Bovine Follicular Fluid Derived Extracellular Vesicles Modulate the Viability, Capacitation and Acrosome Reaction of Bull Spermatozoa
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Bovine FF and Isolation of EVs
2.2. Human Choriocarcinoma Cell Line (JAr) Cell Culture and Purification of JAr EVs
2.3. Nanoparticle Tracking Analysis (NTA)
2.4. Western Blot
2.5. Transmission Electron Microscopy
2.6. Washing of Spermatozoa
2.7. Assessment of the Viability of Spermatozoa
2.8. Assessment of Sperm Capacitation
2.9. Assessment of Acrosomal Reaction
2.10. Surface Modification of EVs
2.11. Determination of Progesterone Concentration of FF and EVs
2.12. Statistical Analysis
2.13. Experimental Design
2.13.1. Determining the Effects of FF EVs and Non-EV Fractions on the Viability, Capacitation and Acrosomal Reaction of Spermatozoa
2.13.2. Determining the Minimum Concentration of FF EVs Required for Modifying the Viability, Capacitation and Acrosomal Reaction of Spermatozoa
2.13.3. Studying the Specificity of the Effects of FF EVs on the Maintenance of Sperm Viability, Induction of Capacitation and the Acrosomal Reaction of Spermatozoa
2.13.4. Studying the Effect of FF EV Surface-Modification on the Viability, Capacitation and Acrosomal Reaction of Spermatozoa
2.13.5. Studying the Efficiency and Synergistic Effects of Progesterone and FF EVs on Sperm Viability, Capacitation and Acrosome Reaction
3. Results
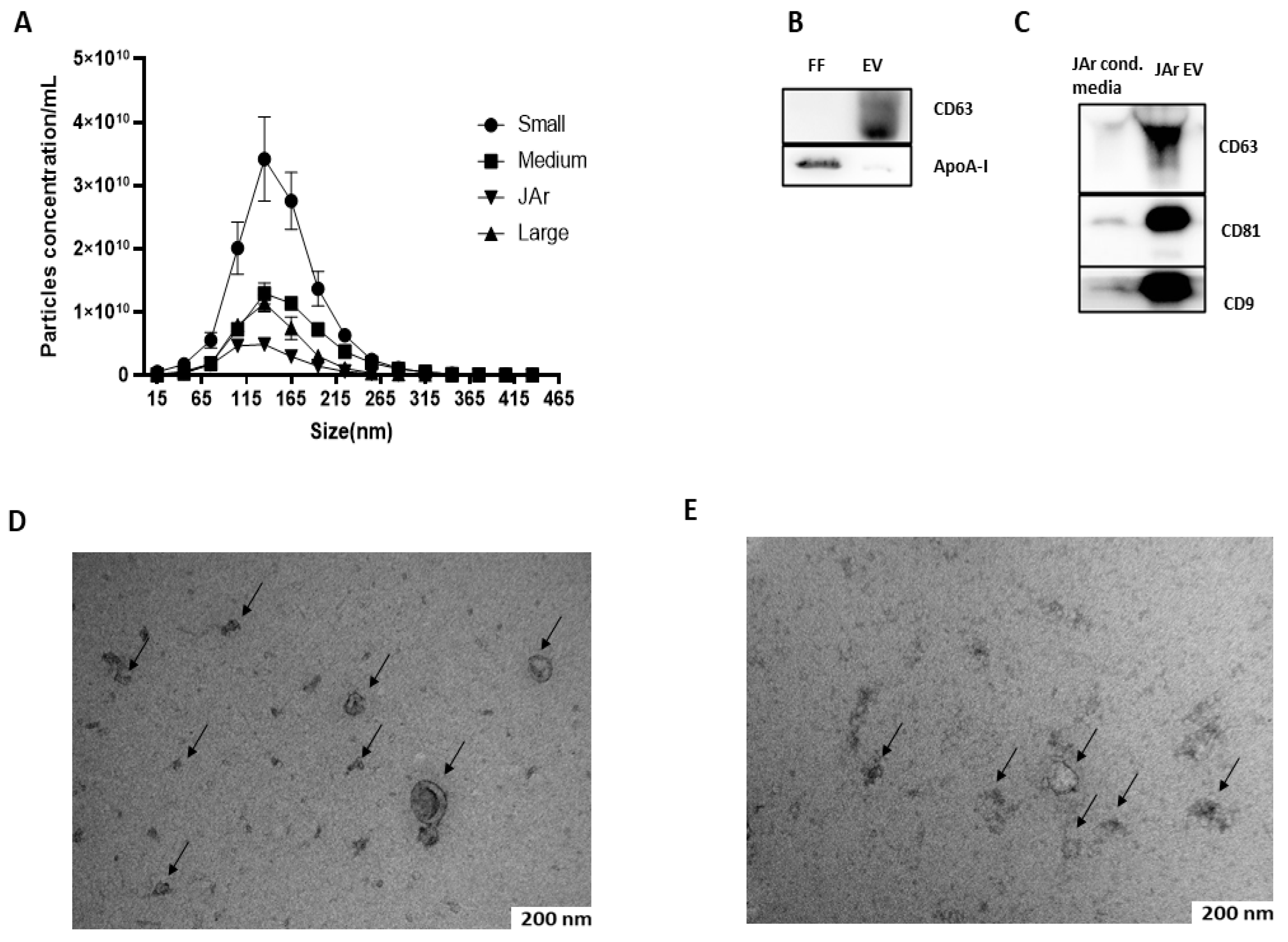
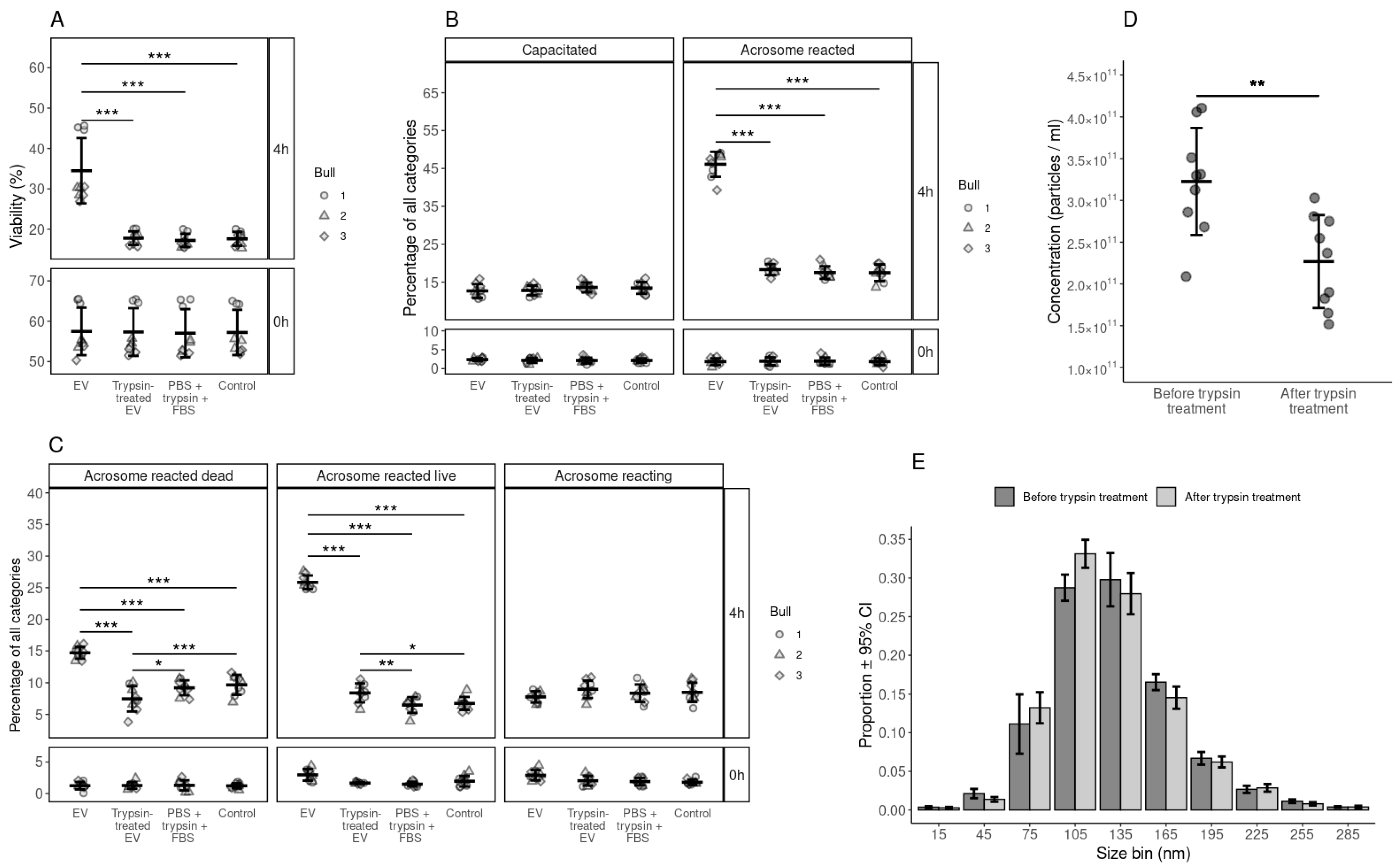
3.1. Characterization of EVs
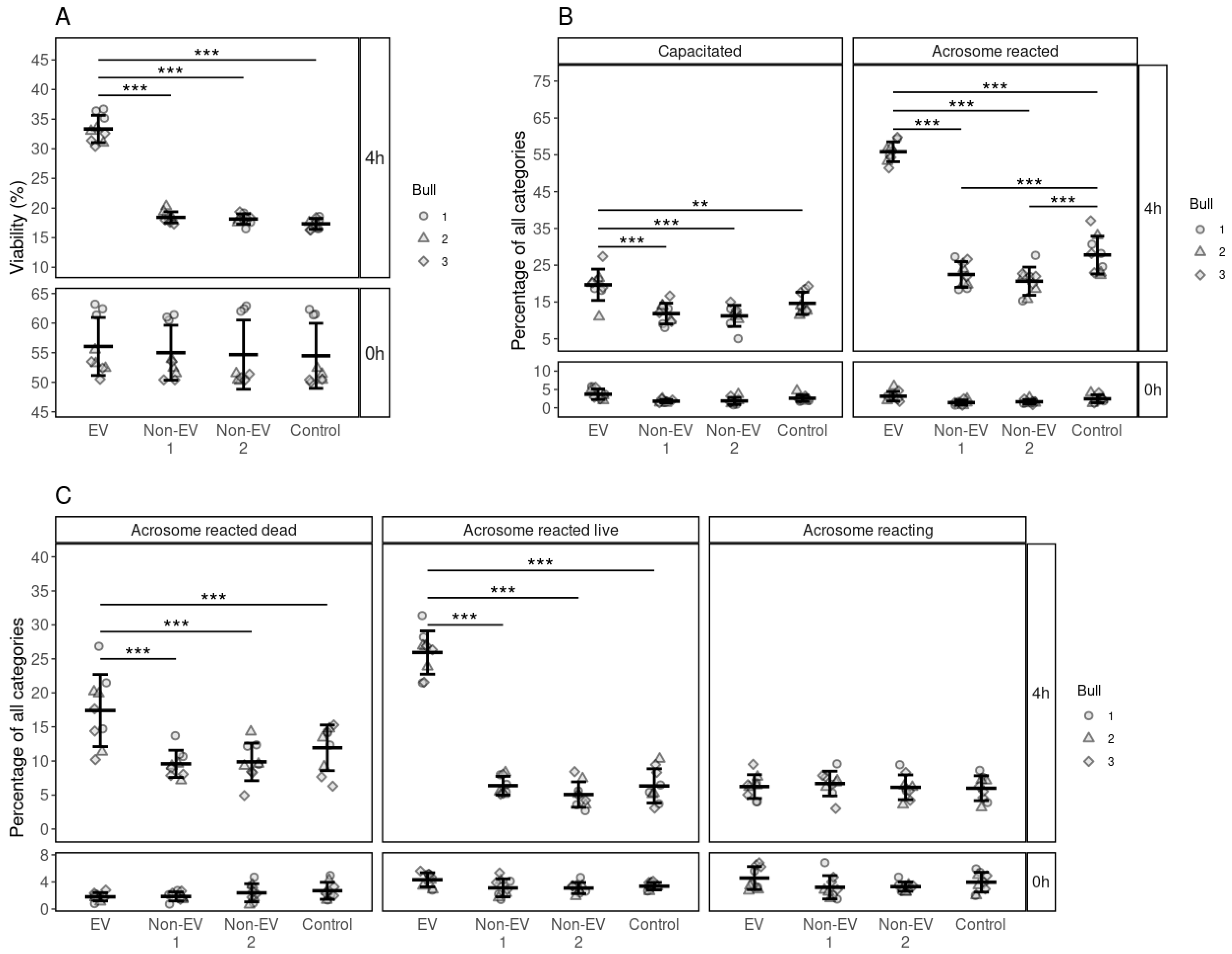
3.2. Effects of FF EV and Non-EV Fractions of SEC on the Viability of Spermatozoa
3.3. Effects of FF EV and Non-EV Fractions of SEC on the Capacitation and the Acrosome Reaction
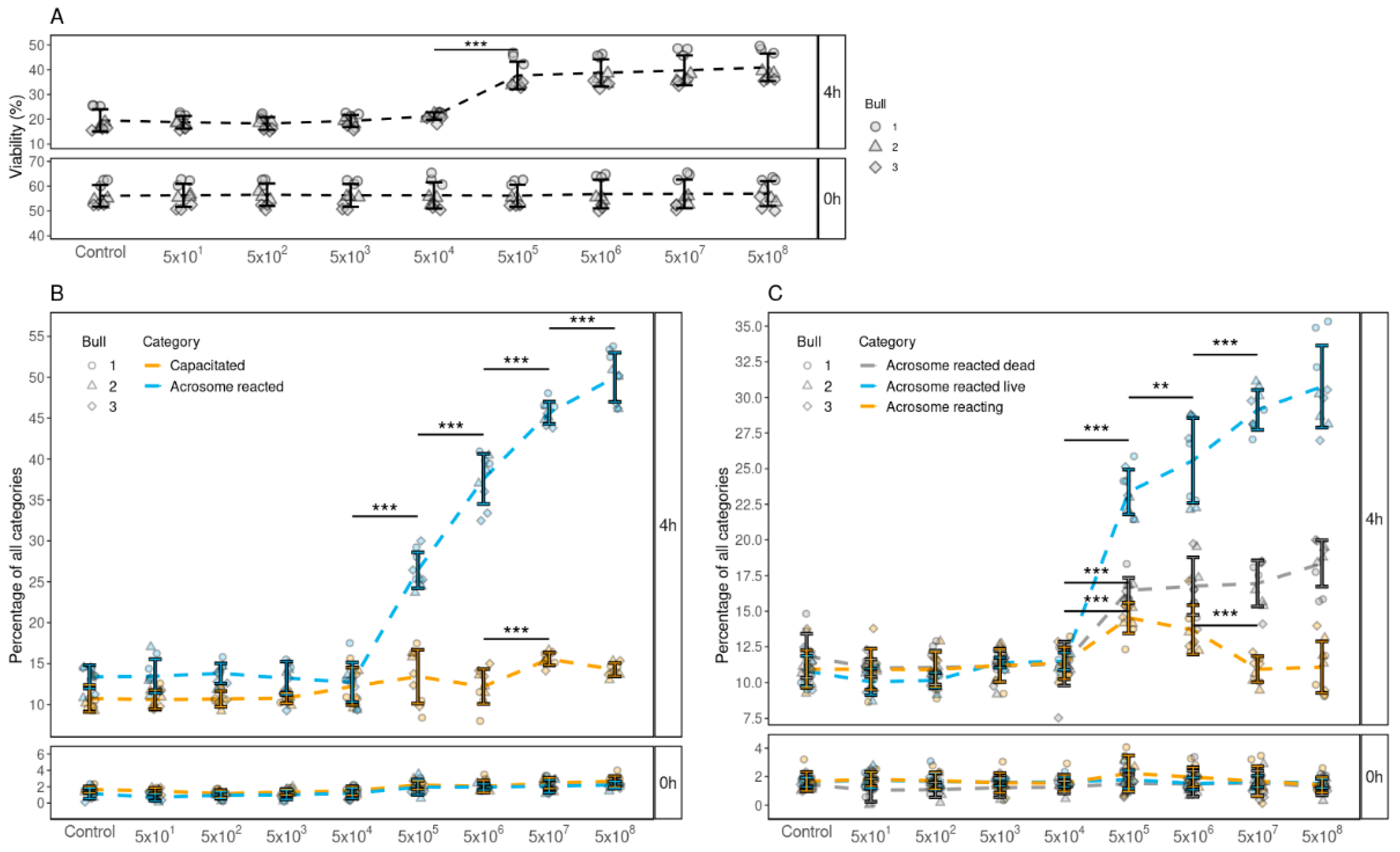
3.4. Determining the Minimum Concentration of FF EVs Required to Improve the Viability, Capacitation and Acrosomal Reaction of Spermatozoa
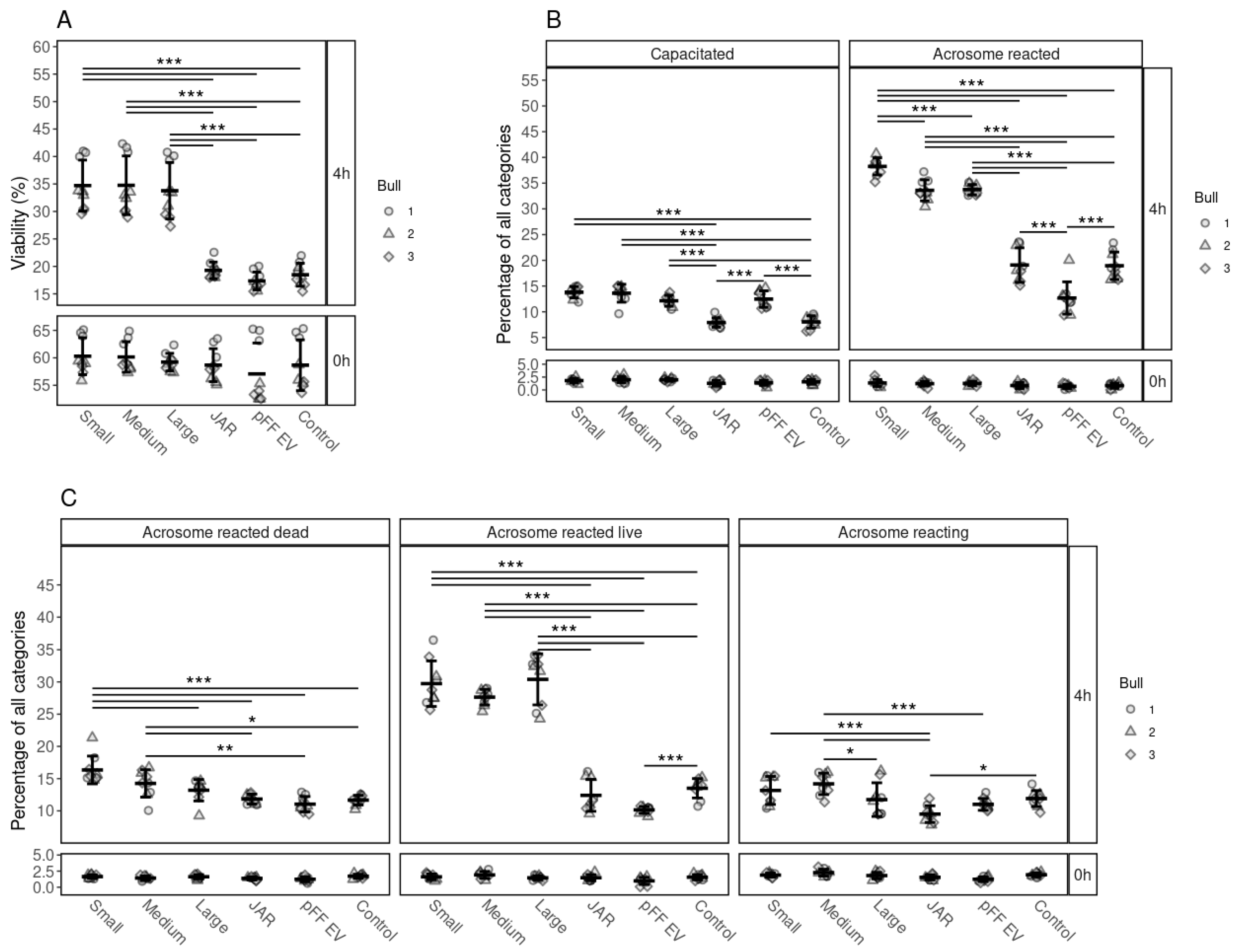
3.5. The Specificity of the Effects of FF EVs on the Viability, Capacitation and Acrosomal Reaction of Spermatozoa
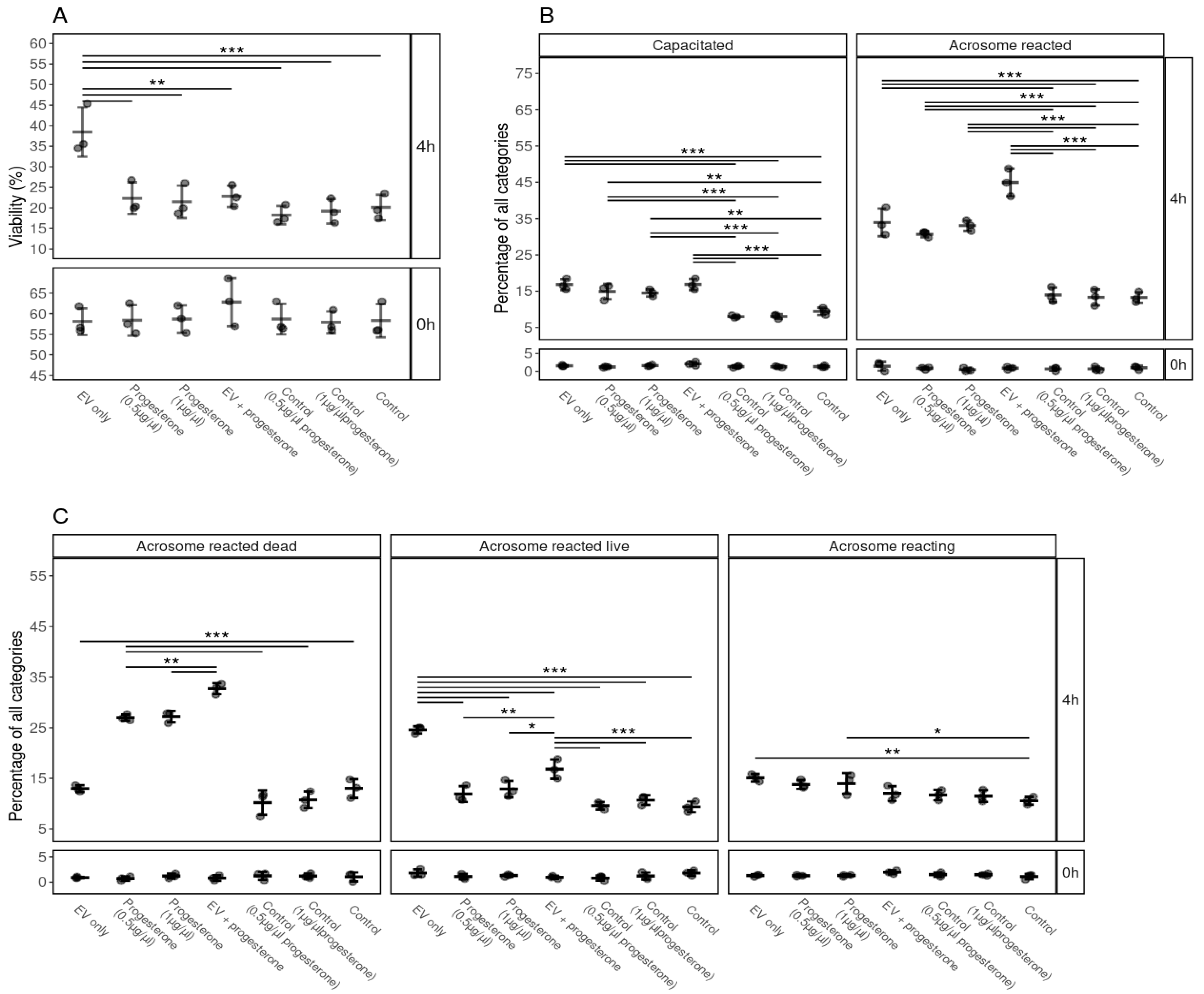
3.6. The Effect of Surface-Modified FF EVs on the Viability, Capacitation and Acrosomal Reaction of Spermatozoa
3.7. The Efficiency and Synergistic Effects of Progesterone and FF EVs on Sperm Viability, Capacitation and Acrosome Reaction
