MiRNAs Expression Profiling in Raw264.7 Macrophages after Nfatc1-Knockdown Elucidates Potential Pathways Involved in Osteoclasts Differentiation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. RAW264.7 Cell Culture
2.2. Small Interfering RNA (siRNA) Transfection
2.3. miRNAs Profiler PCR Array Analysis
2.4. pMirGLO Transfection and Dual-Luciferase Reporter Assay
2.5. MiRNA Transfection
2.6. Quantitative RT-PCR (qPCR) Analysis of mRNA and miRNA Expression
2.7. Western Blot Analysis
2.8. DIANA-miRPath v3.0 Database, KEGG Enrichment Analyses, and Target Gene Prediction
2.9. Statistical Analysis
3. Results
3.1. miRNAs Expression in RANKL-Treated RAW264.7 Cells
3.2. Expression of miRNAs in NFATc1-siRNA/RANKL-Treated Cells
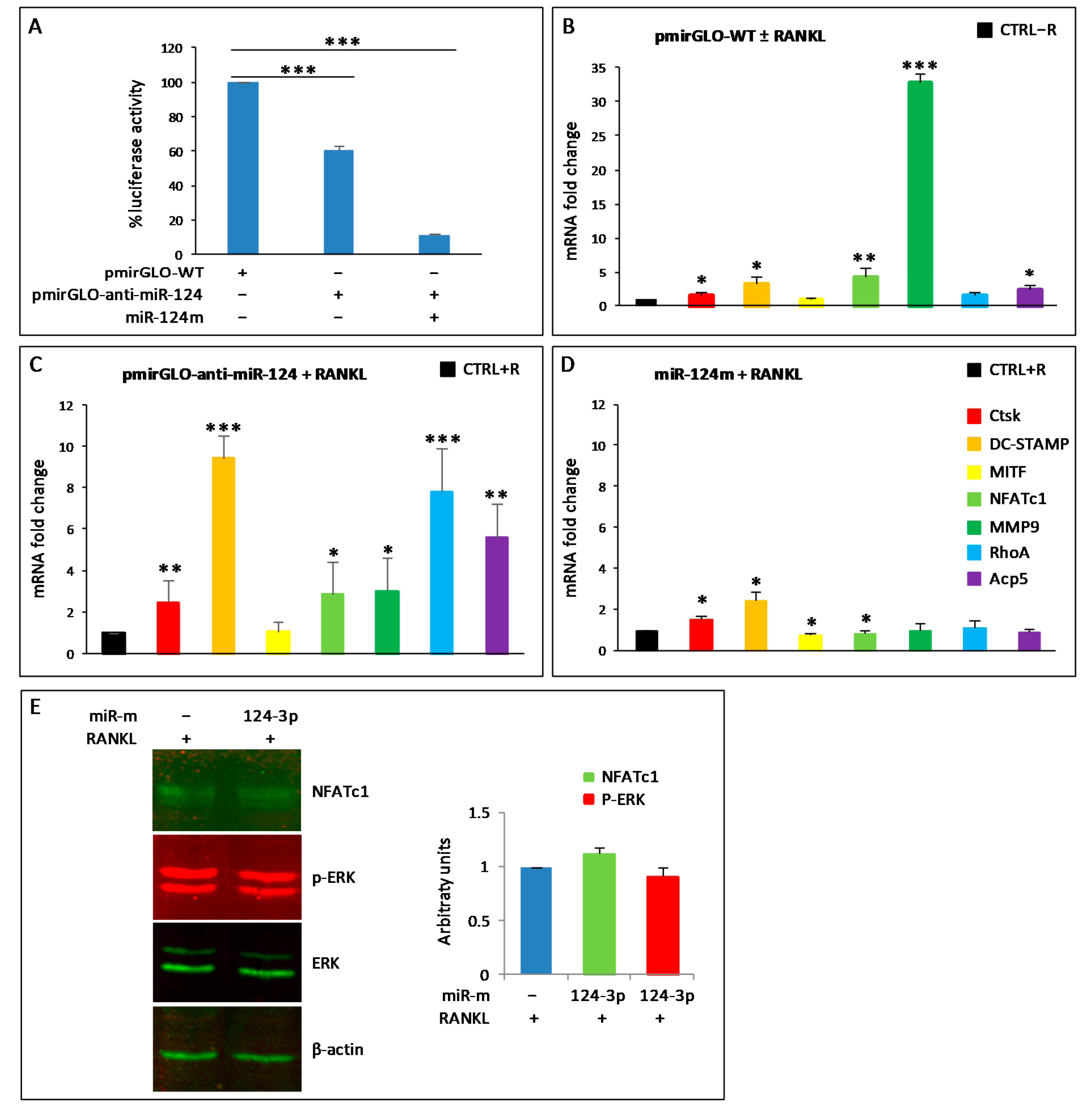
3.3. Validation of PCR Array Results
3.4. KEGG Enrichment Analysis of Differentially Expressed miRNAs
3.5. miR-124-3p Expression in RAW264.7 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.I.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Hirotani, H.; Tuohy, N.A.; Woo, J.T.; Stern, P.H.; Clipstone, N.A. The Calcineurin/Nuclear Factor of Activated T Cells Signaling Pathway Regulates Osteoclastogenesis in RAW264.7 Cells. J. Biol. Chem. 2004, 279, 13984–13992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, R.; Mallia, S.; Zito, F.; Lampiasi, N. Long-lasting activity of erk kinase depends on nfatc1 induction and is involved in cell migration-fusion in murine macrophages raw264.7. Int. J. Mol. Sci. 2020, 21, 8965. [Google Scholar] [CrossRef] [PubMed]
- Lampiasi, N.; Russo, R.; Kireev, I.; Strelkova, O.; Zhironkina, O.; Zito, F. Osteoclasts differentiation from murine RAW 264.7 cells stimulated by RANKL: Timing and behavior. Biology 2021, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, C.M.; Heist, E.K.; Beals, C.R.; Crabtree, G.R.; Gardner, P. Protein kinase A negatively modulates the nuclear accumulation of NF-ATc1 by priming for subsequent phosphorylation by glycogen synthase kinase-3. J. Biol. Chem. 2002, 277, 48664–48676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Ha, J.; Kim, H.J.; Kim, Y.S.; Chang, E.J.; Song, W.J.; Kim, H.H. Negative feedback inhibition of NFATc1 by DYRK1A regulates bone homeostasis. J. Biol. Chem. 2009, 284, 33343–33351. [Google Scholar] [CrossRef] [Green Version]
- Arron, J.R.; Winslow, M.M.; Polleri, A.; Chang, C.-P.; Wu, H.; Gao, X.; Neilson, J.R.; Chen, L.; Heit, J.J.; Kim, S.K.; et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature 2006, 441, 595–600. [Google Scholar] [CrossRef]
