The Long Non-Coding RNA SAMMSON Is a Regulator of Chemosensitivity and Metabolic Orientation in MCF-7 Doxorubicin-Resistant Breast Cancer Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Cell Culture
2.2. Small Interference RNA
2.3. RNA Extraction, RT, qPCR
2.4. Glucose and Lactate Measurements
2.5. Mitochondrial Respiration Rates
2.6. Mitochondrial ROS Production
2.7. Mitochondrial Enzymatic Activities
2.8. Western Blotting
2.9. RNA Sequencing
2.10. Data Processing and Analysis
2.11. Cellular Growth Monitoring
2.12. Senescence Escape
2.13. Statistical Analysis
3. Results
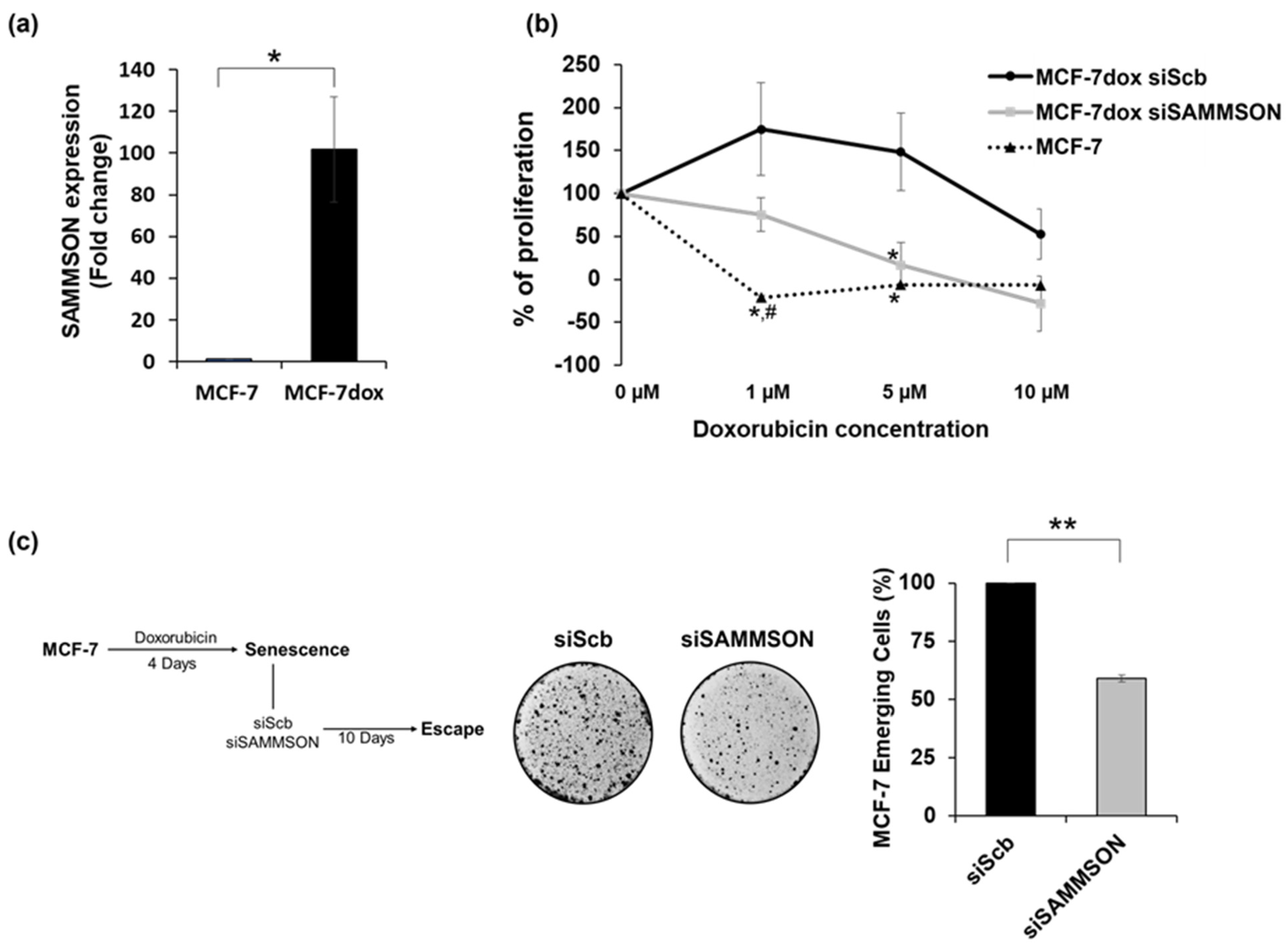
3.1. SAMMSON Expression Increases the Resistance of MCF-7dox Cells to Doxorubicin and Promotes the Senescence Escape of MCF-7 Cells after Doxorubicin Treatment
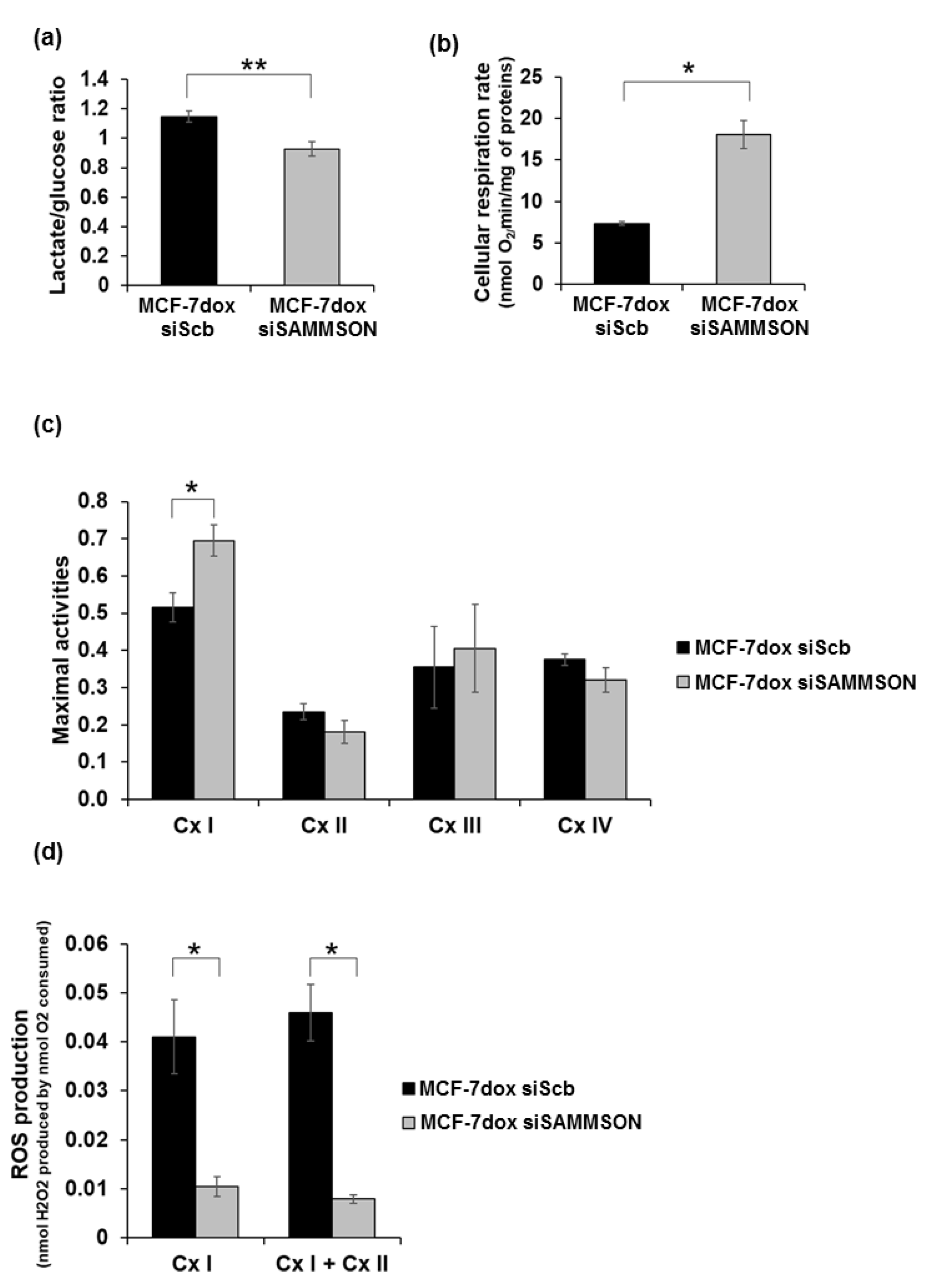
3.2. SAMMSON Modulates Complex I Activity and ROS Production in MCF-7dox Cells
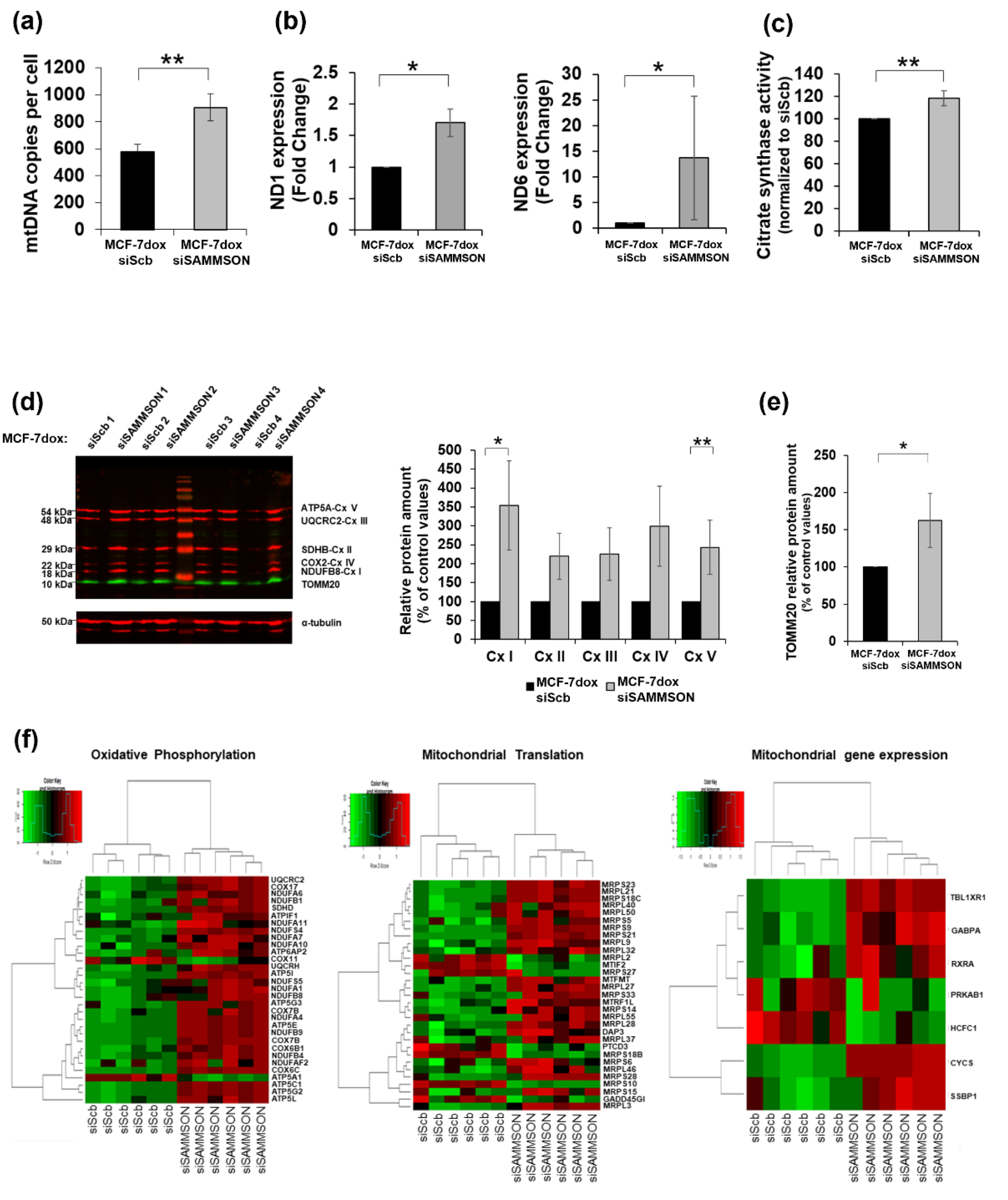
3.3. SAMMSON Participates in Mitochondrial Replication, Transcription and Translation in MCF-7dox Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Gatti, L.; Zunino, F. Overview of tumor cell chemoresistance mechanisms. Methods Mol. Med. 2005, 111, 127–148. [Google Scholar] [PubMed]
- Lim, M.M.K.; Wee, J.W.K.; Soong, J.C.; Chua, D.; Tan, W.R.; Lizwan, M.; Li, Y.; Teo, Z.; Goh, W.W.B.; Zhu, P.; et al. Targeting metabolic flexibility via angiopoietin-like 4 protein sensitizes metastatic cancer cells to chemotherapy drugs. Mol. Cancer 2018, 17, 152. [Google Scholar] [CrossRef] [PubMed]
- Fendt, S.M.; Frezza, C.; Erez, A. Targeting metabolic plasticity and flexibility dynamics for cancer therapy. Cancer Discov. 2020, 10, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.M.; Yeung, S.C.; Lee, M.H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014, 11, 1–19. [Google Scholar]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.; Kroemer, G.; Galluzzi, L. Mitochondrial metabolism and cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef]
