Demonstrative Experiment on the Favorable Effects of Static Electric Field Treatment on Vitamin D3-Induced Hypercalcemia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Procedures
2.3. Serum Biochemistry
2.4. Histopathology
2.5. Statistical Evaluation
3. Results
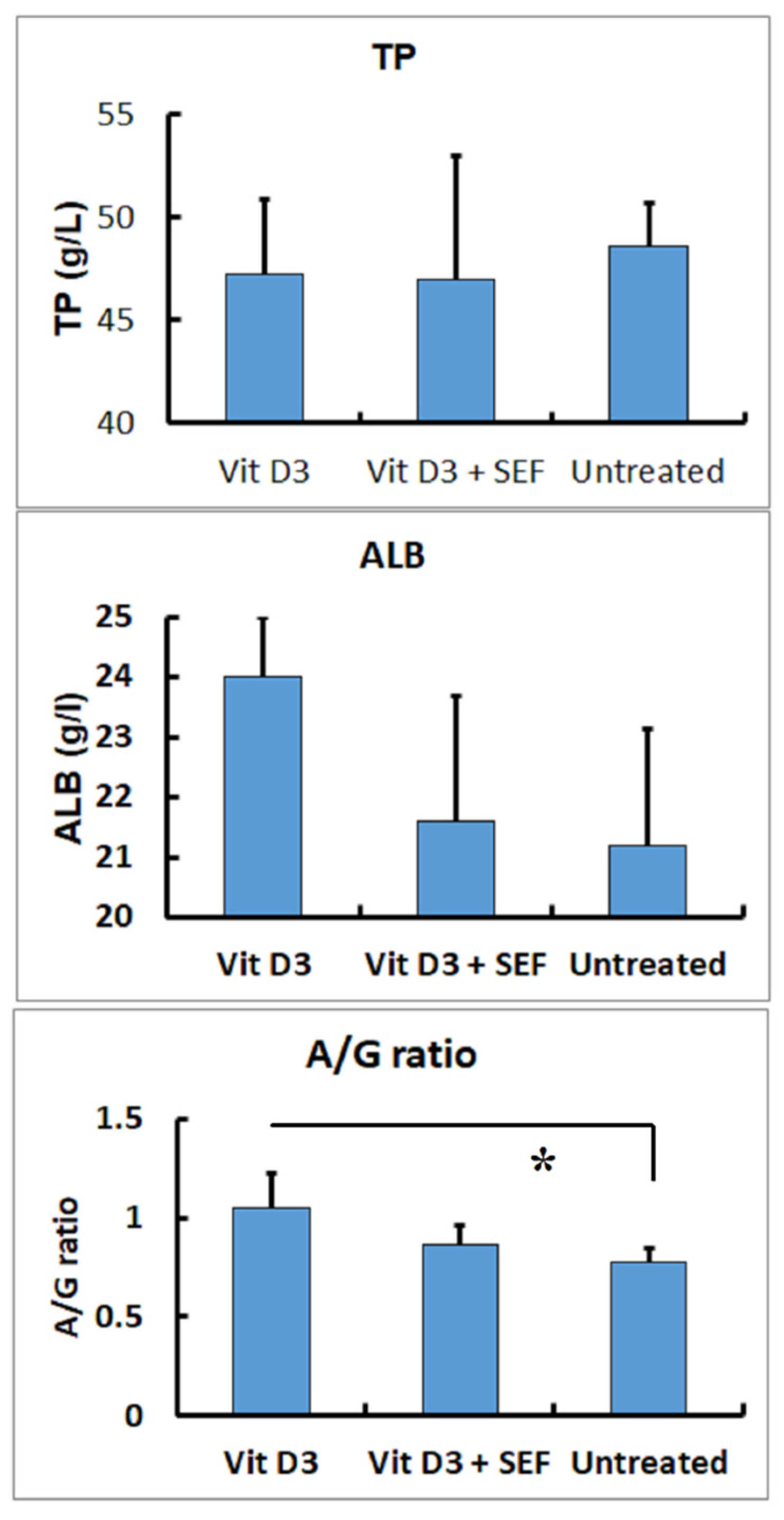
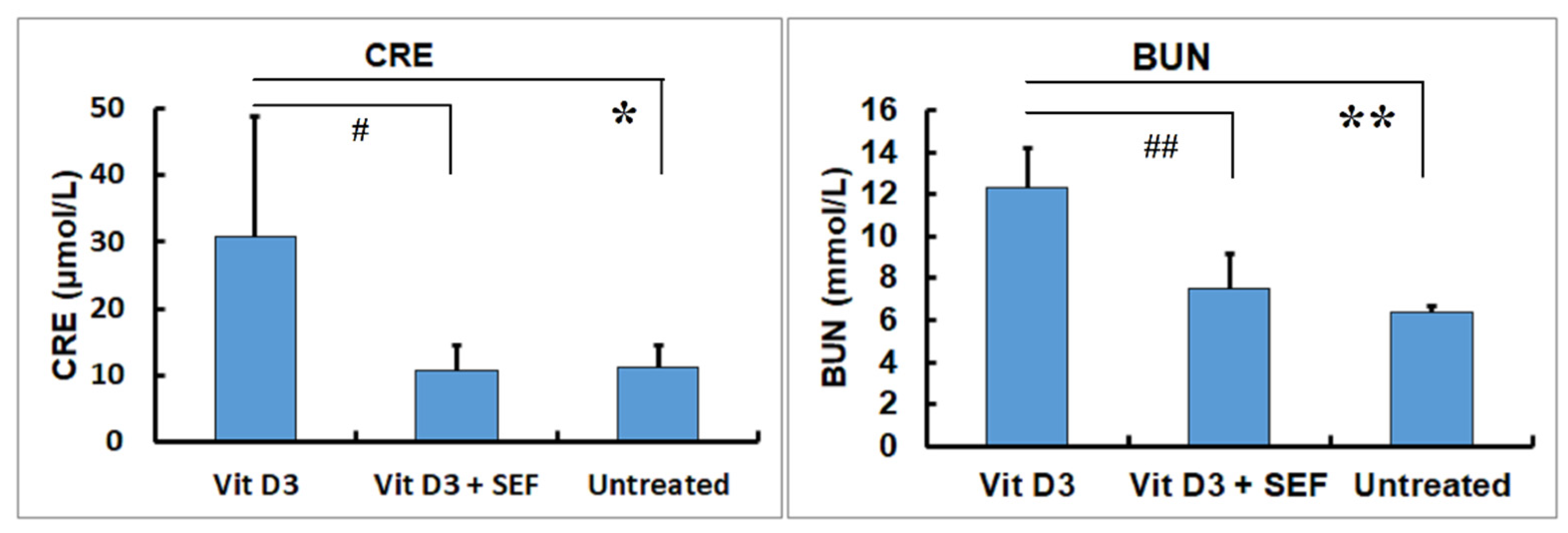
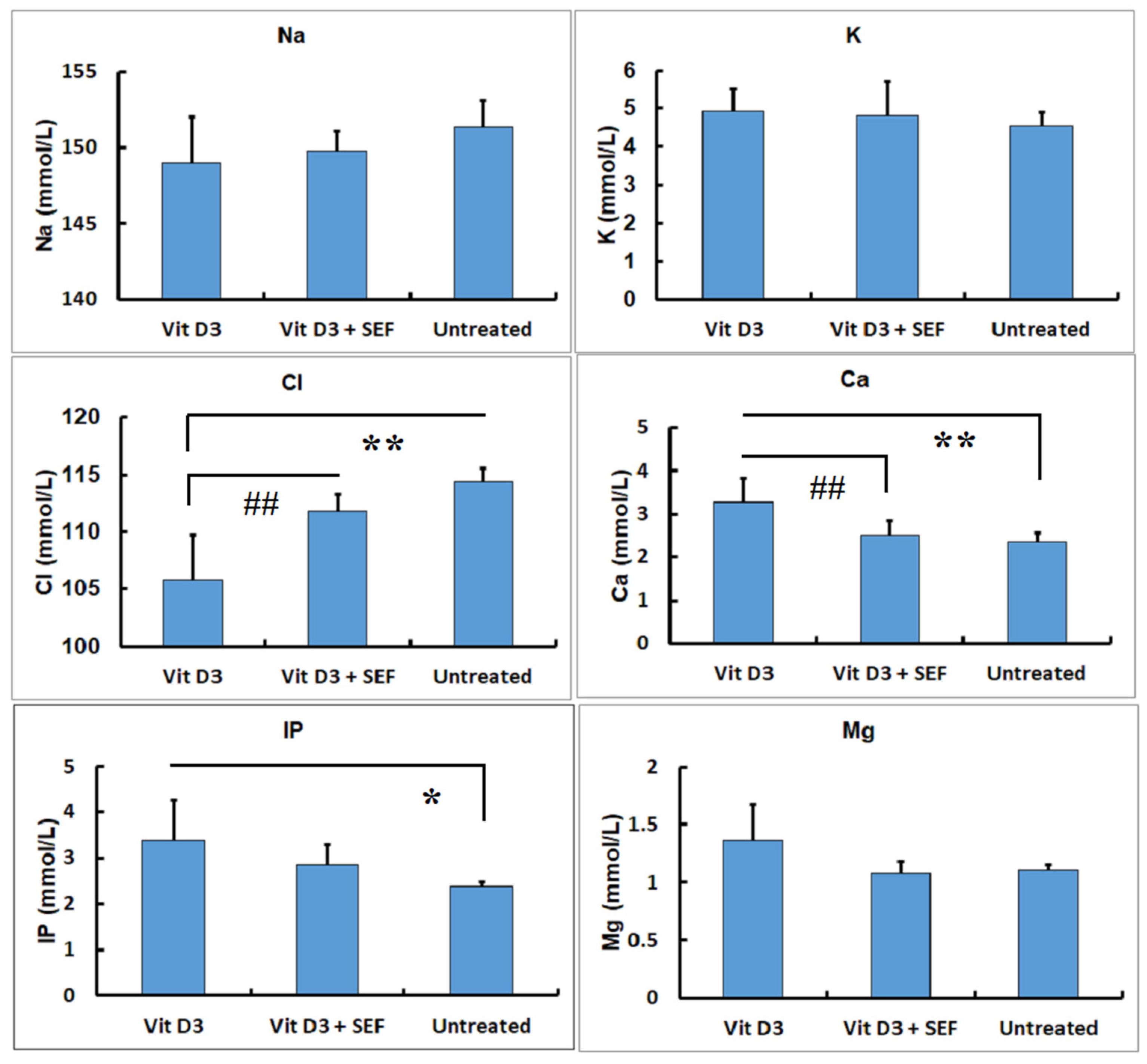
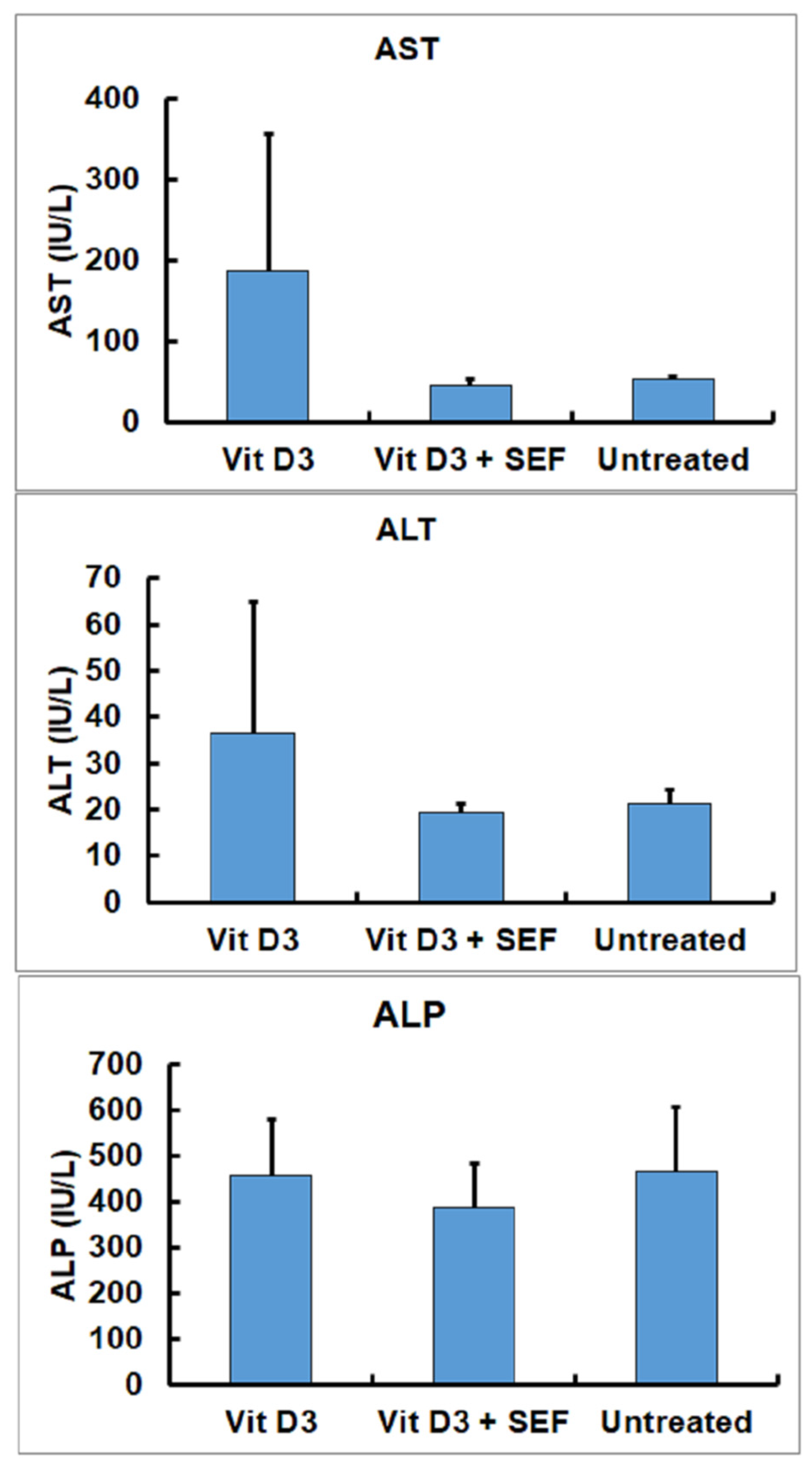
3.1. Serum Biochemical Findings
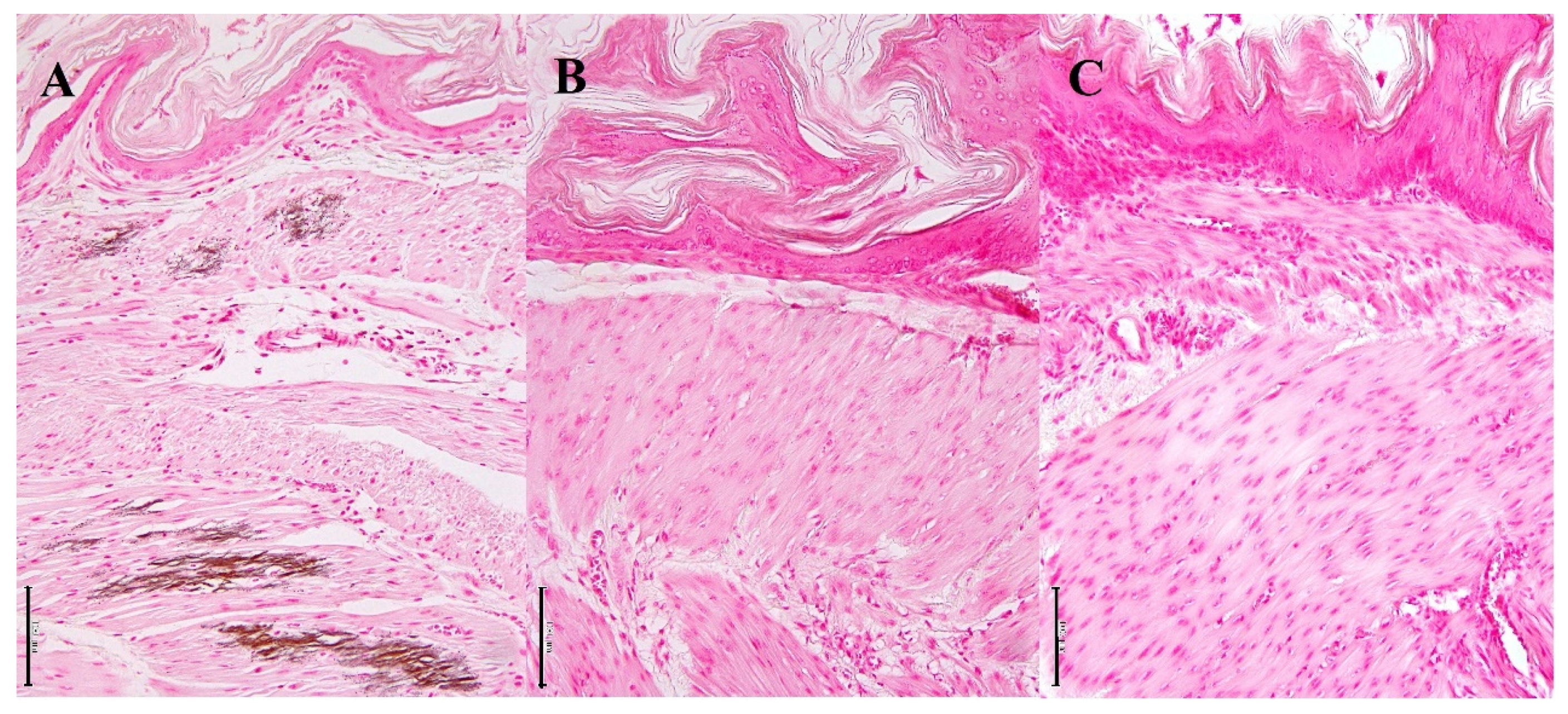
3.2. Histopathological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colbert, A.P.; Markov, M.S.; Soulder, J.S. Static magnetic field therapy: Dosimetry considerations. J. Altern. Complement. Med. 2008, 14, 577–582. [Google Scholar] [CrossRef]
- Wang, S.; Luo, J.; Lv, H.; Zhang, Z.; Yang, J.; Dong, D.; Fang, Y.; Hu, L.; Liu, J.; Fang, Z.; et al. Safety of exposure to high static magnetic fields (2 T–12 T): A study on mice. Eur. Radiol. 2019, 29, 6029–6037. [Google Scholar] [CrossRef]
- Hara, H. On the effect of AC Electrostatic high voltage potential load upon the blood-electrolytes. Jpn. J. 1961, 75, 265–273. (In Japanese) [Google Scholar]