- Franceschetti, T.; Dole, N.S.; Kessler, C.B.; Lee, S.K.; Delany, A.M. Pathway analysis of microRNA expression profile during murine osteoclastogenesis. PLoS ONE 2014, 9, 1–13. [Google Scholar] [CrossRef]
- Sugatani, T.; Hruska, K.A. MicroRNA-223 is a key factor in osteoclast differentiation. J. Cell. Biochem. 2007, 101, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Zito, F.; Lampiasi, N.; Kireev, I.; Russo, R. United we stand: Adhesion and molecular mechanisms driving cell fusion across species. Eur. J. Cell Biol. 2016, 95, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Krzeszinski, J.Y.; Wei, W.; Huynh, H.; Jin, Z.; Wang, X.; Chang, T.C.; Xie, X.J.; He, L.; Mangala, L.S.; Lopez-Berestein, G.; et al. MiR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature 2014, 512, 431–435. [Google Scholar] [CrossRef] [PubMed]
- M’Baya-Moutoula, E.; Louvet, L.; Metzinger-Le Meuth, V.; Massy, Z.A.; Metzinger, L. High inorganic phosphate concentration inhibits osteoclastogenesis by modulating miR-223. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 2202–2212. [Google Scholar] [CrossRef] [Green Version]
- Ell, B.; Mercatali, L.; Ibrahim, T.; Campbell, N.; Schwarzenbach, H.; Pantel, K.; Amadori, D.; Kang, Y. Tumor-Induced Osteoclast miRNA Changes as Regulators and Biomarkers of Osteolytic Bone Metastasis. Cancer Cell 2013, 24, 542–556. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Ma, X.; Sung, D.; Li, M.; Kosti, A.; Lin, G.; Chen, Y.; Pertsemlidis, A.; Hsiao, T.H.; Du, L. MicroRNA-449a functions as a tumor suppressor in neuroblastoma through inducing cell differentiation and cell cycle arrest. RNA Biol. 2015, 12, 538–554. [Google Scholar] [CrossRef] [Green Version]
- Dou, C.; Zhang, C.; Kang, F.; Yang, X.; Jiang, H.; Bai, Y.; Xiang, J.; Xu, J.; Dong, S. MiR-7b directly targets DC-STAMP causing suppression of NFATc1 and c-Fos signaling during osteoclast fusion and differentiation. Biochim. Biophys. Acta Gene Regul. Mech. 2014, 1839, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, T.; Vacher, J.; Hruska, K.A. A microRNA expression signature of osteoclastogenesis. Blood 2011, 117, 3648–3657. [Google Scholar] [CrossRef] [PubMed]