- Wilson, S.; Filipp, F.V. A network of epigenomic and transcriptional cooperation encompassing an epigenomic master regulator in cancer. NPJ Syst. Biol. Appl. 2018, 4, 24. [Google Scholar] [CrossRef]
- Puvvula, P.K. Lncrnas regulatory networks in cellular senescence. Int. J. Mol. Sci. 2019, 20, 2615. [Google Scholar] [CrossRef] [Green Version]
- Mercer, T.R.; Mattick, J.S. Structure and function of long noncoding rnas in epigenetic regulation. Nat. Struct. Mol. Biol. 2013, 20, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Montes, M.; Lund, A.H. Emerging roles of lncrnas in senescence. FEBS J. 2016, 283, 2414–2426. [Google Scholar] [CrossRef] [PubMed]
- Leucci, E.; Coe, E.A.; Marine, J.C.; Vance, K.W. The emerging role of long non-coding rnas in cutaneous melanoma. Pigment Cell Melanoma Res. 2016, 29, 619–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Tian, X.; Zhang, Q.; Shi, P.; Li, S. Long non-coding rna sammson as a novel potential diagnostic and prognostic biomarker for oral squamous cell carcinoma. J. Dent. Sci. 2020, 15, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cai, H.; Hu, B.; Tu, J. Lncrna sammson negatively regulates mir-9-3p in hepatocellular carcinoma cells and has prognostic values. Biosci. Rep. 2019, 39, BSR20190615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.B.; Lin, S.X.; Cao, H.L.; Xiao, Z.Q. Values of long noncoding rna sammson in the clinicopathologic features and the prognostic implications of human gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6080–6087. [Google Scholar] [PubMed]
- Shao, L.; Sun, W.; Wang, Z.; Dong, W.; Qin, Y. Long noncoding rna sammson promotes papillary thyroid carcinoma progression through p300/sp1 axis and serves as a novel diagnostic and prognostic biomarker. IUBMB Life 2020, 72, 237–246. [Google Scholar] [CrossRef]