3.8. The Concentration of Progesterone in FF and FF EVs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yao, Y.; Ho, P.; Yeung, W.S. Effects of human follicular fluid on the capacitation and motility of human spermatozoa. Fertil. Steril. 2000, 73, 680–686. [Google Scholar] [CrossRef]
- Revelli, A.; Piane, L.D.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 2009, 7, 7–40. [Google Scholar] [CrossRef]
- Basuino, L.; Silveira, C.F. Human follicular fluid and effects on reproduction. JBRA Assist. Reprod. 2016, 20, 38–40. [Google Scholar] [CrossRef]
- Kodithuwakku, S.; Miyamoto, A.; Wijayagunawardane, M.P.B. Spermatozoa stimulate prostaglandin synthesis and secretion in bovine oviductal epithelial cells. Reprod. Camb. Engl. 2007, 133, 1087–1094. [Google Scholar] [CrossRef]
- Fazeli, A.; Affara, N.A.; Hubank, M.; Holt, W.V. Sperm-Induced Modification of the Oviductal Gene Expression Profile after Natural Insemination in Mice1. Biol. Reprod. 2004, 71, 60–65. [Google Scholar] [CrossRef] [PubMed]
- López-Úbeda, R.; Vazquez, F.G.; Romar, R.; Gadea, J.; Muñoz, M.; Hunter, R.H.F.; Coy, P. Oviductal Transcriptome Is Modified after Insemination during Spontaneous Ovulation in the Sow. PLoS ONE 2015, 10, e0130128. [Google Scholar] [CrossRef]
- Aitken, R.J.; Nixon, B. Sperm capacitation: A distant landscape glimpsed but unexplored. Mol. Hum. Reprod. 2013, 19, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, A.; Holt, W.V. Cross talk during the periconception period. Theriogenology 2016, 86, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K.; Rajesh, P.B. Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod. Biol. Endocrinol. 2004, 2, 75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gervasi, M.G.; Visconti, P.E. Chang’s meaning of capacitation: A molecular perspective. Mol. Reprod. Dev. 2016, 83, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Krapf, D.; de la Vega-Beltrán, J.L.; Acevedo, J.J.; Darszon, A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J. Androl. 2011, 13, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Vigil, P.; Orellana, R.F.; Cortés, M.E. Modulation of spermatozoon acrosome reaction. Biol. Res. 2011, 44, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.R.; Bishop, M.W. Role of the rodent acrosome and perforatorium in fertilization. Proc. R. Soc. Lond. Ser. B Boil. Sci. 1958, 149, 241–248. [Google Scholar] [CrossRef]
