Toluene Can Disrupt Rat Ovarian Follicullogenesis and Steroidogenesis and Induce Both Autophagy and Apoptosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Treatment and Sampling
2.2. Histology
2.3. Hormones Analysis
2.4. TUNEL Assay
2.5. Immunostaining
2.6. Analysis of Gene Expression
2.7. Statistical Analysis
3. Results
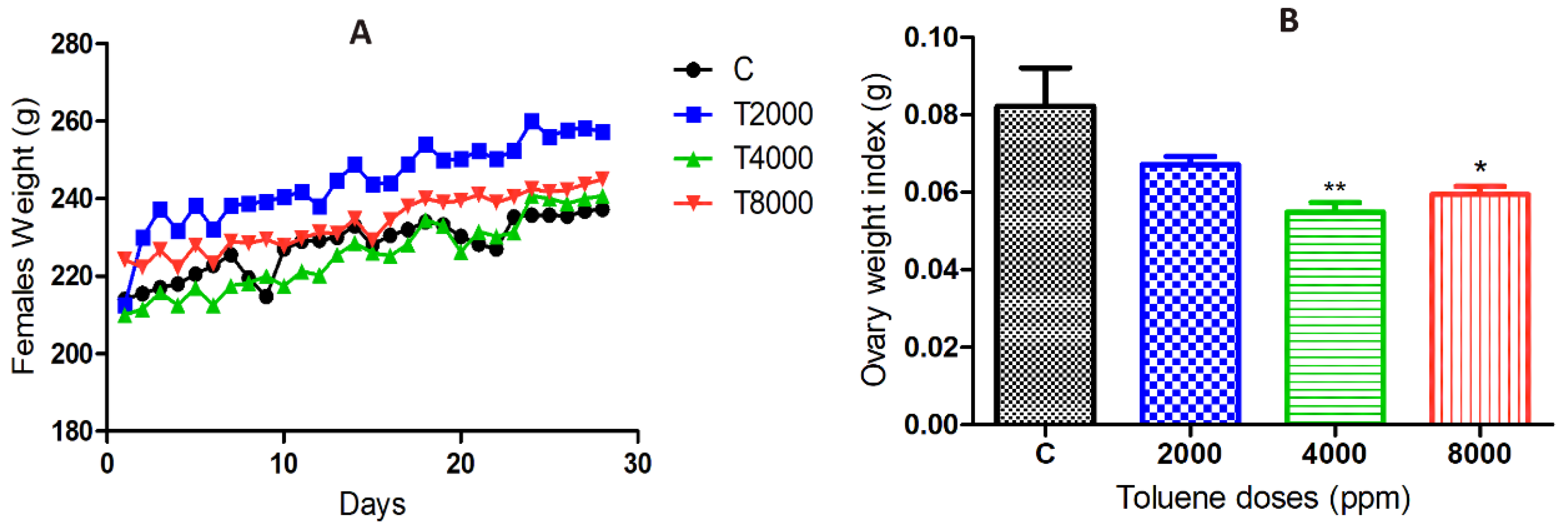
3.1. Effect of Toluene on Body Weight
3.2. Effect of Toluene on Ovary Weight
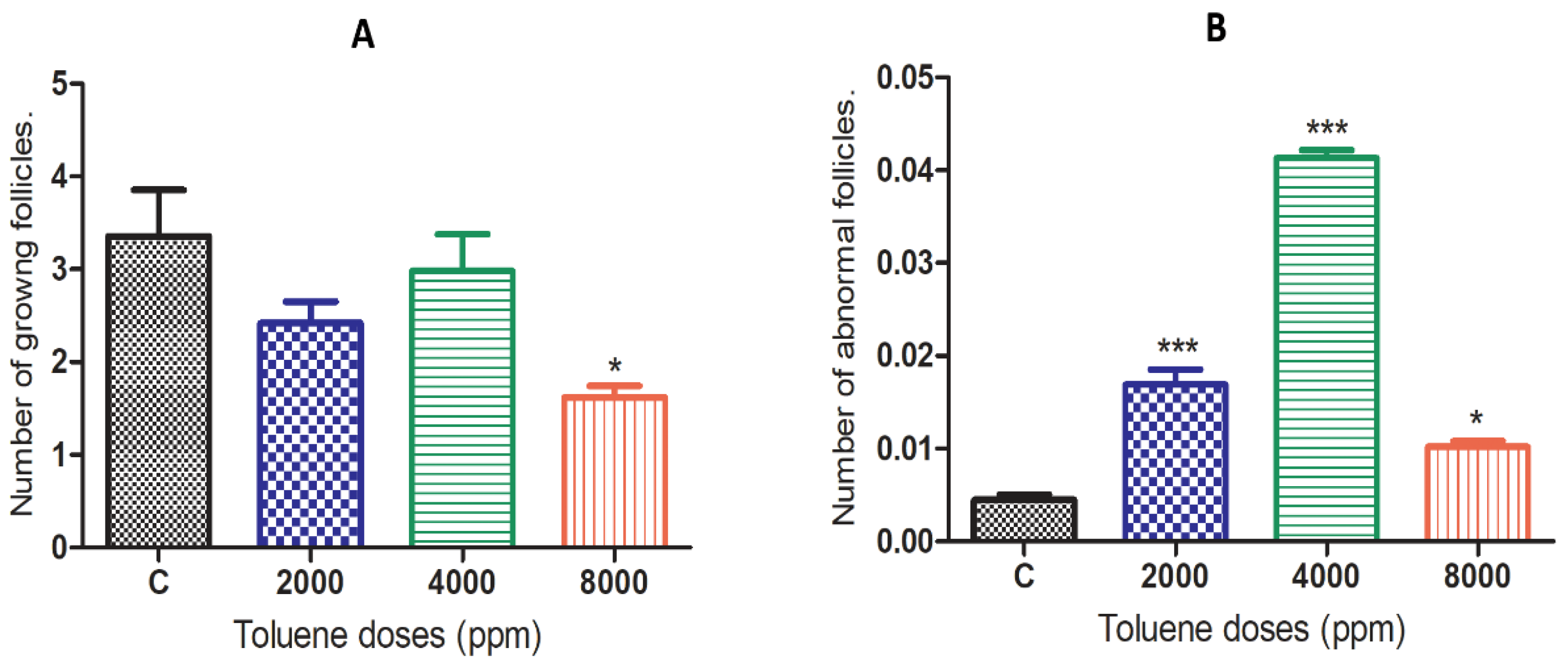
3.3. Effect of Toluene on the Number of Follicles
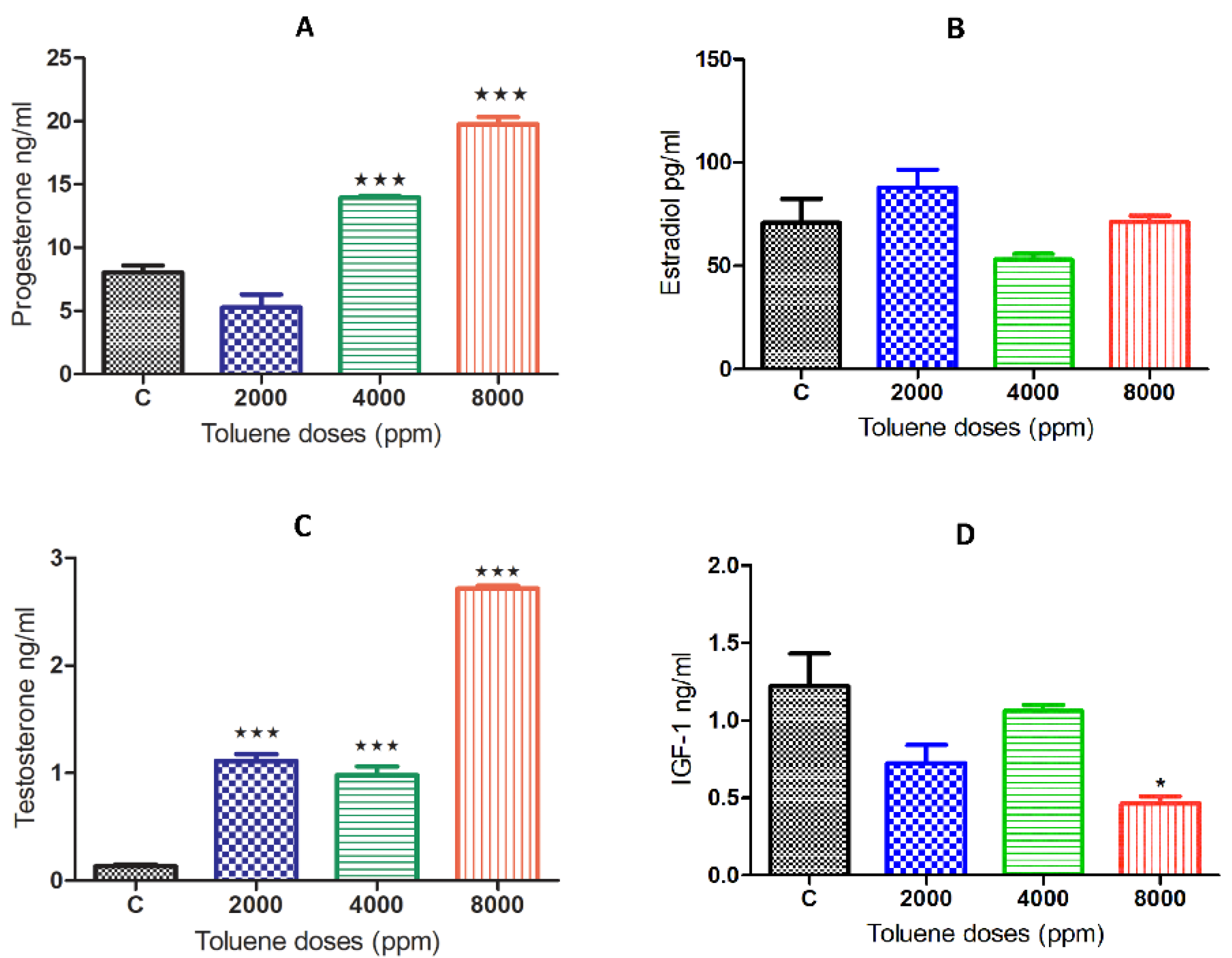
3.4. Effect of Toluene on Steroid Hormone Levels
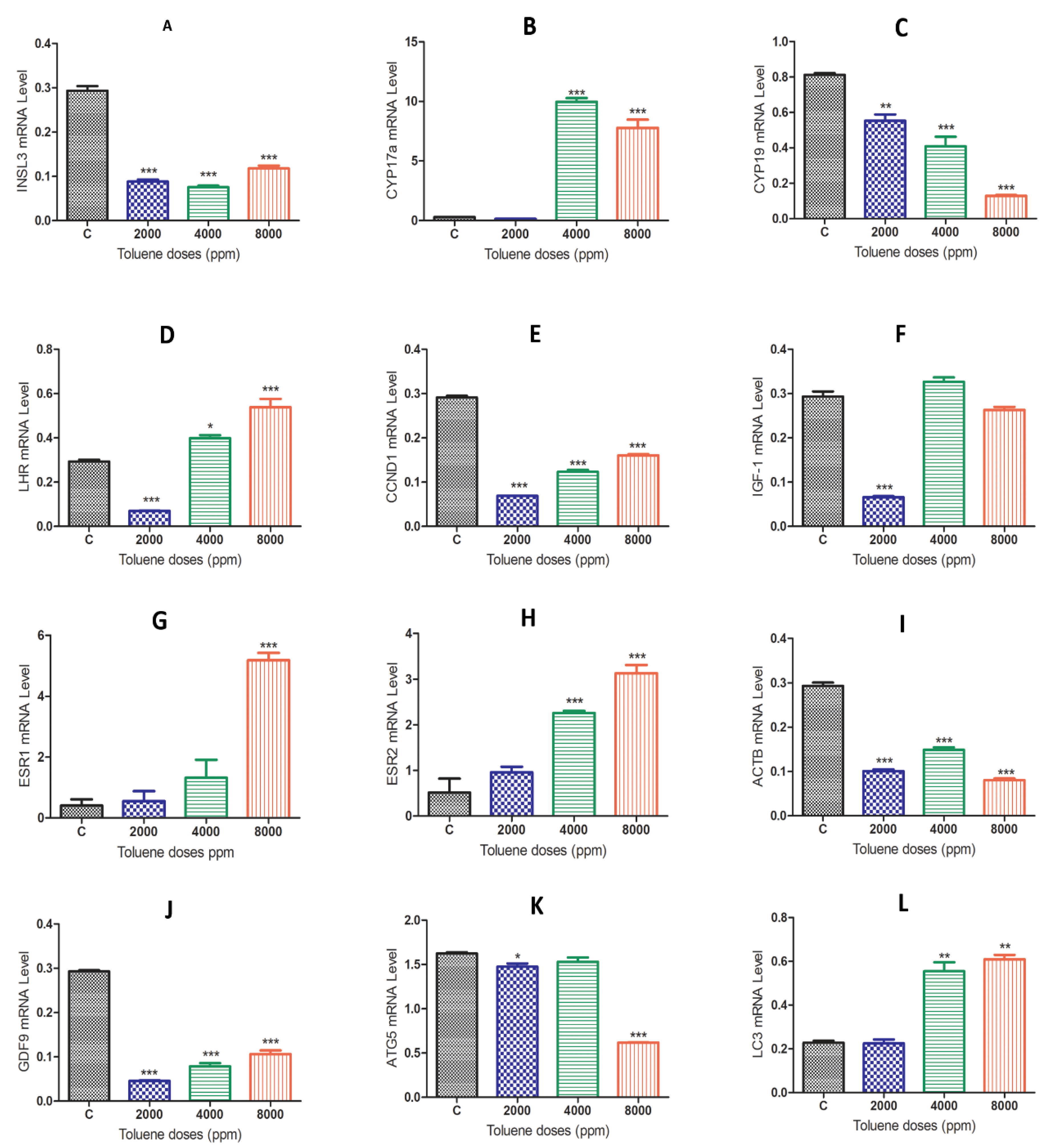
3.5. Effect of Toluene on Gene Expression Levels
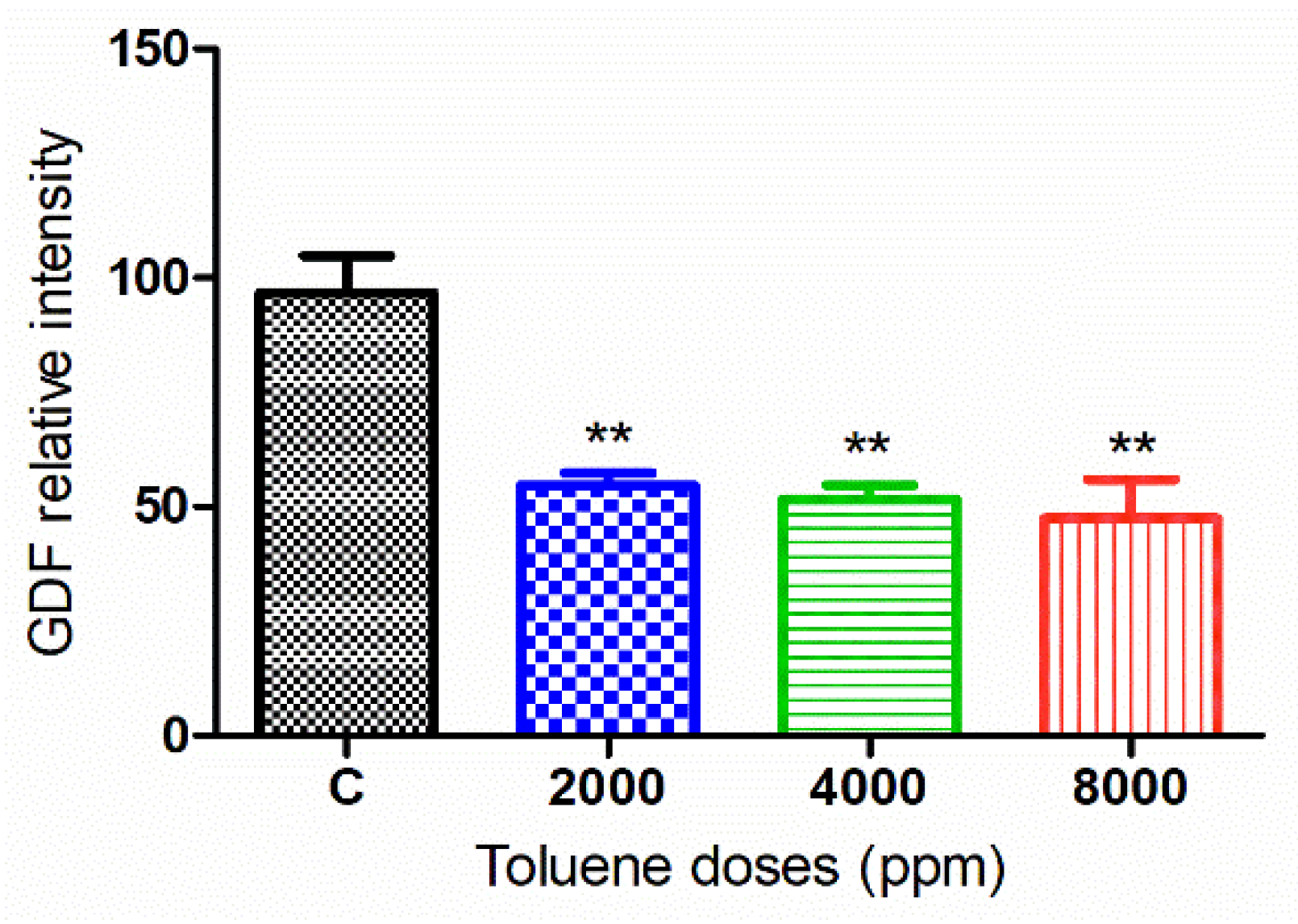
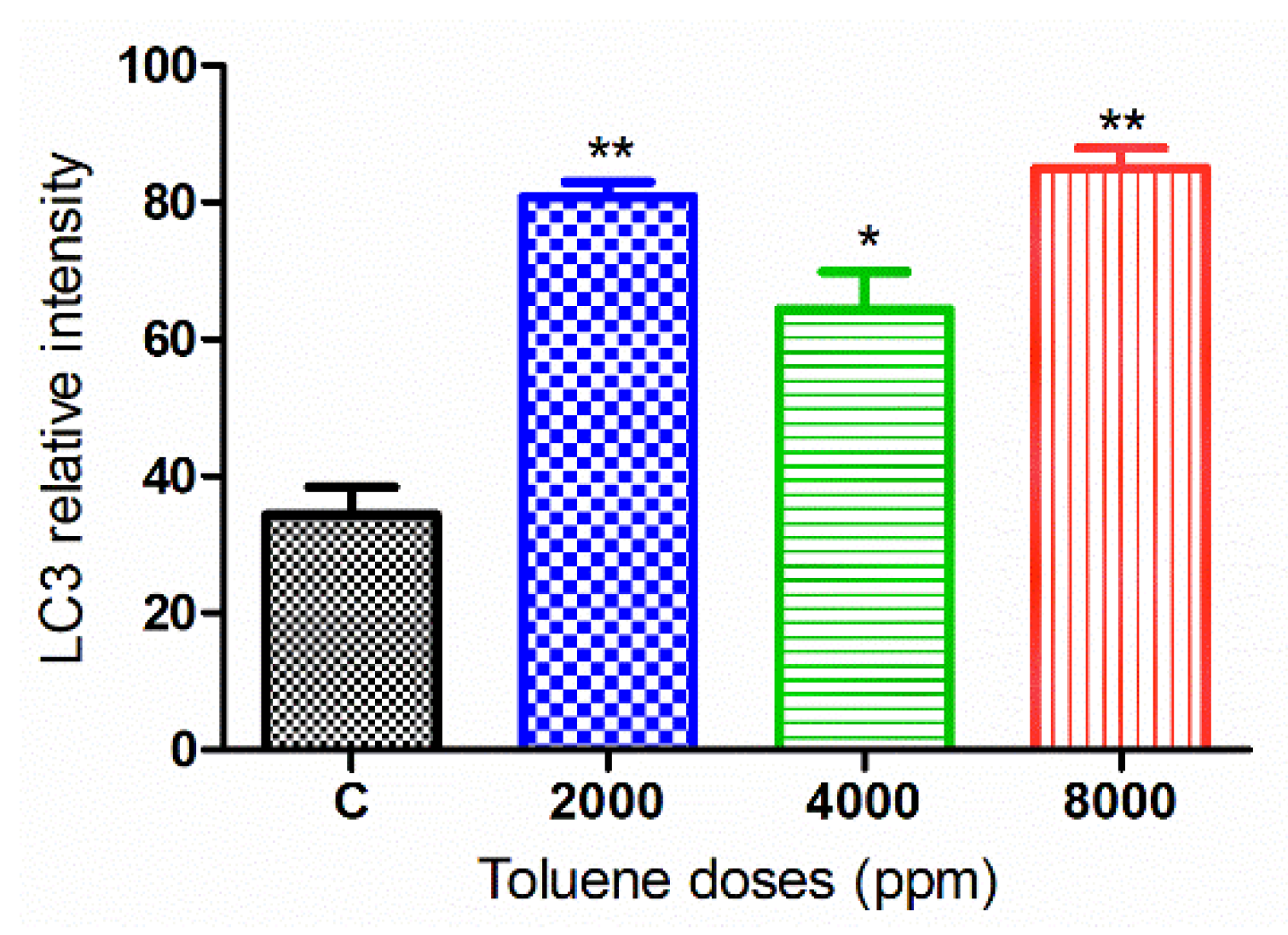
3.6. Effect of Toluene on LC3 and GDF9 Protein Expression
3.6.1. GDF9 Protein
3.6.2. LC3 Protein
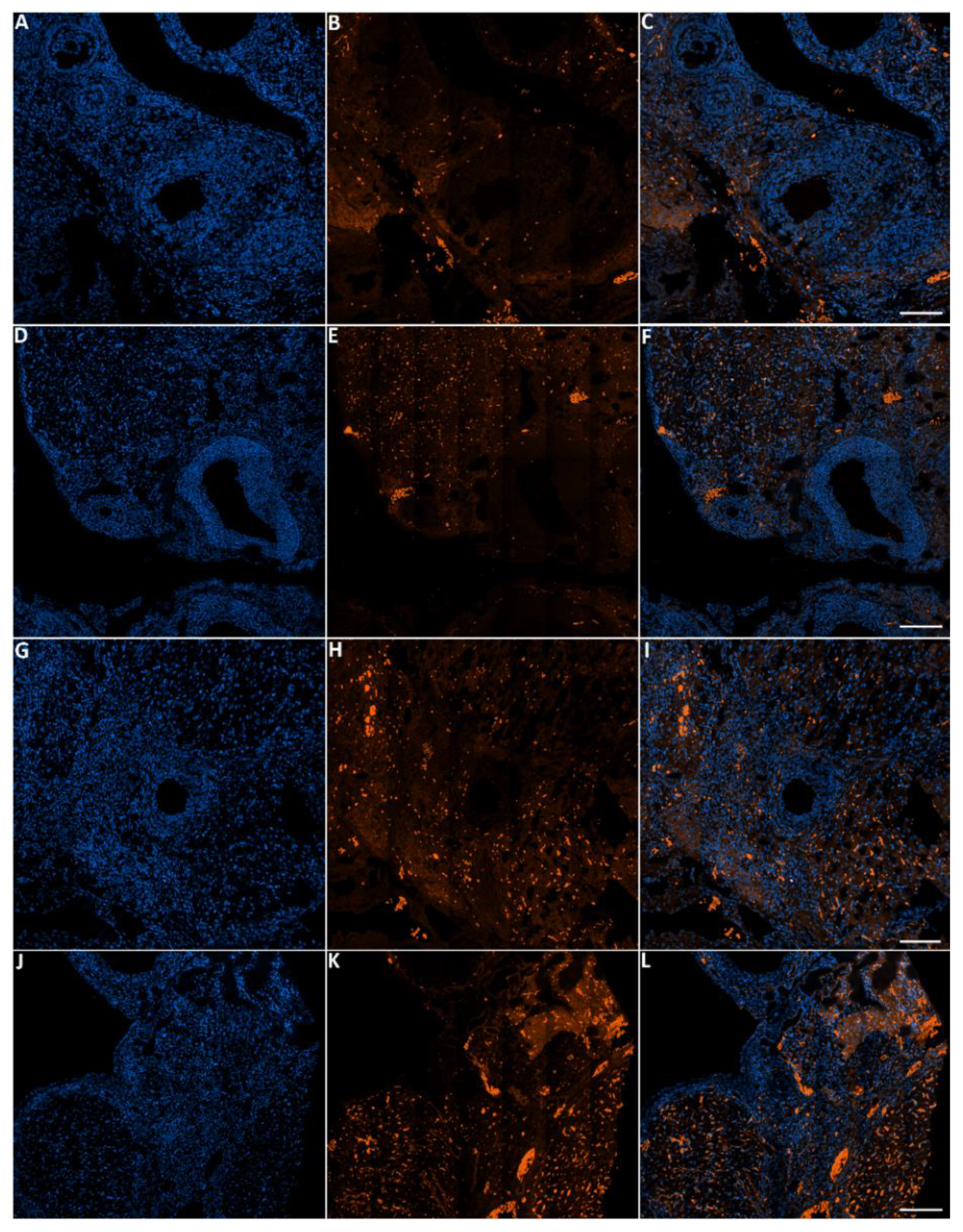
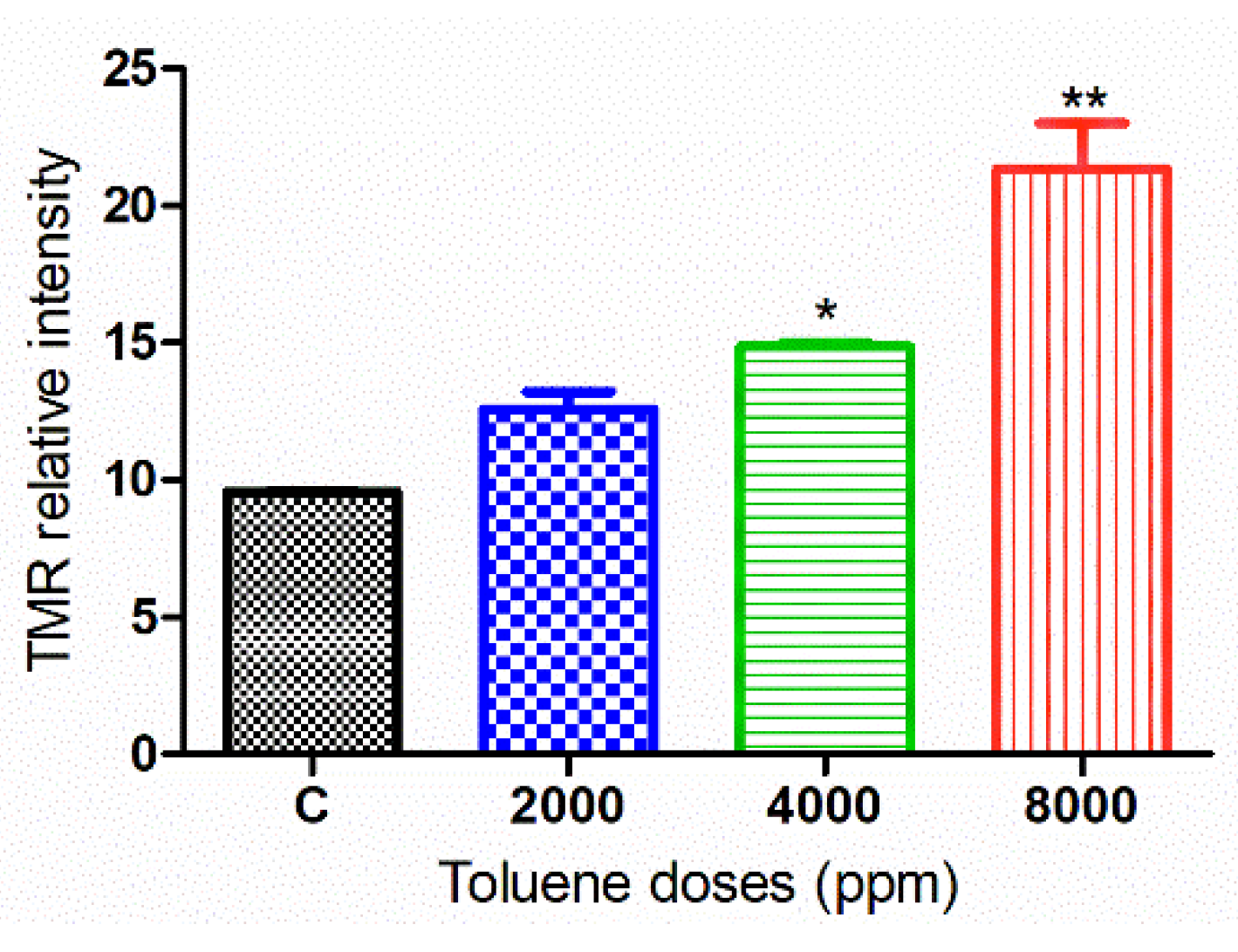
3.7. DNA Damage in Cells Treated with Toluene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Actb | Actin Beta |
| Atg5 | Autophagy related 5 |
| BSA | Bovine serum albumin |
| cDNA | Complementary DNA |
| Ccnd1 | Cyclin D1 |
| Ccnd2 | Cyclin D2 |
| Cdk | Cyclin-dependent kinases |
| Cyp17a | Cytochrome P450 family 17 subfamily A |
| Cyp19 | Cytochrome P450 family 19 |
| Esr1 | Estrogen receptor 1 |
| Esr2 | Estrogen receptor 2 |
| FITC | fluorescein isothiocyanate |
| Gdf-9 | Growth differentiation factor 9 |
| Igf-1 | Insulin-like growth factor-1 |
| Insl3 | Insulin-Like Peptide 3 |
| Lhr | Luteinizing hormone receptor |
| LC3 | Microtubule-associated protein 1A/1B-light chain 3 |
| NBF | Neutral buffered formalin |
| PBS | Phosphate-buffered saline |
| RT-PCR | Real-time PCR |
| TUNEL | Terminal deoxynucleotidyl transferase mediated nick end-labeling |
| TdT | Terminal deoxynucleotidyl transferase |
References
- Zhang, J.; An, J.; Qu, Y.; Liu, X.; Chen, Y. Impacts of potential HONO sources on the concentrations of oxidants and secondary organic aerosols in the Beijing-Tianjin-Hebei region of China. Sci. Total. Environ. 2019, 647, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Zeng, L.; Guo, H.; Simpson, I.; Ling, Z.; Wang, Y.; Murray, F.; Louie, P.; Saunders, S.; Lam, S.; et al. Evaluation of the effectiveness of air pollution control measures in Hong Kong. Environ. Pollut. 2017, 220, 87–94. [Google Scholar] [CrossRef]
- Veerakumar, U.; Al Shaali, S.J.; Abbas, A. Subduing Harmful Exposure of BTEX by Improving Facility and Practices. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 8 November 2016. [Google Scholar]