- Kapinas, K.; Delany, A.M. MicroRNA biogenesis and regulation of bone remodeling. Arthritis Res. Ther. 2011, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, J.B.; Stein, G.S.; van Wijnen, A.J.; Stein, J.L.; Hassan, M.Q.; Gaur, T.; Zhang, Y. MicroRNA control of bone formation and homeostasis. Nat. Rev. Endocrinol. 2012, 8, 212–227. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Chen, C.; Chen, P.; Xie, H.; Luo, X. MicroRNAs and their roles in osteoclast differentiation. Front. Med. China 2011, 5, 414–419. [Google Scholar] [CrossRef]
- Mann, M.; Barad, O.; Agami, R.; Geiger, B.; Hornstein, E. miRNA-based mechanism for the commitment of multipotent progenitors to a single cellular fate. Proc. Natl. Acad. Sci. USA 2010, 107, 15804–15809. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Zhang, L.; Gao, Y.; Ge, W.; Tang, P. The multiple roles of microrna-223 in regulating bone metabolism. Molecules 2015, 20, 19433–19448. [Google Scholar] [CrossRef]
- Ma, X.; Helgason, E.; Phung, Q.T.; Quan, C.L.; Iyer, R.S.; Lee, M.W.; Bowman, K.K.; Starovasnik, M.A.; Dueber, E.C. Molecular basis of Tank-binding kinase 1 activation by transautophosphorylation. Proc. Natl. Acad. Sci. USA 2012, 109, 9378–9383. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Kim, H.J.; Park, C.K.; Kim, Y.G.; Lee, H.J.; Kim, J.Y.; Kim, H.H. MicroRNA-124 regulates osteoclast differentiation. Bone 2013, 56, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, P.; Kalaiselvan, S.; Sujitha, S.; Rasool, M. MiR-506-3p alleviates uncontrolled osteoclastogenesis via repression of RANKL/NFATc1 signaling pathway. J. Cell. Physiol. 2020, 235, 9497–9509. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Guo, Z. miR-193-3p ameliorates bone resorption in ovariectomized mice by blocking NFATc1 signaling. Int. J. Clin. Exp. Pathol. 2019, 12, 4077. [Google Scholar]
- Inoue, S.; Fujikawa, K.; Matsuki-Fukushima, M.; Nakamura, M. Repair processes of flat bones formed via intramembranous versus endochondral ossification. J. Oral Biosci. 2020, 62, 52–57. [Google Scholar] [CrossRef]
- Russo, R.; Mallia, S.; Zito, F.; Lampiasi, N. Gene Expression Profiling of NFATc1-Knockdown in RAW 264.7 Cells: An Alternative Pathway for Macrophage Differentiation. Cells 2019, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2^-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef] [PubMed]