- Franklin, L.E.; Barros, C.; Fussell, E.N. The Acrosomal Region and the Acrosome Reaction in Sperm of the Golden Hamster1. Biol. Reprod. 1970, 3, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Srikandakumar, A.; Downey, B.R. Presence of follicular fluid in the porcine oviduct and its contribution to the acrosome reaction. Mol. Reprod. Dev. 1991, 30, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Thérien, I.; Bergeron, A.; Bousquet, D.; Manjunath, P. Isolation and characterization of glycosaminoglycans from bovine follicular fluid and their effect on sperm capacitation. Mol. Reprod. Dev. 2005, 71, 97–106. [Google Scholar] [CrossRef]
- Funahashi, H.; Day, B.N. Effects of follicular fluid at fertilization in vitro on sperm penetration in pig oocytes. J. Reprod. Fertil. 1993, 99, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R. In vitro capacitation of hamster spermatozoa by follicular fluid. J. Reprod. Fertil. 1969, 18, 275–286. [Google Scholar] [CrossRef]
- Osman, R.A.; Andria, M.L.; Jones, A.D.; Meizel, S. Steroid induced exocytosis: The human sperm acrosome reaction. Biochem. Biophys. Res. Commun. 1989, 160, 828–833. [Google Scholar] [CrossRef]
- Morales, P.; Llanos, M.; Gutierrez, G.; Kohen, P.; Vigil, P.; Vantman, D. The acrosome reaction-inducing activity of individual human follicular fluid samples is highly variable and is related to the steroid content. Hum. Reprod. 1992, 7, 646–651. [Google Scholar] [CrossRef]
- Calogero, A.E.; Burrello, N.; Barone, N.; Palermo, I.; Grasso, U.; D’Agata, R. Effects of progesterone on sperm function: Mechanisms of action. Hum. Reprod. 2000, 15, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Viil, J.; Lättekivi, F.; Ord, J.; Reshi, Q.; Jääger, K.; Velthut-Meikas, A.; Andronowska, A.; Jaakma, U.; Salumets Fazeli, A. Bovine Follicular Fluid and Extracellular Vesicles Derived from Follicular Fluid Alter the Bovine Oviductal Epithelial Cells Transcriptome. Int. J. Mol. Sci. 2020, 21, 5365. [Google Scholar] [CrossRef]