- Harakawa, S.; Inoue, N.; Saito, A.; Doge, F.; Nagasawa, H.; Suzuki, N.; Martin, D.E. 60 Hz electric field upregulates cytosolic Ca2+ level in mouse splenocytes simulated by lectin. Bioelectromagnetics 2004, 25, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Aoi, W.; Inoue, M.; Takagi, T.; Akagiri, S.; Mizushima, K.; Handa, O.; Kokura, S.; Ichikawa, H.; Karita, M.; et al. Static electric field by high voltage alternating current ameliorates collagen-induced arthritis in mice via the inhibition of IL-1β expression. J. Complement. Integr. Med. 2009, 6, 30. [Google Scholar] [CrossRef]
- Marcinowska-Suchowierska, E.; Kupisz-Urbanska, M.; Lukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D toxicity-a clinical perspective. Front. Endocrinol. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Kim, S.; Stephens, L.D.; Fitzgerald, R. How much is too much? Two contrasing cases of excessive vitamin D supplementation. Clin. Chim. Acta 2017, 473, 35–38. [Google Scholar] [CrossRef]
- Ravindran, R.; Witczac, J.; Bahl, S.; Premawardhana, L.D.K.E. Myositis, rhabdomyolysis and severehypercalcaemia in a body builder. Endocrinol. Diabetes Metab. Case Rep. 2020, EDM-20-0032, 1–5. [Google Scholar]
- Ketha, H.; Wadamas, H.; Lteif, A.; Singh, J. Iatrogenic vitamin D toxicity in an infant—A case report and review of literature. J. Steroid. Biochem. Mol. Biol. 2015, 145, 14–18. [Google Scholar] [CrossRef] [PubMed]
