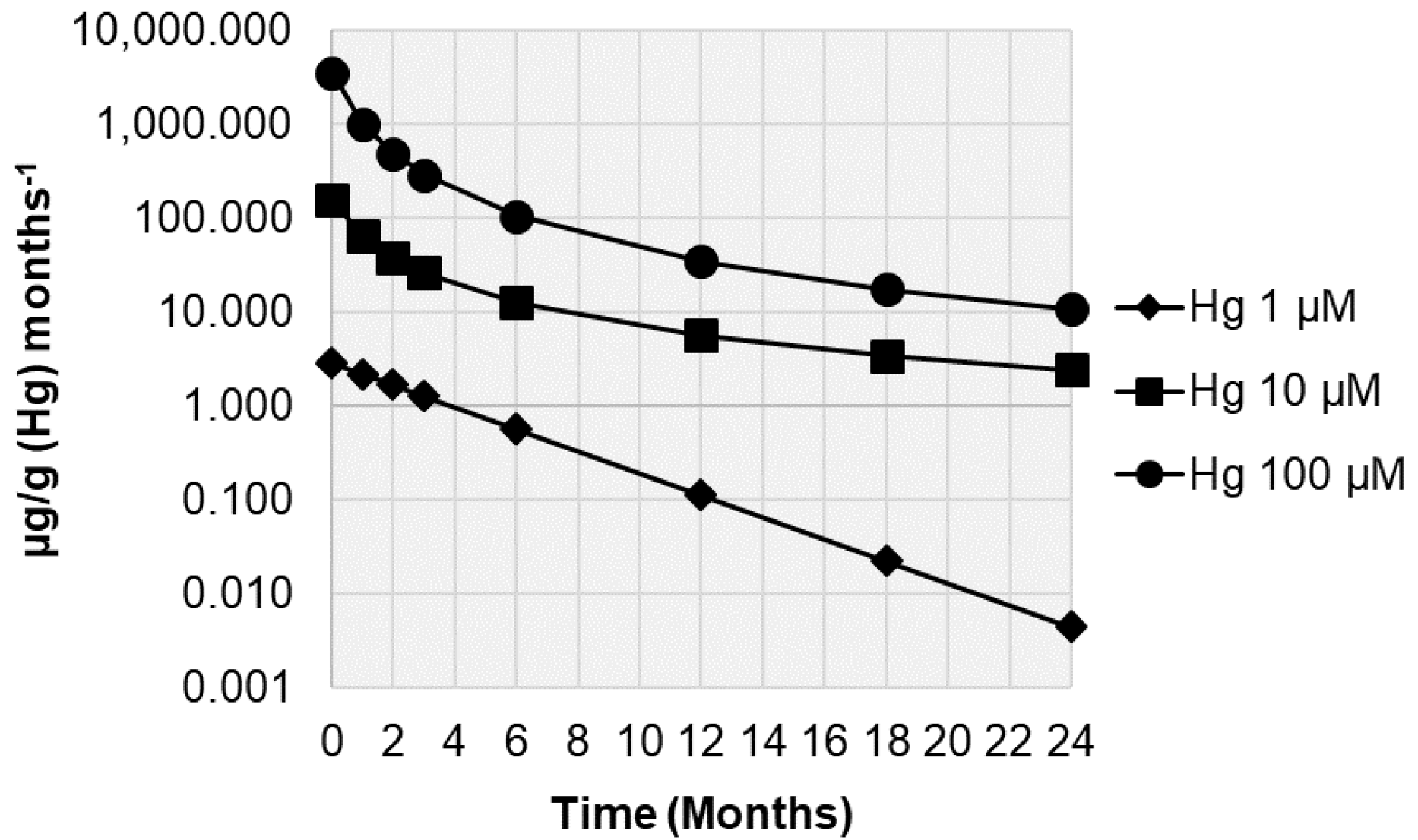
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization, WHO. 2017. Mercury and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/mercury-and-health (accessed on 12 September 2021).
- Aarhus Protocol on Heavy Metals. 1988. Available online: https://unece.org/environment-policy/air/protocol-heavy-metals (accessed on 21 September 2021).