- Rooda, I.; Hasan, M.M.; Roos, K.; Viil, J.; Andronowska, A.; Smolander, O.-P.; Jaakma, Ü.; Salumets, A.; Fazeli, A.; Velthut-Meikas, A. Cellular, Extracellular and Extracellular Vesicular miRNA Profiles of Pre-Ovulatory Follicles Indicate Signaling Disturbances in Polycystic Ovaries. Int. J. Mol. Sci. 2020, 21, 9550. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Probert, C.; Dottorini, T.; Speakman, A.; Hunt, S.; Nafee, T.; Fazeli, A.; Wood, S.; Brown, J.E.; James, V. Communication of prostate cancer cells with bone cells via extracellular vesicle RNA A potential mechanism of metastasis. Oncogene 2018, 38, 1751–1763. [Google Scholar] [CrossRef]
- Simeone, P.; Bologna, G.; Lanuti, P.; Pierdomenico, L.; Guagnano, M.T.; Pieragostino, D.; del Boccio, P.; Vergara, D.; Marchisio, M.; Miscia, S.; et al. Extracellular Vesicles as Signaling Mediators and Disease Biomarkers across Biological Barriers. Int. J. Mol. Sci. 2020, 21, 2514. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-T.; Hong, X.; Christenson, L.K.; McGinnis, L.K. Extracellular Vesicles from Bovine Follicular Fluid Support Cumulus Expansion. Biol. Reprod. 2015, 93, 117. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.D.A.M.M.; Fujihara, M.; Nagashima, J.B.; Noonan, M.J.; Inoue-Murayama, M.; Songsasen, N. Follicular extracellular vesicles enhance meiotic resumption of domestic cat vitrified oocytes. Sci. Rep. 2020, 10, 8619. [Google Scholar] [CrossRef]
- Aalberts, M.; Sostaric, E.; Wubbolts, R.; Wauben, M.; Hoen, E.N.N.; Gadella, B.M.; Stout, T.A.; Stoorvogel, W. Spermatozoa recruit prostasomes in response to capacitation induction. Biochim. Biophys. Acta 2013, 1834, 2326–2335. [Google Scholar] [CrossRef]
- Ronquist, G. Prostasomes: Their characterisation: Implications for human reproduction. Male Role Pregnancy Loss Embryo Implant. Fail. 2015, 868, 191–209. [Google Scholar] [CrossRef]
- Ferraz, M.D.A.M.M.; Carothers, A.; Dahal, R.; Noonan, M.J.; Songsasen, N. Oviductal extracellular vesicles interact with the spermatozoon’s head and mid-piece and improves its motility and fertilizing ability in the domestic cat. Sci. Rep. 2019, 9, 9484. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.D.A.M.M.; Nagashima, J.B.; Noonan, M.J.; Crosier, A.E.; Songsasen, N. Oviductal Extracellular Vesicles Improve Post-Thaw Sperm Function in Red Wolves and Cheetahs. Int. J. Mol. Sci. 2020, 21, 3733. [Google Scholar] [CrossRef]