- The European Agency for the Evaluation of Medicinal Products. Veterinary Medicines Evaluation Unit. In Committee for Veterinary Medicinal Products; Alfacalcidol Summary Report: EMEA/MRL/381/98-FINAL; EMEA: London, UK, 1998. [Google Scholar]
- Pakkala, S.; de Vos, S.; Elstner, E.; Rude, R.K.; Uskokovic, M.; Binderup, L.; Koeffler, P. Vitamin D3 analogs: Effect on leukemic clonal growth and differentiation, and on serum calcium levels. Leuk. Res. 1995, 19, 65–72. [Google Scholar] [CrossRef]
- Harakawa, S.; Inoue, N.; Hori, T.; Tochio, K.; Kariya, T.; Takahashi, K.; Doge, F.; Martin, D.E.; Saito, A.; Suzuki, H.; et al. Effects of exposure to a 50 Hz electric field on plasma levels of lactate, glucose, free fatty acids, triglycerides and creatine phosphokinase activity in hind-limb ischemic rats. J. Vet. Med. Sci. 2004, 67, 969–974. [Google Scholar] [CrossRef][Green Version]
- Naito, Y.; Yamaguchi, S.; Mori, Y.; Nakajima, K.; Hashimoto, S.; Tamaru, M.; Satoh, Y.; Hitomi, Y.; Karita, M.; Hiwatashi, T.; et al. A randomized, double-blind, sham-controlled study of static electric field therapy by high voltage alternating current for active rheumatoid arthritis. J. Clin. Biochem. Nutr. 2013, 53, 63–67. [Google Scholar] [CrossRef]