- Prokopec, S.D.; Watson, J.D.; Waggott, D.M.; Smith, A.B.; Wu, A.H.; Okey, A.B.; Pohjanvirta, R.; Boutros, P.C. Systematic evaluation of medium-throughput mRNA abundance platforms. RNA 2013, 19, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschetti, T.; Kessler, C.B.; Lee, S.K.; Delany, A.M. MiR-29 promotes murine osteoclastogenesis by regulating osteoclast commitment and migration. J. Biol. Chem. 2013, 288, 33347–33360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, M.; Pitari, M.R.; Amodio, N.; Di Martino, M.T.; Conforti, F.; Leone, E.; Botta, C.; Paolino, F.M.; Del Giudice, T.; Iuliano, E.; et al. MiR-29b negatively regulates human osteoclastic cell differentiation and function: Implications for the treatment of multiple myeloma-related bone disease. J. Cell. Physiol. 2013, 228, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Peng, F.; Li, M.L.; Duan, W.B.; Peng, C.Q.; Wu, S.J. LncRNA-SMILR modulates RhoA/ROCK signaling by targeting miR-141 to regulate vascular remodeling in pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H377–H391. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, C.; Li, Y.; Wang, L.; Nie, G.; Peng, J.; Wang, A.; Zhang, P.; Tian, W.; Li, Q.; et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016, 2, 1–23. [Google Scholar] [CrossRef]
- Sujitha, S.; Rasool, M. Berberine coated mannosylated liposomes curtail RANKL stimulated osteoclastogenesis through the modulation of GSK3β pathway via upregulating miR-23a. Int. Immunopharmacol. 2019, 74, 105703. [Google Scholar] [CrossRef]
- Cui, X.S.; Shen, X.H.; Sun, S.C.; Cho, S.W.; Heo, Y.T.; Kang, Y.K.; Wakayama, T.; Kim, N.H. Identifying MicroRNA and mRNA Expression Profiles in Embryonic Stem Cells Derived from Parthenogenetic, Androgenetic and Fertilized Blastocysts. J. Genet. Genom. 2013, 40, 189–200. [Google Scholar] [CrossRef]
- de Almeida, D.C.; Bassi, Ê.J.; Azevedo, H.; Anderson, L.; Origassa, C.S.T.; Cenedeze, M.A.; de Andrade-Oliveira, V.; Felizardo, R.J.F.; da Silva, R.C.; Hiyane, M.I.; et al. A regulatory miRNA-mRNA network is associated with tissue repair induced by mesenchymal stromal cells in acute kidney injury. Front. Immunol. 2017, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Lampiasi, N.; Russo, R.; Zito, F. The Alternative Faces of Macrophage Generate Osteoclasts. BioMed Res. Int. 2016, 2016, 9089610. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, K.; Chen, S.; Sun, Q.; Zhang, Y.; Chen, L.; Sun, X. NFATc1 phosphorylation by DYRK1A increases its protein stability. PLoS ONE 2017, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gwack, Y.; Sharma, S.; Nardone, J.; Tanasa, B.; Iuga, A.; Srikanth, S.; Okamura, H.; Bolton, D.; Feske, S.; Hogan, P.G.; et al. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature 2006, 441, 646–650. [Google Scholar] [CrossRef]
