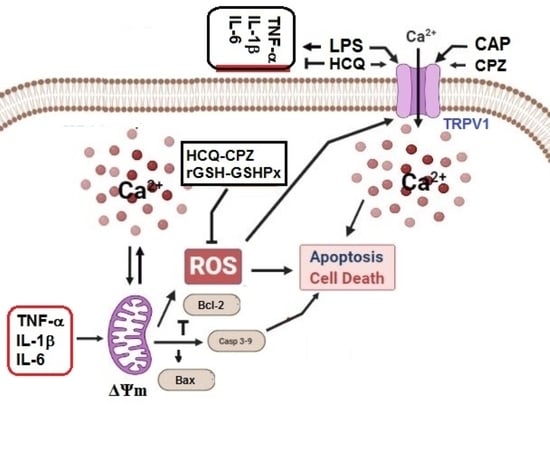
Hydroxychloroquine Attenuates Acute Inflammation (LPS)-Induced Apoptosis via Inhibiting TRPV1 Channel/ROS Signaling Pathways in Human Monocytes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. The Experimental Groups
2.3. The Analyses of Cell Viability, Cell Count, Debris Amount, and Cell Volume
2.4. Western Blot Bands
2.5. The Determination of Apoptosis, Cell Viability, CAS3, and CAS9
2.6. The Measurements of Mitochondria Membrane Potential (ΔΨm) and Cytosolic ROS (cROS) Levels
2.7. The Analysis of Reduced Glutathione (rGSH), Glutathione Peroxidase (GSHPx), and Lipid Peroxidation (MDA)