- Güler, G.; Seyhan, N.; Aricioğlu, A. Effects of static and 50 Hz alternating electric fields on superoxide dismutase activities and TBARS levels in guinea pigs. Gen. Physiol. Biophys. 2006, 25, 177–193. [Google Scholar]
- Aïda, L.; Frédéric, L.; Soumaya, G.; Philippe, H.; Mohsen, S.; Hafedh, A. Static magnetic field induced hypovitaminosis D in rat. J. Vet. Med. Sci. 2013, 75, 1181–1185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lahbib, A.; Miryam, E.; Ghodbane, S.; Belguith, H.; Chater, S.; Sakly, M.; Abdelmelek, H. Time-dependent effects of exposure to static magnetic field on glucose and lipid metabolism in rat. Gen. Physiol. Biophys. 2010, 29, 390–395. [Google Scholar] [CrossRef][Green Version]
- Lahbib, A.; Ghodbane, S.; Sakly, M.; Abdelmelek, H. Vitamins and glucose metabolism: The role of static magnetic fields. Int. J. Radiat. Biol. 2014, 90, 1240–1245. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Its role in cancer prevention and treatment. Prog. Biophys. Mol. Biol. 2006, 92, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Chavhan, S.G.; Brar, R.S.; Banga, H.S.; Sandhu, H.S.; Sodhi, S.; Gadhave, P.D.; Kothule, V.R.; Kammon, A.M. Clinicopathological studies on vitamin D3 toxicity and therapeutic evaluation of Aloe vena in rats. Toxicol. Int. 2011, 18, 35–43. [Google Scholar] [CrossRef]