- Vendramin, R.; Verheyden, Y.; Ishikawa, H.; Goedert, L.; Nicolas, E.; Saraf, K.; Armaos, A.; Delli Ponti, R.; Izumikawa, K.; Mestdagh, P.; et al. Sammson fosters cancer cell fitness by concertedly enhancing mitochondrial and cytosolic translation. Nat. Struct. Mol. Biol. 2018, 25, 1035–1046. [Google Scholar] [CrossRef]
- Fogal, V.; Richardson, A.D.; Karmali, P.P.; Scheffler, I.E.; Smith, J.W.; Ruoslahti, E. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol. Cell. Biol. 2010, 30, 1303–1318. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Colleoni, M.; Sun, Z.; Price, K.N.; Karlsson, P.; Forbes, J.F.; Thurlimann, B.; Gianni, L.; Castiglione, M.; Gelber, R.D.; Coates, A.S.; et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: Results from the international breast cancer study group trials i to v. J. Clin. Oncol. 2016, 34, 927–935. [Google Scholar] [CrossRef]
- Desquiret-Dumas, V.; Leman, G.; Wetterwald, C.; Chupin, S.; Lebert, A.; Khiati, S.; Le Mao, M.; Geffroy, G.; Kane, M.S.; Chevrollier, A.; et al. Warburg-like effect is a hallmark of complex i assembly defects. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2475–2489. [Google Scholar] [CrossRef]
- Zhang, Y.; Parmigiani, G.; Johnson, W.E. Combat-seq: Batch effect adjustment for rna-seq count data. NAR Genom. Bioinform. 2020, 2, lqaa078. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for rna-seq data with deseq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, A.; Ibrahim, J.G.; Love, M.I. Heavy-tailed prior distributions for sequence count data: Removing the noise and preserving large differences. Bioinformatics 2019, 35, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Petit, C.; Moreau, M.; Toutain, B.; Henry, C.; Roche, H.; Bonichon-Lamichhane, N.; Salmon, J.P.; Lemonnier, J.; Campone, M.; et al. Regulation of senescence escape by tsp1 and cd47 following chemotherapy treatment. Cell Death Dis. 2019, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Jonchere, B.; Vetillard, A.; Toutain, B.; Lam, D.; Bernard, A.C.; Henry, C.; De Carne Trecesson, S.; Gamelin, E.; Juin, P.; Guette, C.; et al. Irinotecan treatment and senescence failure promote the emergence of more transformed and invasive cells that depend on anti-apoptotic mcl-1. Oncotarget 2015, 6, 409–426. [Google Scholar] [CrossRef] [Green Version]