2.8. The Fluorescence Intensity Determination of cCa2+ Concentration
2.9. The Current Records of Electrophysiology (Patch-Clamp)
2.10. The Analyses of Cytokines using ELISA
2.11. Statistical Analysis
3. Results
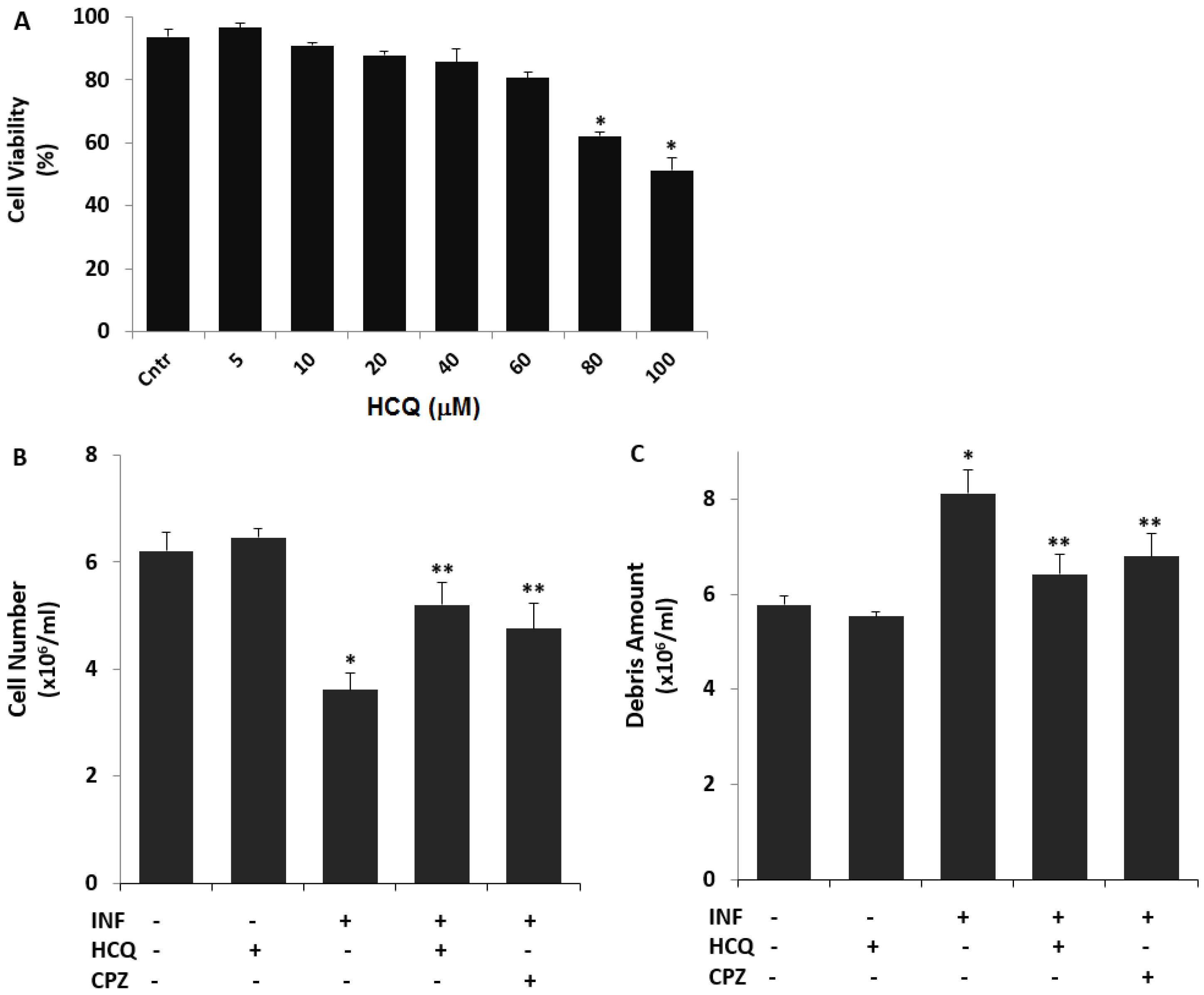
3.1. The Nontoxic Concentration of HCQ in the U937 Cell Line
3.2. The Acute INF-Induced Changes of Cell Number, Debris Amount, and Cell Volume Levels in the U937 Cells were Modulated by the Treatment of HCQ
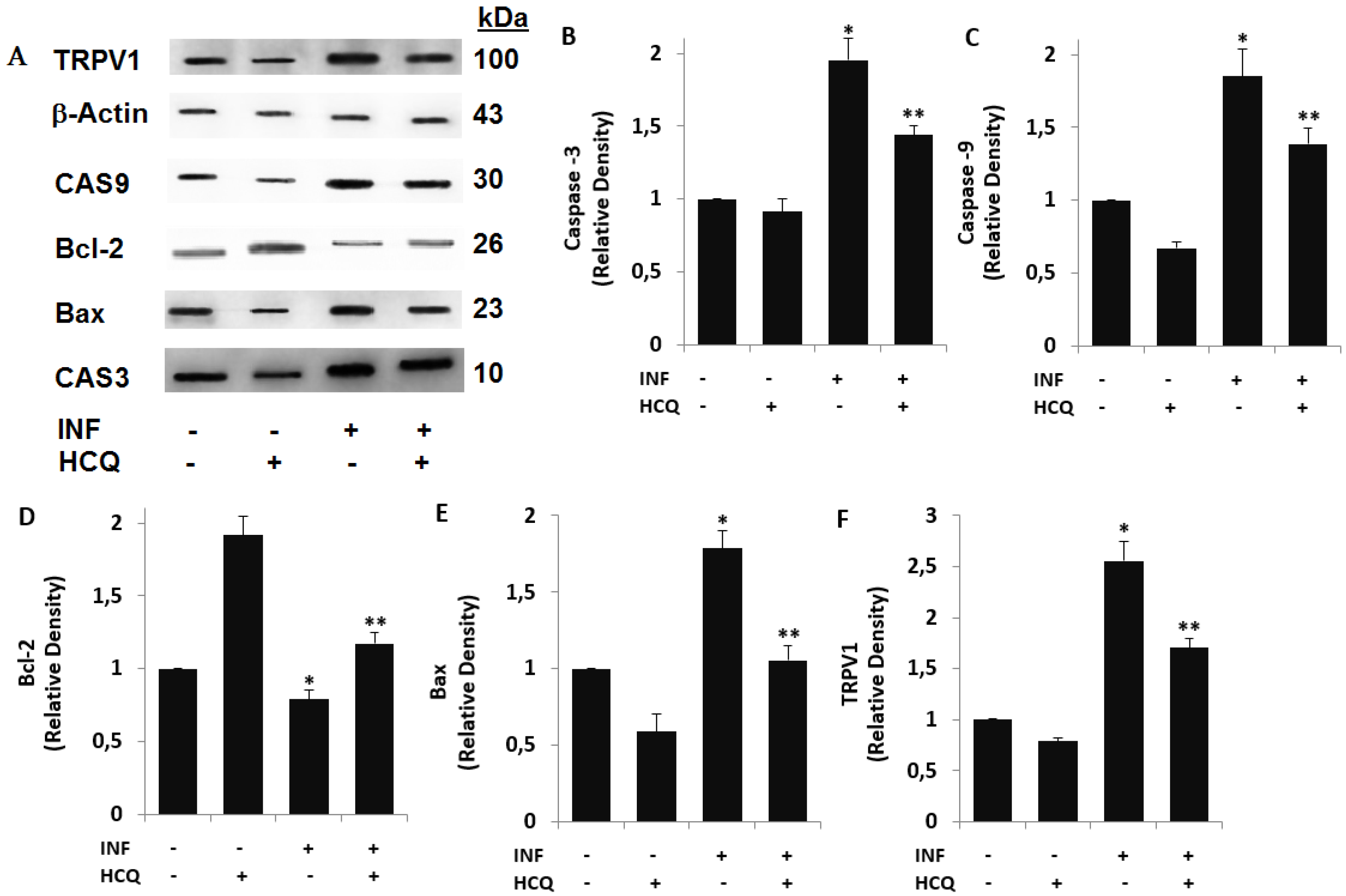
3.3. Acute INF-Induced Expression Changes of Apoptotic Markers and TRPV1 in U937 were Modulated by the HCQ Treatment