- Takahashi, K.; Kuroki, M.; Doge, F.; Sawasaki, Y.; Yoshioka, M. Effects of low-frequency electric fields on the intracellular Ca2+ response induced in human vascular endothelial cells by vasoactive substances. Electromagn. Biol. Med. 2002, 21, 279–286. [Google Scholar] [CrossRef]
- Rosen, A.D. Inhibition of calcium channel activation in GH3 cells by static magnetic fields. Biochim. Biophys. Acta 1996, 1282, 149–155. [Google Scholar] [CrossRef]
- Morris, C.E.; Skalak, T.C. Acute exposure to a moderate strength static magnetic field reduces edema formation in rats. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H50–H57. [Google Scholar] [CrossRef] [PubMed]
- Miryam, E.; Aida, L.; Samira, M.; Mohsen, S.; Hafedh, A. Effects of acute exposure to static magnetic field on ionic composition of rat spinal cord. Gen. Physiol. Biophys. 2010, 29, 288–294. [Google Scholar] [CrossRef]
- Kramann, R.; Kusaba, T.; Humphreys, B.D. Who regenerates the kidney tubule? Nephrol. Dial. Transplant. 2015, 30, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Benov, L.C.; Anteonov, P.A.; Ribarov, S.R. Oxidative damage of the membrane lipids after electroproration. Gen. Physiol. Biophys. 1994, 13, 85–97. [Google Scholar] [PubMed]