- Environmental Protection Agency, EPA. Minamata Convention on Mercury. 2021. Available online: https://www.epa.gov/international-cooperation/minamata-convention-mercury (accessed on 7 September 2021).
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the Terrestrial Environment: A Review. Environ. Sci. Eur. 2020, 32, 128. [Google Scholar] [CrossRef]
- Carpi, A. Mercury from combustion sources: A review of the chemical species emitted and their transport in the atmosphere. Water Air Soil Pollut. 1997, 98, 241–254. [Google Scholar] [CrossRef]
- Munthe, J.; Wangberg, I.; Pirrone, N.; Iverfeld, A.; Ferrara, R.; Ebinghaus, R.; Feng, X.; Gårdfelt, K.; Keeler, G.; Lanzillotta, E.; et al. Intercomparison of methods for sampling and analysis of atmospheric mercury species. Atmos. Environ. 2001, 35, 3007–3017. [Google Scholar] [CrossRef]
- Evers, D.C.; Keane, S.E.; Basu, N.; Buck, D. Evaluating the Effectiveness of the Minamata Convention on Mercury: Principles and Recommendations for next Steps. Sci. Total Environ. 2016, 569–570, 888–903. [Google Scholar] [CrossRef]
- Bargagli, R. Moss and Lichen Biomonitoring of Atmospheric Mercury: A Review. Sci. Total Environ. 2016, 572, 216–231. [Google Scholar] [CrossRef]
- Loppi, S.; Paoli, L.; Gaggi, C. Diversity of Epiphytic Lichens and Hg Contents of Xanthoria Parietina Thalli as Monitors of Geothermal Air Pollution in the Mt. Amiata Area (Central Italy). J. Atmos. Chem. 2006, 53, 93–105. [Google Scholar] [CrossRef]
- Ljubič Mlakar, T.; Horvat, M.; Kotnik, J.; Jeran, Z.; Vuk, T.; Mrak, T.; Fajon, V. Biomonitoring with Epiphytic Lichens as a Complementary Method for the Study of Mercury Contamination near a Cement Plant. Environ. Monit. Assess. 2011, 181, 225–241. [Google Scholar] [CrossRef]
- Si, L.; Ariya, P. Recent Advances in Atmospheric Chemistry of Mercury. Atmosphere 2018, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Pisani, T.; Munzi, S.; Paoli, L.; Bačkor, M.; Kováčik, J.; Piovár, J.; Loppi, S. Physiological Effects of Mercury in the Lichens Cladonia Arbuscula Subsp. Mitis (Sandst.) Ruoss and Peltigera Rufescens (Weiss) Humb. Chemosphere 2011, 82, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Nicolardi, V.; Bargagli, R.; Loppi, S. Estimating Atmospheric Mercury Concentrations with Lichens. Environ. Sci. Technol. 2014, 48, 8754–8759. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.; Rodriguez, E. Phytotoxicity of Mercury in Plants: A Review. J. Bot. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Rimondi, V.; Benesperi, R.; Beutel, M.W.; Chiarantini, L.; Costagliola, P.; Lattanzi, P.; Medas, D.; Morelli, G. Monitoring of airborne mercury: Comparison of different techniques in the Monte Amiata District, Southern Tuscany, Italy. IJERPH 2020, 17, 2353. [Google Scholar] [CrossRef] [Green Version]
- Viso, S.; Rivera, S.; Martinez-Coronado, A.; Esbrí, J.M.; Moreno, M.M.; Higueras, P. Biomonitoring of Hg0, Hg2 and Particulate Hg in a Mining Context Using Tree Barks+. IJERPH 2021, 18, 5191. [Google Scholar] [CrossRef] [PubMed]