- Chauhan, S.K.; Saini, N.; Yadav, V.B. Recent trends of volatile organic compounds in ambient air and its health impacts: A review. Int. J. Technol. Res. Eng. 2014, 1, 667. [Google Scholar]
- Administration OSaH, Toluene. Available online: https://www.osha.gov/SLTC/toluene/exposure_limits.html (accessed on 14 November 2013).
- Lubman, D.I.; Yücel, M.; Lawrence, A.J. Inhalant abuse among adolescents: Neurobiological considerations. Br. J. Pharmacol. 2008, 154, 316–326. [Google Scholar] [CrossRef]
- Duncan, J.R.; Dick, A.L.W.; Egan, G.; Kolbe, S.; Gavrilescu, M.; Wright, D.; Lubman, D.I.; Lawrence, A.J. Adolescent toluene inhalation in rats affects white matter maturation with the potential for recovery following abstinence. PLoS ONE 2012, e44790. [Google Scholar] [CrossRef] [PubMed]
- Bingham, E.; Cohrssen, B. Patty’s Toxicology, 6 Volume Set; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Ballantyne, B. Perspectives in Basic and Applied Toxicology, 1987 ed.; Butterworth & Co.: London, UK, 1988. [Google Scholar]
- Ayan, M.; Tas, U.; Sogut, E.; Kuloglu, T.; Cayli, S.; Kocaman, N.; Karaca, Z.I.; Sahin, M. The apoptotic effect of a high dose of toluene on liver tissue during the acute phase: An experimental study. Toxicol. Ind. Health 2013, 29, 728–736. [Google Scholar] [CrossRef]
- Tas, U.; Ogeturk, M.; Kuloglu, T.; Sapmaz, H.I.; Kocaman, N.; Zararsiz, I.; Sarsilmaz, M. HSP70 immune reactivity and TUNEL positivity in the liver of toluene-inhaled and melatonin-treated rats. Toxicol. Ind. Health 2013, 29, 514–522. [Google Scholar] [CrossRef]
- Filley, C.M.; Halliday, W.; Kleinschmidt-DeMasters, B.K. The Effects of Toluene on the Central Nervous System. J. Neuropathol. Exp. Neurol. 2004, 63, 1–12. [Google Scholar] [CrossRef]
- Soares, M.V.; Charão, M.F.; Jacques, M.T.; dos Santos, A.L.A.; Luchese, C.; Pinton, S.; Ávila, D.S. Airborne toluene exposure causes germline apoptosis and neuronal damage that promotes neurobehavioural changes in Caenorhabditis elegans. Environ. Pollut. 2020, 256, 113406. [Google Scholar] [CrossRef]
- Balster, R.L. Neural basis of inhalant abuse. Drug Alcohol Depend. 1998, 51, 207–214. [Google Scholar] [CrossRef]
- Berenguer, P.; Soulage, C.; Perrin, D.; Pequignot, J.-M.; Abraini, J.H. Behavioral and neurochemical effects induced by subchronic exposure to 40 ppm toluene in rats. Pharmacol. Biochem. Behav. 2003, 74, 997–1003. [Google Scholar] [CrossRef]
- Kondo, H.; Huang, J.; Ichihara, G.; Kamijima, M.; Saito, I.; Shibata, E.; Ono, Y.; Hisanaga, N.; Takeuchi, Y.; Nakahara, D. Toluene induces behavioral activation without affecting striatal dopamine metabolism in the rat: Behavioral and microdialysis studies. Pharmacol. Biochem. Behav. 1995, 51, 97–101. [Google Scholar] [CrossRef]