- Le Duff, M.; Gouju, J.; Jonchere, B.; Guillon, J.; Toutain, B.; Boissard, A.; Henry, C.; Guette, C.; Lelievre, E.; Coqueret, O. Regulation of senescence escape by the cdk4-ezh2-ap2m1 pathway in response to chemotherapy. Cell Death Dis. 2018, 9, 199. [Google Scholar] [CrossRef]
- Desbats, M.A.; Giacomini, I.; Prayer-Galetti, T.; Montopoli, M. Metabolic plasticity in chemotherapy resistance. Front. Oncol. 2020, 10, 281. [Google Scholar] [CrossRef]
- Guerra, F.; Arbini, A.A.; Moro, L. Mitochondria and cancer chemoresistance. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 686–699. [Google Scholar] [CrossRef]
- Ma, L.; Zong, X. Metabolic symbiosis in chemoresistance: Refocusing the role of aerobic glycolysis. Front. Oncol. 2020, 10, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyon, R.C.; Cohen, J.S.; Faustino, P.J.; Megnin, F.; Myers, C.E. Glucose metabolism in drug-sensitive and drug-resistant human breast cancer cells monitored by magnetic resonance spectroscopy. Cancer Res. 1988, 48, 870–877. [Google Scholar] [PubMed]
- Varghese, E.; Samuel, S.M.; Liskova, A.; Samec, M.; Kubatka, P.; Busselberg, D. Targeting glucose metabolism to overcome resistance to anticancer chemotherapy in breast cancer. Cancers (Basel) 2020, 12, 2252. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, S.T.; Desquiret-Dumas, V.; Yikilmaz, U.; Dartier, J.; Domingo, I.; Wetterwald, C.; Orre, C.; Gueguen, N.; Brisson, L.; Mahéo, K.; et al. Doxorubicin-induced autophagolysosome formation is partly prevented by mitochondrial ros elimination in dox-resistant breast cancer cells. Int. J. Mol. Sci. 2021, 22, 9283. [Google Scholar] [CrossRef] [PubMed]