- Bargagli, R.; Barghigiani, C. Lichen Biomonitoring of Mercury Emission and Deposition in Mining, Geothermal and Volcanic Areas of Italy. Environ Monit. Assess. 1991, 16, 265–275. [Google Scholar] [CrossRef]
- Grangeon, S.; Guédron, S.; Asta, J.; Sarret, G.; Charlet, L. Lichen and Soil as Indicators of an Atmospheric Mercury Contamination in the Vicinity of a Chlor-Alkali Plant (Grenoble, France). Ecol. Indic. 2012, 13, 178–183. [Google Scholar] [CrossRef]
- Kłos, A.; Rajfur, M.; Šrámek, I.; Wacławek, M. Mercury Concentration in Lichen, Moss and Soil Samples Collected from the Forest Areas of Praded and Glacensis Euroregions (Poland and Czech Republic). Environ. Monit. Assess. 2012, 184, 6765–6774. [Google Scholar] [CrossRef] [Green Version]
- Fortuna, L.; Candotto Carniel, F.; Capozzi, F.; Tretiach, M. Congruence evaluation of mercury pollution patterns around a waste incinerator over a 16-year-long period using different biomonitors. Atmosphere 2019, 10, 183. [Google Scholar] [CrossRef] [Green Version]
- Fantozzi, L.; Guerrieri, N.; Manca, G.; Orrù, A.; Marziali, L. An Integrated Investigation of Atmospheric Gaseous Elemental Mercury Transport and Dispersion Around a Chlor-Alkali Plant in the Ossola Valley (Italian Central Alps). Toxics 2021, 9, 172. [Google Scholar] [CrossRef]
- Klapstein, S.J.; Walker, A.K.; Saunders, C.H.; Cameron, R.P.; Murimboh, J.D.; O’Driscoll, N.J. Spatial Distribution of Mercury and Other Potentially Toxic Elements Using Epiphytic Lichens in Nova Scotia. Chemosphere 2020, 241, 125064. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.A.; Ramelow, G.J.; Beck, J.N.; Young, J.C.; Callahan, J.D.; Maroon, M.F. Temporal changes in metal levels of the lichens Parmotrema praesorediosum and Ramalina stenospora, Southwest Louisiana. Water Air Soil Pollut. 1990, 53, 189–200. [Google Scholar] [CrossRef]
- Godinho, R.M.; Verburg, T.G.; Freitas, M.D.; Wolterbeek, H.T. Dynamics of Element Accumulation and Release of Flavoparmelia Caperata during a Long-Term Field Transplant Experiment. IJENVH 2011, 5, 49. [Google Scholar] [CrossRef]
- Vannini, A.; Paoli, L.; Fedeli, R.; Kangogo, S.K.; Guarnieri, M.; Ancora, S.; Monaci, F.; Loppi, S. Modeling Heavy Metal Release in the Epiphytic Lichen Evernia Prunastri. Environ. Sci. Pollut. Res. 2021, 28, 27392–27397. [Google Scholar] [CrossRef]
- Tretiach, M.; Candotto Carniel, F.; Loppi, S.; Carniel, A.; Bortolussi, A.; Mazzilis, D.; Del Bianco, C. Lichen transplants as a suitable tool to identify mercury pollution from waste in-cinerators: A case study from NE Italy. Environ Monit Assess. 2011, 175, 589–600. [Google Scholar] [CrossRef]
- Rinino, S.; Bombardi, V.; Giordani, P.; Tretiach, M.; Crisafulli, P.; Monaci, F.; Modenesi, P. New histochemical techniques for the localization of metal ions in the lichen thallus. Lichenologist 2005, 37, 463–466. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 27 August 2021).