- Reshi, Q.U.A.; Hasan, M.M.; Dissanayake, K.; Fazeli, A. Isolation of extracellular vesicles (EVs) using benchtop size exclusion chromatography (SEC) columns. In Next Generation Culture Platforms for Reliable In Vitro Models; Springer: Berlin/Heidelberg, Germany, 2021; pp. 201–206. [Google Scholar]
- Es-Haghi, M.; Godakumara, K.; Häling, A.; Lättekivi, F.; Lavrits, A.; Viil, J.; Andronowska, A.; Nafee, T.; James, V.; Jaakma, U.; et al. Specific trophoblast transcripts transferred by extracellular vesicles affect gene expression in endometrial epithelial cells and may have a role in embryo-maternal crosstalk. Cell Commun. Signal. 2019, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Reshi, Q.U.A.; Viil, J.; Ord, J.; Lättekivi, F.; Godakumara, K.; Hasan, M.M.; Nõmm, M.; Jääger, K.; Velthut-Meikas, A.; Jaakma, U.; et al. Spermatozoa induce transcriptomic alterations in bovine oviductal epithelial cells prior to initial contact. J. Cell Commun. Signal. 2020, 14, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.R.; McDermott, C.A. Ca2+-related changes in the mouse sperm capacitation state: A possible role for Ca2+-ATPase. Reproduction 1992, 96, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Kitiyanant, Y.; Chaisalee, B.; Pavasuthipaisit, K. Evaluation of the acrosome reaction and viability in buffalo spermatozoa using two staining methods: The effects of heparin and calcium ionophore A23187. Int. J. Androl. 2002, 25, 215–222. [Google Scholar] [CrossRef]
- Skliar, M.; Chernyshev, V.S.; Belnap, D.M.; Sergey, G.V.; Al-Hakami, S.M.; Stijleman, I.J.; Rachamadugu, R.; Bernard, P.S. Membrane Proteins Significantly Restrict Exosome Mobility. bioRxiv 2017, 196691. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, Aka Least-Squares Means; R Package Version; R Foundation for Statistical Computing: Vienna, Austria, 2018; Volume 1, p. 3. [Google Scholar]
- Wickham, H. Build a plot layer by layer. In Ggplot2: Elegant Graphics for Data Analysis; Wickham, H., Ed.; Spinger: New York, NY, USA, 2009; pp. 41–64. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Kekäläinen, J.; Evans, J.P. Gamete-mediated mate choice: Towards a more inclusive view of sexual selection. Proc. R. Soc. B Boil. Sci. 2018, 285, 20180836. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.J.; Christianson, H.C.; Wittrup, A.; Bourseau-Guilmain, E.; Lindqvist, E.; Svensson, L.M.; Mörgelin, M.; Belting, M. Exosome Uptake Depends on ERK1/2-Heat Shock Protein 27 Signaling and Lipid Raft-mediated Endocytosis Negatively Regulated by Caveolin-1. J. Biol. Chem. 2013, 288, 17713–17724. [Google Scholar] [CrossRef]