- Zhang, Q.; Muller, M.; Chen, C.H.; Zeng, L.; Farooq, A.; Zhou, M.M. New insights into the catalytic activation of the MAPK phosphatase PAC-1 induced by its substrate MAPK ERK2 binding. J. Mol. Biol. 2005, 354, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, K.L.; Brummer, T.; Rolph, M.S.; Liu, S.M.; Callejas, N.A.; Grumont, R.J.; Gillieron, C.; Mackay, F.; Grey, S.; Camps, M.; et al. Positive regulation of immune cell function and inflammatory responses by phosphatase PAC-1. Nat. Immunol. 2006, 7, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Kagiya, T.; Nakamura, S. Expression profiling of microRNAs in RAW264.7 cells treated with a combination of tumor necrosis factor alpha and RANKL during osteoclast differentiation. J. Periodontal Res. 2013, 48, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shan, Z.; Ma, J.; Wang, Q.; Chu, J.; Xu, P.; Qin, A.; Fan, S. Validation of downregulated microRNAs during osteoclast formation and osteoporosis progression. Mol. Med. Rep. 2016, 13, 2273–2280. [Google Scholar] [CrossRef] [Green Version]
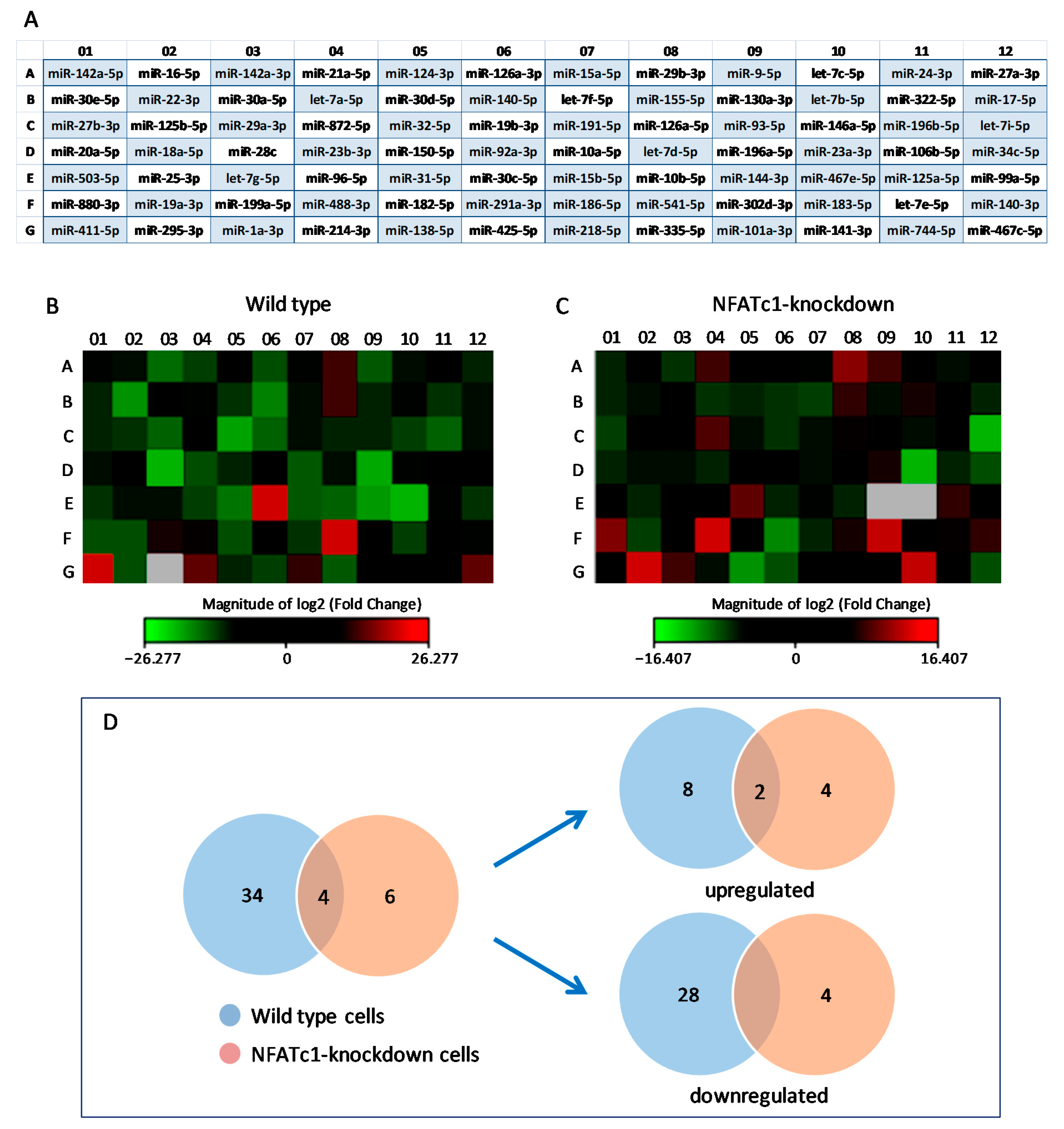


| Upregulated (Group 1) | Downregulated (Group 2) | ||
|---|---|---|---|
| miRNA | Fold Change | miRNA | Fold Change |
| miR-30c-5p | +690.085 | miR-142a-3p | −3.285 |
| miR-541-5p | +128.86 | miR-21a-5p | −2.045 |
| miR-411-5p | +169889.4 | miR-126a-3p | −2.355 |
| miR-214-3p | +3.45 | miR-9-5p | −2.385 |
| miR-467c-5p | +3.53 | miR-22-3p | −8.17 |
| miR-29b-3p | +3.23 | miR-140-5p | −4.94 |
| miR-199a-5p | +2.46 | miR-29a-3p | −2.87 |
| miR-488-3p | +2.02 | miR-32-5p | −14.34 |
| miR-218-5p | +2.83 | miR-19b-3p | −2.905 |
| miR-155-5p | +3.33 | miR-146a-5p | −2.01 |
| miR-196b-5p | −2.92 | ||
| miR-28c | −3,252,400 | ||
| miR-23b-3p | −2.135 | ||
| miR-10a-5p | −2.7 | ||
| miR-196a-5p | −18.89 | ||
| miR-96-5p | −2.01 | ||
| miR-31-5p | −3.91 | ||
| miR-15b-5p | −2.395 | ||
| miR-10b-5p | −3.01 | ||
| miR-144-3p | −10.48 | ||
| miR-467e-5p | −15,364,809 | ||
| miR-880-3p | −2.13 | ||
| miR-19a-3p | −2.28 | ||
| miR-182-5p | −2.15 | ||
| miR-183-5p | −2.035 | ||
| miR-295-3p | −2.33 | ||
| miR-425-5p | −2.01 | ||
| miR-335-5p | −2.56 | ||
| 10 | 28 | ||
| Upregulated (Group 3) | Downregulated (Group 4) | ||
|---|---|---|---|
| miRNA | Fold Change | miRNA | Fold Change |
| miR-29b-3p | +2.78 | miR-7i-5p | −10,459.28 |