- Bargagli, R. Trace Elements in Terrestrial Plants: An Ecophysiological Approach to Biomonitoring and Biorecovery; Springer. R.G. Landes Company: Georgetown, TX, USA, 1998; p. 190. [Google Scholar]
- Cecconi, E.; Fortuna, L.; Benesperi, R.; Bianchi, E.; Brunialti, G.; Contardo, T.; Di Nuzzo, L.; Frati, L.; Monaci, F.; Munzi, S.; et al. New Interpretative Scales for Lichen Bioaccumulation Data: The Italian Proposal. Atmosphere 2019, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- Paoli, L.; Vannini, A.; Fačkovcová, Z.; Guarnieri, M.; Bačkor, M.; Loppi, S. One Year of Transplant: Is It Enough for Lichens to Reflect the New Atmospheric Conditions? Ecol. Indic. 2018, 88, 495–502. [Google Scholar] [CrossRef]
- Reis, M.A.; Alves, L.C.; Freitas, M.C.; Van Os, B.; Wolterbeek, H.T. Lichens (Parmelia sulcata) time re-sponse model to environmental elemental availability. Sci. Total Environ. 1999, 232, 105–115. [Google Scholar] [CrossRef]
- Bargagli, R.; Mikhailova, I. Accumulation of Inorganic Contaminants. In Monitoring with Lichens—Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; NATO Science Series (Series IV: Earth and Environmental Sciences); Springer: Dordrecht, The Netherlands, 2002; Volume 7, pp. 65–84. [Google Scholar]
- Nieboer, E.; Richardson, D.H.S. Lichens as Monitors of Atmospheric Deposition; Ann Arbor Science Publishers: Ann Arbor, MI, USA, 1981; pp. 339–388. [Google Scholar]
- Paoli, L.; Vannini, A.; Monaci, F.; Loppi, S. Competition between heavy metal ions for binding sites in lichens: Implications for biomonitoring studies. Chemosphere 2018, 199, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Sarret, G.; Manceau, A.; Cuny, D.; Van Haluwyn, C.; Déruelle, S.; Hazemann, J.-L.; Soldo, Y.; Eybert-Bérard, L.; Menthonnex, J.-J. Mechanisms of Lichen Resistance to Metallic Pollution. Environ. Sci. Technol. 1998, 32, 3325–3330. [Google Scholar] [CrossRef]
- Ariya, P.A.; Amyot, M.; Dastoor, A.; Deeds, D.; Feinberg, A.; Kos, G.; Poulain, A.; Ryjkov, A.; Semeniuk, K.; Subir, M.; et al. Mercury Physicochemical and Biogeochemical Transformation in the Atmosphere and at Atmospheric Interfaces: A Review and Future Directions. Chem. Rev. 2015, 115, 3760–3802. [Google Scholar] [CrossRef]
- Reis, M.A.; Alves, L.C.; Freitas, M.C.; Van Os, B.; de Goeij, J.; Wolterbeek, H.T. Calibration of Lichen Transplants Considering Faint Memory Effects. Environ. Pollut. 2002, 120, 87–95. [Google Scholar] [CrossRef]
- Boulyga, S.F.; Desideri, D.; Meli, M.A.; Testa, C.; Becker, J.S. Plutonium and Americium Determination in Mosses by Laser Ablation ICP-MS Combined with Isotope Dilution Technique. Int. J. Mass Spectrom. 2003, 226, 329–339. [Google Scholar] [CrossRef]
- Dalvand, A.; Jahangiri, A.; Iranmanesh, J. Introduce Lichen Lepraria Incana as Biomonitor of Cesium-137 from Ramsar, Northern Iran. J. Environ. Radioact. 2016, 160, 36–41. [Google Scholar] [CrossRef]
- Loppi, S.; Di Lucia, A.; Vannini, A.; Ancora, S.; Monaci, F.; Paoli, L. Uptake and Release of Copper Ions in Epiphytic Lichens. Biologia 2020, 75, 1547–1552. [Google Scholar] [CrossRef]
- Gupta, M.; Chandra, P. Bioaccumulation and Toxicity of Mercury in Rooted-Submerged Macrophyte Vallisneria Spiralis. Environ. Pollut. 1998, 103, 327–332. [Google Scholar] [CrossRef]