- Leucci, E.; Vendramin, R.; Spinazzi, M.; Laurette, P.; Fiers, M.; Wouters, J.; Radaelli, E.; Eyckerman, S.; Leonelli, C.; Vanderheyden, K.; et al. Melanoma addiction to the long non-coding rna sammson. Nature 2016, 531, 518–522. [Google Scholar] [CrossRef]
- Marinello, J.; Delcuratolo, M.; Capranico, G. Anthracyclines as topoisomerase ii poisons: From early studies to new perspectives. Int. J. Mol. Sci. 2018, 19, 3480. [Google Scholar] [CrossRef] [Green Version]
- Khiati, S.; Dalla Rosa, I.; Sourbier, C.; Ma, X.; Rao, V.A.; Neckers, L.M.; Zhang, H.; Pommier, Y. Mitochondrial topoisomerase i (top1mt) is a novel limiting factor of doxorubicin cardiotoxicity. Clin. Cancer Res. 2014, 20, 4873–4881. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.K.; Jang, M.; Song, M.J.; Kim, D.; Kim, Y.; Jang, H.H. Redox-mediated mechanism of chemoresistance in cancer cells. Antioxidants (Basel) 2019, 8, 471. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Kumari, S.; Badana, A.K.; Murali Mohan, G.; Shailender, G.; Malla, R. Reactive oxygen species: A key constituent in cancer survival. Biomark Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-H.; Yu, N.-S.; Deng, Q.; Zhang, Y.; Hu, Y.-Y.; Liu, G.; Huang, K. Lncrna snhg7 mediates the chemoresistance and stemness of breast cancer by sponging mir-34a. Front. Oncol. 2020, 10, 2506. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shoorei, H.; Bahroudi, Z.; Abak, A.; Taheri, M. The role of h19 lncrna in conferring chemoresistance in cancer cells. Biomed. Pharmacother. 2021, 138, 111447. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Hu, Z.; Zhou, Z.; Zhang, H. Linc00518 contributes to multidrug resistance through regulating the mir-199a/mrp1 axis in breast cancer. Cell Physiol. Biochem. 2018, 48, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, P.; Liu, J.; Liu, W.; Wu, X.; Ding, J.; Kang, J.; Li, J.; Lu, J.; Pan, G. Hypermethylation of lncrna meg3 impairs chemosensitivity of breast cancer cells. J. Clin. Lab. Anal. 2020, 34, e23369. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Lai, H.; Li, S.; You, Q.; Fang, Y.; Li, Q.; Liu, Y. Long non-coding rna crala is associated with poor response to chemotherapy in primary breast cancer. Thorac. Cancer 2017, 8, 582–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Li, Y.; Song, X.; Zhang, N.; Sang, Y.; Zhang, H.; Liu, Y.; Chen, B.; Zhao, W.; Wang, L.; et al. Long noncoding rna linp1 acts as an oncogene and promotes chemoresistance in breast cancer. Cancer Biol. Ther. 2018, 19, 120–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, V.Y.; Chen, J.; Cheuk, I.W.; Siu, M.T.; Ho, C.W.; Wang, X.; Jin, H.; Kwong, A. Long non-coding rna neat1 confers oncogenic role in triple-negative breast cancer through modulating chemoresistance and cancer stemness. Cell Death Dis. 2019, 10, 270. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Cheng, Y.; She, Q.; Jiang, Y.; Chen, Y.; Yang, W.; Li, Y. Long non-coding rna tug1 sponges mir-197 to enhance cisplatin sensitivity in triple negative breast cancer. Biomed. Pharmacother. 2018, 107, 338–346. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, P.; Li, P.; Yang, F.; Gao, X.Q. Long non-coding rna h19 regulates proliferation and doxorubicin resistance in mcf-7 cells by targeting parp1. Bioengineered 2020, 11, 536–546. [Google Scholar] [CrossRef]
- Wu, H.; Gu, J.; Zhou, D.; Cheng, W.; Wang, Y.; Wang, Q.; Wang, X. Linc00160 mediated paclitaxel-and doxorubicin-resistance in breast cancer cells by regulating tff3 via transcription factor c/ebpbeta. J. Cell. Mol. Med. 2020, 24, 8589–8602. [Google Scholar] [CrossRef]
- Xu, S.; Wan, L.; Yin, H.; Xu, H.; Zheng, W.; Shen, M.; Zhang, Z.; Pang, D. Long noncoding rna linc00152 functions as a tumor propellant in pan-cancer. Cell. Physiol. Biochem. 2017, 44, 2476–2490. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Jin, T.; Zhang, T. Suppression of long non-coding rna metastasis-associated lung adenocarcinoma transcript 1 (malat1) potentiates cell apoptosis and drug sensitivity to taxanes and adriamycin in breast cancer. Med. Sci. Monit. 