| miR-880-3p | +2.54 | miR-23a-3p | −86,904.84 |
| miR-488-3p | +16.21 | miR-291a-3p | −3.65 |
| miR-302d-3p | +7.36 | miR-138-5p | −4.23 |
| miR-295-3p | +18.97 | ||
| miR-141-3p | +6.83 | ||
| 6 | 4 | ||
| KEGG Pathway | p-Value | # Genes | # miRNAs |
|---|---|---|---|
| Signal Transduction | |||
| Group 1 | |||
| MAPK signaling pathway (04010) | 0.013507337 | 63 | 8 |
| Group 2 | |||
| MAPK signaling pathway (04010) | 0.000171124 | 105 | 26 |
| Group 3 | |||
| ECM–receptor interaction (04512) | 2.38994017785 × 10−14 | 4 | 4 |
| MAPK signaling pathway (04010) | 0.013613431 | 50 | 6 |
| Group 4 | |||
| MAPK signaling pathway (04010) | 2.61806457963 × 10−5 | 70 | 4 |
| Organismal systems | |||
| Group 1 | |||
| Osteoclast differentiation (04380) | 0.02547096 | 36 | 6 |
| Group 2 | |||
| Osteoclast differentiation (04380) | 0.034318254 | 50 | 23 |
| Cellular processes | |||
| Group 1 | |||
| Regulation of actin cytoskeleton (04810) | 0.000242141 | 64 | 7 |
| Focal adhesion (04510) | 0.022609292 | 53 | 7 |
| Group 2 | |||
| Regulation of actin cytoskeleton (04810) | 8.6120987 ×10−6 | 93 | 24 |
| Focal adhesion (04510) | 0.000412213 | 89 | 24 |
| Group 3 | |||
| Focal adhesion (04510) | 2.43074477409 ×10−5 | 53 | 6 |
| Group 4 | |||
| Regulation of actin cytoskeleton (04810) | 0.004173186 | 57 | 4 |
| Focal adhesion (04510) | 0.026505552 | 52 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, R.; Zito, F.; Lampiasi, N. MiRNAs Expression Profiling in Raw264.7 Macrophages after Nfatc1-Knockdown Elucidates Potential Pathways Involved in Osteoclasts Differentiation. Biology 2021, 10, 1080. https://doi.org/10.3390/biology10111080
Russo R, Zito F, Lampiasi N. MiRNAs Expression Profiling in Raw264.7 Macrophages after Nfatc1-Knockdown Elucidates Potential Pathways Involved in Osteoclasts Differentiation. Biology. 2021; 10(11):1080. https://doi.org/10.3390/biology10111080
Chicago/Turabian StyleRusso, Roberta, Francesca Zito, and Nadia Lampiasi. 2021. "MiRNAs Expression Profiling in Raw264.7 Macrophages after Nfatc1-Knockdown Elucidates Potential Pathways Involved in Osteoclasts Differentiation" Biology 10, no. 11: 1080. https://doi.org/10.3390/biology10111080
APA StyleRusso, R., Zito, F., & Lampiasi, N. (2021). MiRNAs Expression Profiling in Raw264.7 Macrophages after Nfatc1-Knockdown Elucidates Potential Pathways Involved in Osteoclasts Differentiation. Biology, 10(11), 1080. https://doi.org/10.3390/biology10111080