- Reid, A.T.; Redgrove, K.; Aitken, R.J.; Nixon, B. Cellular mechanisms regulating sperm-zona pellucida interaction. Asian J. Androl. 2010, 13, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Murdica, V.; Giacomini, E.; Alteri, A.; Bartolacci, A.; Cermisoni, G.C.; Zarovni, N.; Papaleo, E.; Montorsi, F.; Salonia, A.; Viganò, P.; et al. Seminal plasma of men with severe asthenozoospermia contain exosomes that affect spermatozoa motility and capacitation. Fertil. Steril. 2019, 111, 897–908.e2. [Google Scholar] [CrossRef] [PubMed]
- Crozet, N. Acrosome reaction and fertilization. Contracept. Fertil. Sex. 1994, 1992, 22. [Google Scholar]
- Ferramosca, A.; Zara, V. Bioenergetics of Mammalian Sperm Capacitation. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Gerth, K.; Kodidela, S.; Mahon, M.; Haque, S.; Verma, N.; Kumar, S. Circulating Extracellular Vesicles Containing Xenobiotic Metabolizing CYP Enzymes and Their Potential Roles in Extrahepatic Cells Via Cell–Cell Interactions. Int. J. Mol. Sci. 2019, 20, 6178. [Google Scholar] [CrossRef]
- Margolis, L.; Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef]
- Inoue, N.; Satouh, Y.; Ikawa, M.; Okabe, M.; Yanagimachi, R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc. Natl. Acad. Sci. USA 2011, 108, 20008–20011. [Google Scholar] [CrossRef]
- Austin, C.R. Observations on the Penetration of the Sperm into the Mammalian Egg. Aust. J. Biol. Sci. 1951, 4, 581–596. [Google Scholar] [CrossRef]
- Chang, H.; Suarez, S.S. Two Distinct Ca2+ Signaling Pathways Modulate Sperm Flagellar Beating Patterns in Mice1. Biol. Reprod. 2011, 85, 296–305. [Google Scholar] [CrossRef]
- Morales, P.; Cross, N.L.; Overstreet, J.W.; Hanson, F.W. Acrosome intact and acrosome-reacted human sperm can initiate binding to the zona pellucida. Dev. Biol. 1989, 133, 385–392. [Google Scholar] [CrossRef]