- Riegel, A.C.; French, E.D. An Electrophysiological Analysis of Rat Ventral Tegmental Dopamine Neuronal Activity During Acute Toluene Exposure. Pharmacol. Toxicol. 1999, 85, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-S.; Yang, M.; Song, M.-S.; Kim, J.-S.; Kim, S.-H.; Kim, J.-C.; Kim, H.; Shin, T.; Wang, H.; Moon, C. Toluene inhibits hippocampal neurogenesis in adult mice. Pharmacol. Biochem. Behav. 2010, 94, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Von Euler, G.; Fuxe, K.; Hansson, T.; Ögren, S.O.; Agnati, L.F.; Eneroth, P.; Härfstrand, A.; Gustafsson, J.Å. Effects of chronic toluene exposure of central monoamine and peptide receptors and their interactions in the adult male rat. Toxicology 1988, 52, 103–126. [Google Scholar] [CrossRef]
- Eisenberg, D.P. Neurotoxicity and mechanism of toluene abuse. Einstein QJ Biol. Med. 2003, 19, 150–159. [Google Scholar]
- Cruz, S.L.; Soberanes-Chávez, P.; Páez-Martinez, N.; López-Rubalcava, C. Toluene has antidepressant-like actions in two animal models used for the screening of antidepressant drugs. Psychopharmacology 2009, 204, 279–286. [Google Scholar] [CrossRef]
- Cruz, S.L.; Rivera-García, M.T.; Woodward, J.J. Review of Toluene Actions: Clinical Evidence, Animal Studies, and Molecular Targets. J. Drug Alcohol Res. 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Kodavanti, P.R.S.; Royland, J.E.; Richards, J.E.; Besas, J.; MacPhail, R.C. Toluene effects on oxidative stress in brain regions of young-adult, middle-age, and senescent Brown Norway rats. Toxicol. Appl. Pharmacol. 2011, 256, 386–398. [Google Scholar] [CrossRef]
- Zhvania, M.G.; Chilachava, L.R.; Japaridze, N.J.; Gelazonia, L.K.; Lordkipanidze, T. Immediate and persisting effect of toluene chronic exposure on hippocampal cell loss in adolescent and adult rats. Brain Res. Bull. 2012, 87, 187–192. [Google Scholar] [CrossRef]
- Huerta-Rivas, A.; López-Rubalcava, C.; Sánchez-Serrano, S.L.; Valdez-Tapia, M.; Lamas, M.; Cruz, S.L. Toluene impairs learning and memory, has antinociceptive effects, and modifies histone acetylation in the dentate gyrus of adolescent and adult rats. Pharmacol. Biochem. Behav. 2012, 102, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Revilla, A.S.; Pestana, C.; Pardo-Andreu, G.L.; Santos, A.C.; Uyemura, S.A.; Gonzales, M.E.; Curti, C. Potential toxicity of toluene and xylene evoked by mitochondrial uncoupling. Toxicol. Vitr. 2007, 21, 782–788. [Google Scholar] [CrossRef]