- De Filippis, L.F.; Hampp, R.; Ziegler, H. The effects of sublethal concentrations of zinc, cadmium and mercury on Euglena. Arch. Microbiol. 1981, 128, 407–411. [Google Scholar] [CrossRef]
- Cecconi, E.; Fortuna, L.; Peplis, M.; Tretiach, M. Element Accumulation Performance of Living and Dead Lichens in a Large-Scale Transplant Application. Environ. Sci. Pollut. Res. 2021, 28, 16214–16226. [Google Scholar] [CrossRef]
- Tyler, G. Uptake, Retention and Toxicity of Heavy Metals in L Ichens: A Brief Review. Water Air Soil Pollut. 1989, 47, 321–333. [Google Scholar] [CrossRef]
- Boquete, M.T.; Fernández, J.Á.; Carballeira, A.; Aboal, J.R. Assessing the Tolerance of the Terrestrial Moss Pseudoscleropodium Purum to High Levels of Atmospheric Heavy Metals: A Reciprocal Transplant Study. Sci. Total Environ. 2013, 461–462, 552–559. [Google Scholar] [CrossRef] [PubMed]
- McLagan, D.S.; Monaci, F.; Huang, H.; Lei, Y.D.; Mitchell, C.P.; Wania, F. Characterization and quantification of atmospheric mercury sources using passive air samplers. J. Geophys. 2019, 124, 2351–2362. [Google Scholar] [CrossRef]

| Time (Months) | Photosynthetic Efficiency (FV/FM) | Chlorophyll a Content | ||||
|---|---|---|---|---|---|---|
| 1 µM | 10 µM | 100 µM | 1 µM | 10 µM | 100 µM | |
| 0 | 0.95 ± 0.03 (ab) | 0.97 ± 0.02 (ab) | 0.13 ± 0.07 (a) | 0.99 ± 0.07 (a) | 1.01 ± 0.08 (a) | 0.09 ± 0.02 (a) |
| 1 | 1.04 ± 0.01 (a) | 0.95 ± 0.03 (ab) | 0.23 ± 0.04 (ab) | 1.23 ± 0.11 (b) | 0.92 ± 0.07 (ab) | 0.02 ± 0.01 (b) |
| 2 | 0.93 ± 0.02 (b) | 0.96 ± 0.03 (ab) | 0.31 ± 0.03 (ab) | 0.93 ± 0.04 (ac) | 0.93 ± 0.07 (ab) | 0.01 ± 0.01 (b) |
| 3 | 1.02 ± 0.06 (ab) | 1.05 ± 0.02 (ab) | 0.35 ± 0.05 (b) | 0.97 ± 0.04 (a) | 0.92 ± 0.06 (ab) | 0.01 ± 0.01 (b) |
| 6 | 0.98 ± 0.03 (ab) | 0.94 ± 0.02 (a) | 0.38 ± 0.05 (b) | 1.09 ± 0.16 (ab) | 0.95 ± 0.9 (ab) | 0.01 ± 0.01 (b) |
| 12 | 0.96 ± 0.01 (ab) | 0.96 ± 0.01 (ab) | 0.56 ± 0.02 (c) | 0.99 ± 0.07 (a) | 0.90 ± 0.01 (b) | 0.02 ± 0.00 (b) |
| 18 | 1.00 ± 0.01 (ab) | 1.08 ± 0.04 (b) | 0.57 ± 0.01 (c) | 1.05 ± 0.08 (ab) | 1.05 ± 0.06 (a) | 0.03 ± 0.01 (b) |
| 24 | 1.01 ± 0.01 (ab) | 0.97 ± 0.03 (ab) | 0.63 ± 0.04 (c) | 1.08 ± 0.04 (ab) | 0.98 ± 0.02 (ab) | 0.11 ± 0.02 (a) |
| T24 | |
|---|---|
| 1 µM | 6 |
| 10 µM | 79 |
| 100 µM | 230 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vannini, A.; Jamal, M.B.; Gramigni, M.; Fedeli, R.; Ancora, S.; Monaci, F.; Loppi, S. Accumulation and Release of Mercury in the Lichen Evernia prunastri (L.) Ach. Biology 2021, 10, 1198. https://doi.org/10.3390/biology10111198
Vannini A, Jamal MB, Gramigni M, Fedeli R, Ancora S, Monaci F, Loppi S. Accumulation and Release of Mercury in the Lichen Evernia prunastri (L.) Ach. Biology. 2021; 10(11):1198. https://doi.org/10.3390/biology10111198
Chicago/Turabian StyleVannini, Andrea, Muhammad Bilal Jamal, Margherita Gramigni, Riccardo Fedeli, Stefania Ancora, Fabrizio Monaci, and Stefano Loppi. 2021. "Accumulation and Release of Mercury in the Lichen Evernia prunastri (L.) Ach" Biology 10, no. 11: 1198. https://doi.org/10.3390/biology10111198
APA StyleVannini, A., Jamal, M. B., Gramigni, M., Fedeli, R., Ancora, S., Monaci, F., & Loppi, S. (2021). Accumulation and Release of Mercury in the Lichen Evernia prunastri (L.) Ach. Biology, 10(11), 1198. https://doi.org/10.3390/biology10111198