- Huang, T.T.F.; Fleming, A.D.; Yanagimachi, R. Only acrosome-reacted spermatozoa can bind to and penetrate zona pellucida: A study using the guinea pig. J. Exp. Zool. 1981, 217, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Kuzan, F.B.; Fleming, A.D.; Seidel, G.E. Successful fertilization in vitro of fresh intact oocytes by perivitelline (acrosome-reacted) spermatozoa of the rabbit. Fertil. Steril. 1984, 41, 766–770. [Google Scholar] [CrossRef]
- Navakanitworakul, R.; Hung, W.-T.; Gunewardena, S.; Davis, J.S.; Chotigeat, W.; Christenson, L.K. Characterization and Small RNA Content of Extracellular Vesicles in Follicular Fluid of Developing Bovine Antral Follicles. Sci. Rep. 2016, 6, 25486. [Google Scholar] [CrossRef]
- Wirleitner, B.; Okhowat, J.; Vištejnová, L.; Králíčková, M.; Karlíková, M.; Vanderzwalmen, P.; Ectors, F.; Hradecký, L.; Schuff, M.; Murtinger, M. Relationship between follicular volume and oocyte competence, blastocyst development and live-birth rate: Optimal follicle size for oocyte retrieval. Ultrasound Obstet. Gynecol. 2018, 51, 118–125. [Google Scholar] [CrossRef]
- Nivet, A.; Léveillé, M.; Leader, A.; Sirard, M. Transcriptional characteristics of different sized follicles in relation to embryo transferability: Potential role of hepatocyte growth factor signalling. Mol. Hum. Reprod. 2016, 22, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Bravo, Z.; Valdivia, M. Follicular fluid stimulates capacitation and acrosome reaction in alpaca sperm (Vicugna pacos). Reprod. Domest. Anim. 2018, 53, 629–635. [Google Scholar] [CrossRef]
- El-Shahat, K.; Taysser, M.; Badr, M.; Zaki, K. Effect of oviduct and follicular fluids on ram sperm capacitation and acrosome reaction in vitro. Int. J. Veter Sci. Med. 2018, 6, S57–S62. [Google Scholar] [CrossRef]
- Falcone, L.; Gianni, S.; Piffaretti-Yanez, A.; Marchini, M.; Eppenberger, U.; Balerna, M. Follicular fluid enhances sperm motility and velocity in vitro. Fertil. Steril. 1991, 55, 619–623. [Google Scholar] [CrossRef]
- Jeon, B.-G.; Moon, J.-S.; Kim, K.-C.; Lee, H.-J.; Choe, S.-Y.; Rho, G.-J. Follicular fluid enhances sperm attraction and its motility in human. J. Assist. Reprod. Genet. 2001, 18, 407–412. [Google Scholar] [CrossRef]
- Schuffner, A.A.; Bastiaan, H.S.; Duran, H.E.; Lin, Z.-Y.; Morshedi, M.; Franken, D.R.; Oehninger, S. Zona pellucida-induced acrosome reaction in human sperm: Dependency on activation of pertussis toxin-sensitive Gi protein and extracellular calcium, and priming effect of progesterone and follicular fluid. Mol. Hum. Reprod. 2002, 8, 722–727. [Google Scholar] [CrossRef]