- Wang, D.; Horike, T.; Mizuuchi, H.; Ishii, K.; Zhen, L.; Taketa, K. Liver Function Tests of Workers Exposed to Toluene and Toluene/Dimethylformamide at Low Concentrations. J. Occup. Health 1996, 38, 113–117. [Google Scholar] [CrossRef]
- Pyykkö, K. Time-course of effects of toluene on microsomal enzymes in rat liver, kidney and lung during and after inhalation exposure. Chem. Interact. 1983, 44, 299–310. [Google Scholar] [CrossRef]
- Ullah, E.; Arredondo, D.; Moreno, A.; Campanas, M.; Lopez, F.; Kay, G.; Nguyen, A.; Rosell, R. Increased Toluene Exposure and Toxicity Effects Results in Low Fly Fecundity and Offspring Development in Female Drosophila melanogaster. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Ungváry, G.; Tátrai, E. On the Embryotoxic Effects of Benzene and Its Alkyl Derivatives in Mice, Rats and Rabbits. Arch. Toxicol. 1985, 8, 425–430. [Google Scholar] [CrossRef]
- Webb, E.; Bushkin-Bedient, S.; Cheng, A.; Kassotis, C.; Balise, V.; Nagel, S.C. Developmental and reproductive effects of chemicals associated with unconventional oil and natural gas operations. Rev. Environ. Health 2014, 29, 307–318. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Harrath, A.H. Influence of oil-related environmental pollutants on female reproduction. Reprod. Toxicol. 2017, 71, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Kuczkowski, K.M. The effects of drug abuse on pregnancy. Curr. Opin. Obstet. Gynecol. 2007, 19, 578–585. [Google Scholar] [CrossRef]
- Hannigan, J.H.; Bowen, S.E. Reproductive Toxicology and Teratology of Abused Toluene. Syst. Biol. Reprod. Med. 2010, 56, 184–200. [Google Scholar] [CrossRef]
- Waldner, C.L. The Association Between Exposure to the Oil and Gas Industry and Beef Calf Mortality in Western Canada. Arch. Environ. Occup. Health 2008, 63, 220–240. [Google Scholar] [CrossRef] [PubMed]
- Bowen, S.E.; Batis, J.C.; Paez-Martinez, N.; Cruz, S.L. The last decade of solvent research in animal models of abuse: Mechanistic and behavioral studies. Neurotoxicol. Teratol. 2006, 28, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, P.A.; Fata, E.; Bowen, S.E.; Jen, K.-L.C.; Coscina, D.V. Effects of abuse pattern of gestational toluene exposure on metabolism, feeding and body composition. Physiol. Behav. 2008, 93, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Tarko, A.; Fabová, Z.; Kotwica, J.; Valocký, I.; Alrezaki, A.; Alwasel, S.; Harrath, A.; Sirotkin, A. The inhibitory influence of toluene on mare ovarian granulosa cells can be prevented by fennel. Gen. Comp. Endocrinol. 2020, 295, 113491. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Kadasi, A.; Baláži, A.; Kotwica, J.; Alrezaki, A.; Harrath, A.H. Mechanisms of the direct effects of oil-related contaminants on ovarian cells. Environ. Sci. Pollut. Res. 2020, 27, 5314–5322. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Tarko, A.; Kotwica, J.; Alrezaki, A.; Harrath, A.H. Interrelationships between metabolic hormones, leptin and ghrelin, and oil-related contaminants in control of oxytocin and prostaglandin F release by feline ovaries. Reprod. Biol. 2020, 20, 254–258. [Google Scholar] [CrossRef]