- Blumenthal, N.C.; Ricci, J.; Breger, L.; Zychlinsky, A.; Soloman, H.; Chen, G.G.; Kuznetsov, D.; Dorfman, R. Effects of low-intensity AC and/or DC electromagnetic fields on cell attachment and induction of apoptosis. Bioelectromagnetics 1997, 18, 264–272. [Google Scholar] [CrossRef]
- Barriviera, M.L.; Louro, S.R.; Wajnberg, E.; Hasson-Voloch, A. Denervation alters protein-lipid interactions in membrane fractions from electrocytes of Electopjorus electrius. Biophys. Chem. 2001, 91, 93–104. [Google Scholar] [CrossRef]
- Ivancsites, S.; Diem, E.; Jahn, O.; Rudiger, H.W. Intermittent extremely low frequency electromagnetic fields cause DNA damage in a dose-dependent way. Int. Arch. Occup. Environ. Health 2003, 76, 431–436. [Google Scholar] [CrossRef]
- Liburdy, R.P.; Callahan, D.E.; Harland, J.; Dunham, E.; Sloma, T.R.; Yaswen, P. Experimental evidence for 60 Hz magnetic fields operatingthrough the signal transduction cascade. Effects on calcium influx and c-MYC mRNA induction. FEBS Lett. 1993, 334, 301–308. [Google Scholar] [CrossRef]
- Liburdy, R.P. Calcium signaling in lymphocytes and ELF fields. Evidence for an electric field metric and a site of interaction involving the calcium ion channel. FEBS Lett. 1992, 301, 53–59. [Google Scholar] [CrossRef]