- Blumenfeld, Z.; Nahhas, F. Pretreatment of sperm with human follicular fluid for borderline male infertility. Fertil. Steril. 1989, 51, 863–868. [Google Scholar] [CrossRef]
- Ghetler, Y.; Ben-Nun, I.; Kaneti, H.; Jaffe, R.; Gruber, A.; Fejgin, M. Effect of sperm preincubation with follicular fluid on the fertilization rate in human in vitro fertilization. Fertil. Steril. 1990, 54, 944–946. [Google Scholar] [CrossRef]
| Sample Type | Concentration (nmol/L) |
|---|---|
| Follicular fluid (small) | 9.63 nmol/L |
| Follicular fluid (medium) | 6.64 nmol/L |
| Follicular fluid (large) | 7.64 nmol/L |
| Follicular fluid EVs (small) | <0.67 nmol/L |
| Follicular fluid EVs (medium) | <0.67 nmol/L |
| Follicular fluid EVs (large) | <0.67 nmol/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.M.; Reshi, Q.U.A.; Lättekivi, F.; Viil, J.; Godakumara, K.; Dissanayake, K.; Andronowska, A.; Jaakma, Ü.; Fazeli, A. Bovine Follicular Fluid Derived Extracellular Vesicles Modulate the Viability, Capacitation and Acrosome Reaction of Bull Spermatozoa. Biology 2021, 10, 1154. https://doi.org/10.3390/biology10111154
Hasan MM, Reshi QUA, Lättekivi F, Viil J, Godakumara K, Dissanayake K, Andronowska A, Jaakma Ü, Fazeli A. Bovine Follicular Fluid Derived Extracellular Vesicles Modulate the Viability, Capacitation and Acrosome Reaction of Bull Spermatozoa. Biology. 2021; 10(11):1154. https://doi.org/10.3390/biology10111154
Chicago/Turabian StyleHasan, Mohammad Mehedi, Qurat Ul Ain Reshi, Freddy Lättekivi, Janeli Viil, Kasun Godakumara, Keerthie Dissanayake, Aneta Andronowska, Ülle Jaakma, and Alireza Fazeli. 2021. "Bovine Follicular Fluid Derived Extracellular Vesicles Modulate the Viability, Capacitation and Acrosome Reaction of Bull Spermatozoa" Biology 10, no. 11: 1154. https://doi.org/10.3390/biology10111154
APA StyleHasan, M. M., Reshi, Q. U. A., Lättekivi, F., Viil, J., Godakumara, K., Dissanayake, K., Andronowska, A., Jaakma, Ü., & Fazeli, A. (2021). Bovine Follicular Fluid Derived Extracellular Vesicles Modulate the Viability, Capacitation and Acrosome Reaction of Bull Spermatozoa. Biology, 10(11), 1154. https://doi.org/10.3390/biology10111154