- Harrath, A.H.; Alrezaki, A.; Mansour, L.; Alwasel, S.H.; Palomba, S. Food restriction during pregnancy and female offspring fertility: Adverse effects of reprogrammed reproductive lifespan. J. Ovarian Res. 2017, 10, 77. [Google Scholar] [CrossRef]
- Painter, R.; Osmond, C.; Gluckman, P.; Hanson, M.; Phillips, D.; Roseboom, T. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1243–1249. [Google Scholar] [CrossRef]
- Susiarjo, M.; Hassold, T.J.; Freeman, E.; Hunt, P.A. Bisphenol a Exposure In Utero Disrupts Early Oogenesis in the Mouse. PLoS Genet. 2007, 3, e5. [Google Scholar] [CrossRef]
- Sirotkin, A.; Záhoranska, Z.; Tarko, A.; Fabova, Z.; Alwasel, S.; Harrath, A.H. Plant polyphenols can directly affect ovarian cell functions and modify toluene effects. J. Anim. Physiol. Anim. Nutr. 2021, 105, 80–89. [Google Scholar] [CrossRef]
- Roberts, L.; Nicolich, M.; Schreiner, C. Developmental and reproductive toxicity evaluation of toluene vapor in the rat: II. Developmental toxicity. Reprod. Toxicol. 2007, 23, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Callan, S.; Kott, J.; Cleary, J.; McCarthy, M.K.; Baltes, B.B.; Bowen, S.E. Changes in developmental body weight as a function of toluene exposure: A meta-analysis of animal studies. Hum. Exp. Toxicol. 2016, 35, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Kareem, L.A.; Banna, H.B.; Naoom, K.M. Effects of toluene and formaldehyde on oogenesis in adult female mice. Diyala J. Med. 2014, 6, 33–40. [Google Scholar]
- Chen, H.; Song, L.; Wang, X.; Wang, S. Effect of exposure to low concentration of benzene and its analogues on luteal function of female workers. J. Hyg. Res. 2000, 29, 351–353. [Google Scholar]
- Murray, A.A.; Gosden, R.G.; Allison, V.; Spears, N. Effect of androgens on the development of mouse follicles growing in vitro. J Reprod. Fertil. 1998, 113, 27–33. [Google Scholar] [CrossRef]
- Yang, J.-L.; Zhang, C.-P.; Li, L.; Huang, L.; Ji, S.-Y.; Lu, C.-L.; Fan, C.-H.; Cai, H.; Ren, Y.; Hu, Z.-Y.; et al. Testosterone Induces Redistribution of Forkhead Box-3a and Down-Regulation of Growth and Differentiation Factor 9 Messenger Ribonucleic Acid Expression at Early Stage of Mouse Folliculogenesis. Endocrinology 2010, 151, 774–782. [Google Scholar] [CrossRef]