- Walleczek, J.; Liburdy, R.P. Nonthermal 60 Hz sinusoidal magnetic-field exposure enhance 45Ca2+ uptake in rat thymocytes: De pendence on mitogen activation. FEBS Lett. 1990, 271, 157–160. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, T.; Inaka, K.; Ogiso, N. Demonstrative Experiment on the Favorable Effects of Static Electric Field Treatment on Vitamin D3-Induced Hypercalcemia. Biology 2021, 10, 1116. https://doi.org/10.3390/biology10111116
Kimura T, Inaka K, Ogiso N. Demonstrative Experiment on the Favorable Effects of Static Electric Field Treatment on Vitamin D3-Induced Hypercalcemia. Biology. 2021; 10(11):1116. https://doi.org/10.3390/biology10111116
Chicago/Turabian StyleKimura, Tohru, Kengo Inaka, and Noboru Ogiso. 2021. "Demonstrative Experiment on the Favorable Effects of Static Electric Field Treatment on Vitamin D3-Induced Hypercalcemia" Biology 10, no. 11: 1116. https://doi.org/10.3390/biology10111116
APA StyleKimura, T., Inaka, K., & Ogiso, N. (2021). Demonstrative Experiment on the Favorable Effects of Static Electric Field Treatment on Vitamin D3-Induced Hypercalcemia. Biology, 10(11), 1116. https://doi.org/10.3390/biology10111116