2020, 26, e922672. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Cui, X.; Zhao, L.; Liu, L.; Wang, D. Overexpression of growth-arrest-specific transcript 5 improved cisplatin sensitivity in hepatocellular carcinoma through sponging mir-222. DNA Cell Biol. 2020, 39, 724–732. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orre, C.; Dieu, X.; Guillon, J.; Gueguen, N.; Ahmadpour, S.T.; Dumas, J.-F.; Khiati, S.; Reynier, P.; Lenaers, G.; Coqueret, O.; et al. The Long Non-Coding RNA SAMMSON Is a Regulator of Chemosensitivity and Metabolic Orientation in MCF-7 Doxorubicin-Resistant Breast Cancer Cells. Biology 2021, 10, 1156. https://doi.org/10.3390/biology10111156
Orre C, Dieu X, Guillon J, Gueguen N, Ahmadpour ST, Dumas J-F, Khiati S, Reynier P, Lenaers G, Coqueret O, et al. The Long Non-Coding RNA SAMMSON Is a Regulator of Chemosensitivity and Metabolic Orientation in MCF-7 Doxorubicin-Resistant Breast Cancer Cells. Biology. 2021; 10(11):1156. https://doi.org/10.3390/biology10111156
Chicago/Turabian StyleOrre, Charlotte, Xavier Dieu, Jordan Guillon, Naïg Gueguen, Seyedeh Tayebeh Ahmadpour, Jean-François Dumas, Salim Khiati, Pascal Reynier, Guy Lenaers, Olivier Coqueret, and et al. 2021. "The Long Non-Coding RNA SAMMSON Is a Regulator of Chemosensitivity and Metabolic Orientation in MCF-7 Doxorubicin-Resistant Breast Cancer Cells" Biology 10, no. 11: 1156. https://doi.org/10.3390/biology10111156
APA StyleOrre, C., Dieu, X., Guillon, J., Gueguen, N., Ahmadpour, S. T., Dumas, J.-F., Khiati, S., Reynier, P., Lenaers, G., Coqueret, O., Chevrollier, A., Mirebeau-Prunier, D., & Desquiret-Dumas, V. (2021). The Long Non-Coding RNA SAMMSON Is a Regulator of Chemosensitivity and Metabolic Orientation in MCF-7 Doxorubicin-Resistant Breast Cancer Cells. Biology, 10(11), 1156. https://doi.org/10.3390/biology10111156