- Verma, Y.; Rana, S. Effects of Progesterone on Benzene Toxicity in Rats. Arch. Ind. Hyg. Toxicol. 2008, 59, 1–9. [Google Scholar] [CrossRef][Green Version]
- Black, D.L.; Marks, T.A.; Branstetter, D.G.; Kirton, K.T. Reversal of bropirimine developmental toxicity with progesterone. Toxicol. Appl. Pharmacol. 1991, 108, 121–128. [Google Scholar] [CrossRef]
- Stocco, C.; Telleria, C.; Gibori, G. The Molecular Control of Corpus Luteum Formation, Function, and Regression. Endocr. Rev. 2007, 28, 117–149. [Google Scholar] [CrossRef]
- Salem, R.R.; Kelada, M.N. A Biochemichal and Ultrastructural Study on the Effect of Toluene on the Pars Distalis of Anterior Pituitary Glands of Adult Male Albino Rats. Egypt J. Histol. 2020, 43, 948–959. [Google Scholar] [CrossRef]
- Na Kim, H.; Lee, S.-J.; Koh, J.-Y. The neurosteroids, allopregnanolone and progesterone, induce autophagy in cultured astrocytes. Neurochem. Int. 2012, 60, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Yenuganti, V.R.; Viergutz, T.; Vanselow, J. Oleic acid induces specific alterations in the morphology, gene expression and steroid hormone production of cultured bovine granulosa cells. Gen. Comp. Endocrinol. 2016, 232, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ivell, R.; Anand-Ivell, R. Insulin-like peptide 3 (INSL3) is a major regulator of female reproductive physiology. Hum. Reprod. Update 2018, 24, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Anand-Ivell, R.; Tremellen, K.; Dai, Y.; Heng, K.; Yoshida, M.; Knight, P.G.; Hale, G.E. Circulating insulin-like factor 3 (INSL3) in healthy and infertile women. Hum. Reprod. 2013, 28, 3093–3102. [Google Scholar] [CrossRef]
- Seyam, E.; Hefzy, E. Evaluation of the correlation between insulin like factor 3, polycystic ovary syndrome, and ovarian maldescent. Gynecol. Endocrinol. 2018, 34, 481–488. [Google Scholar] [CrossRef]
- Shimizu, T.; Hirai, Y.; Miyamoto, A. Expression of Cyclins and Cyclin-Dependent Kinase Inhibitors in Granulosa Cells from Bovine Ovary. Reprod. Domest. Anim. 2013, 48, e65–e69. [Google Scholar] [CrossRef]
- Sicinski, P.; Donaher, J.L.; Geng, Y.; Parker, S.B.; Gardner, H.P.; Park, M.Y.; Robker, R.; Richards, J.S.; McGinnis, L.; Biggers, J.D.; et al. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nat. Cell Biol. 1996, 384, 470–474. [Google Scholar] [CrossRef]
- Dong, J.; Albertini, D.F.; Nishimori, K.; Kumar, T.R.; Lu, N.; Matzuk, M.M. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nat. Cell Biol. 1996, 383, 531–535. [Google Scholar] [CrossRef]
- Orisaka, M.; Orisaka, S.; Jiang, J.-Y.; Craig, J.; Wang, Y.; Kotsuji, F.; Tsang, B.K.; Elliott, J. Growth Differentiation Factor 9 Is Antiapoptotic during Follicular Development from Preantral to Early Antral Stage. Mol. Endocrinol. 2006, 20, 2456–2468. [Google Scholar] [CrossRef]
- Ross, D. The Role of Metabolism and Specific Metabolites in Benzene-Induced Toxicity: Evidence and Issues. J. Toxicol. Environ. Health Part A 2000, 61, 357–372. [Google Scholar] [CrossRef]
- Nakai, N.; Murata, M.; Nagahama, M.; Hirase, T.; Tanaka, M.; Fujikawa, T.; Nakao, N.; Nakashima, K.; Kawanishi, S. Oxidative DNA Damage Induced by Toluene is Involved in its Male Reproductive Toxicity. Free Radic. Res. 2003, 37, 69–76. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, Q.; Wang, Y.; Zhu, S. Toxic effects of BTEX in water on Daphnia magna and Limnodrilus hoffmeisteri and safety assessment of the aquatic environment. Acta Sci. Circumstantiae 2009, 29, 1485–1490. [Google Scholar]
- Zhang, M.; Wang, Y.; Wang, Q.; Yang, J.; Yang, D.; Liu, J.; Li, J. Involvement of Mitochondria-Mediated Apoptosis in Ethylbenzene-Induced Renal Toxicity in Rat. Toxicol. Sci. 2010, 115, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Q.; Luo, Y.; Yang, G.; Zhou, T. Joint action and lethal levels of toluene, ethylbenzene, and xylene on midge (Chironomus plumosus) larvae. Environ. Sci. Pollut. Res. 2012, 20, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Neuparth, T.; Capela, R.; Pereira, S.P.P.; Moreira, S.M.; Santos, M.M.; Reis-Henriques, M.A. Toxicity effects of hazardous and noxious substances (HNS) to marine organisms: Acute and chronic toxicity of p-xylene to the amphipod Gammarus locusta. J. Toxicol. Environ. Health Part A 2014, 77, 1210–1221. [Google Scholar] [CrossRef]
- García, G.M.; Mariño, G. Autophagy role in environmental pollutants exposure. Prog. Mol. Biol. Transl. Sci. 2020, 172, 257–291. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yoshino, O.; Nakashima, A.; Ito, M.; Nishio, K.; Ono, Y.; Kusabiraki, T.; Kunitomi, C.; Takahashi, N.; Harada, M.; et al. Inhibition of autophagy in theca cells induces CYP17A1 and PAI-1 expression via ROS/p38 and JNK signalling during the development of polycystic ovary syndrome. Mol. Cell. Endocrinol. 2020, 508, 110792. [Google Scholar] [CrossRef]
- Zecchini, S.; Serafini, F.P.; Catalani, E.; Giovarelli, M.; Coazzoli, M.; Di Renzo, I.; De Palma, C.; Perrotta, C.; Clementi, E.; Buonanno, F.; et al. Dysfunctional autophagy induced by the pro-apoptotic natural compound climacostol in tumour cells. Cell Death Dis. 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Y.; Yuan, Z.; Yi, J.; Chen, J.; Wang, N.; Tian, Y. Autophagy and Apoptosis Interact to Modulate T-2 Toxin-Induced Toxicity in Liver Cells. Toxins 2019, 11, 45. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Park, K.-I.; Kim, S.-H.; Yu, S.-N.; Park, S.-G.; Kim, Y.W.; Seo, Y.-K.; Ma, J.-Y.; Ahn, S.-C. Inhibition of Autophagy Promotes Salinomycin-Induced Apoptosis via Reactive Oxygen Species-Mediated PI3K/AKT/mTOR and ERK/p38 MAPK-Dependent Signaling in Human Prostate Cancer Cells. Int. J. Mol. Sci. 2017, 18, 1088. [Google Scholar] [CrossRef] [PubMed]


| Gene Symbol | Sequences | |
|---|---|---|
| INSL3 | Forward: | CTTCCTCACCAGGCTTCTCA |
| Reverse: | CACCACCTGAGCCCTACAAT | |
| CYP17a1 | Forward: | ACTGAGGGTATCGTGGATGC |
| Reverse: | TCGAACTTCTCCCTGCACTT | |
| CYP19 | Forward: | TGAGTCTCCCAAGGTCATCC |
| Reverse: | GGGTTCAGCATTTCCAAAAA | |
| LHR | Forward: | TTATTCCGCCATCTTTGAGG |
| Reverse: | ACAGGGGTTGAAAGCATCTG | |
| CCND2 | Forward: | CCTCACGACTTCATTGAGCA |
| Reverse: | GGTAGCACACAGAGCGATGA | |
| IGF-I | Forward: | CCGCTGAAGCCTACAAAGTC |
| Reverse: | GGGAGGCTCCTCCTACATTC | |
| ESR1 | Forward: | CATCGATAAGAACCGGAGGA |
| Reverse: | AAGGTTGGCAGCTCTCATGT | |
| ESR2 | Forward: | GAAGCTGAACCACCCAATGT |
| Reverse: | CAGTCCCACCATTAGCACCT | |
| ACTB | Forward: | AGCCATGTACGTAGCCATCC |
| Reverse: | ACCCTCATAGATGGGCACAG | |
| GDF9 | Forward: | GATGTGACCTCCCTCCTTCA |
| Reverse: | GCCTGGGTACTCGTGTCATT | |
| ATG5 | Forward: | CCTGAAGACGGAGAGAAGAAGAG |
| Reverse: | CGGGAAGCAAGGGTGTCAT | |
| LC3 | Forward: | TGTTAGGCTTGCTCTTTTGG |
| Reverse: | GCAGAGGAAATGACCACAGAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrezaki, A.; Aldawood, N.; Mansour, L.; Ahmed, M.; Sirotkin, A.V.; Alwasel, S.; Harrath, A.H. Toluene Can Disrupt Rat Ovarian Follicullogenesis and Steroidogenesis and Induce Both Autophagy and Apoptosis. Biology 2021, 10, 1153. https://doi.org/10.3390/biology10111153
Alrezaki A, Aldawood N, Mansour L, Ahmed M, Sirotkin AV, Alwasel S, Harrath AH. Toluene Can Disrupt Rat Ovarian Follicullogenesis and Steroidogenesis and Induce Both Autophagy and Apoptosis. Biology. 2021; 10(11):1153. https://doi.org/10.3390/biology10111153
Chicago/Turabian StyleAlrezaki, Abdulkarem, Nouf Aldawood, Lamjed Mansour, Mukhtar Ahmed, Alexander V. Sirotkin, Saleh Alwasel, and Abdel Halim Harrath. 2021. "Toluene Can Disrupt Rat Ovarian Follicullogenesis and Steroidogenesis and Induce Both Autophagy and Apoptosis" Biology 10, no. 11: 1153. https://doi.org/10.3390/biology10111153
APA StyleAlrezaki, A., Aldawood, N., Mansour, L., Ahmed, M., Sirotkin, A. V., Alwasel, S., & Harrath, A. H. (2021). Toluene Can Disrupt Rat Ovarian Follicullogenesis and Steroidogenesis and Induce Both Autophagy and Apoptosis. Biology, 10(11), 1153. https://doi.org/10.3390/biology10111153