3.4. The INF-Mediated Upregulation of Caspase, Apoptosis, JC1, cROS, and MDA Levels in the U- 937 Monocytes was Diminished via Ipregulation of rGSH and GSHPx by the HCQ and CPZ Treatments
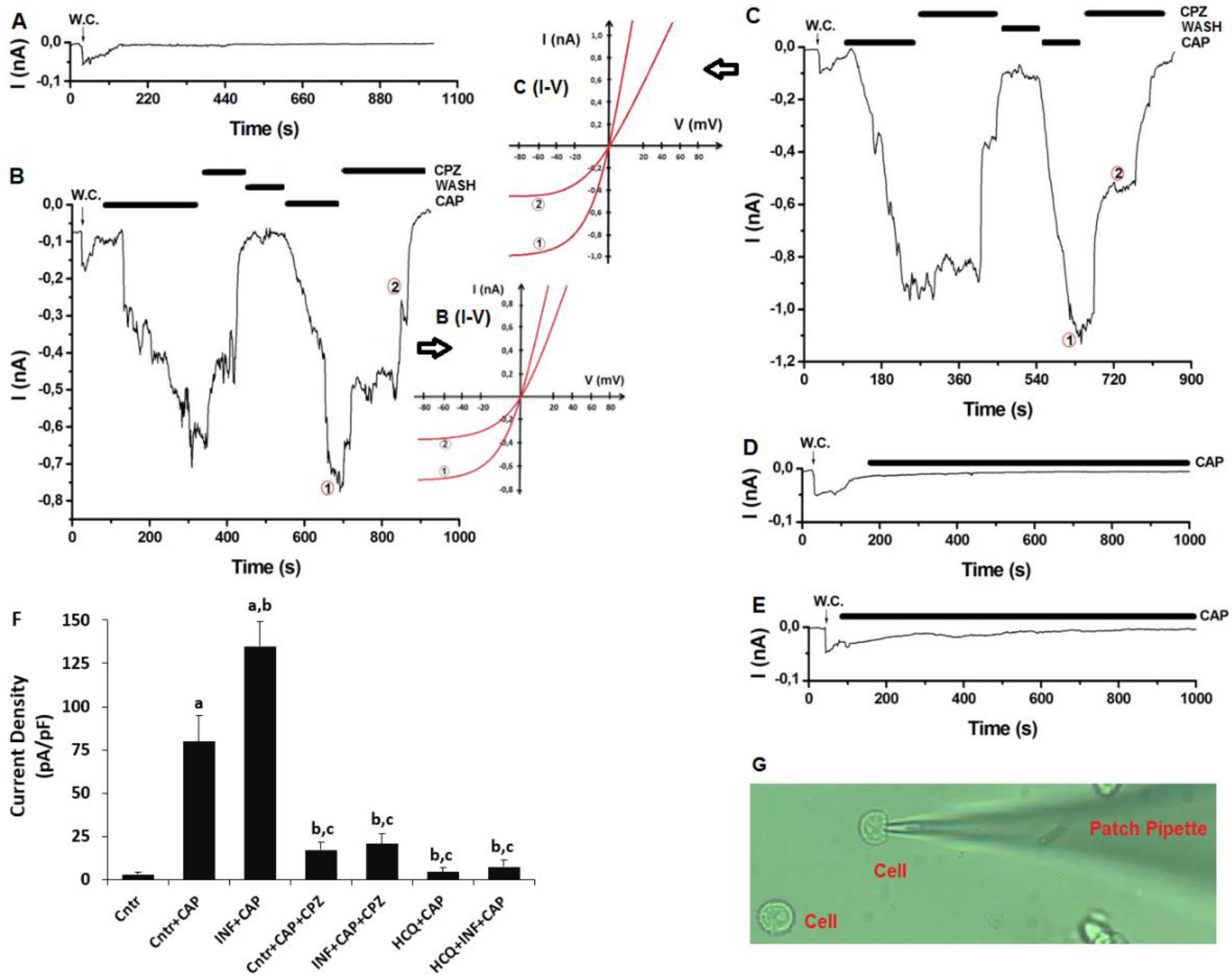
3.5. Acute INF-Mediated Increases in Ca2+ Fluorescence Intensity were Diminished via the Modulation of TRPV1 in U937 by HCQ and CPZ Treatments
3.6. The Acute INF-Caused Upregulation of TRPV1 Current Densities was Downregulated in U937 Monocytes by HCQ Treatment
3.7. Acute INF-Induced TRPV1 Stimulation Results in Cytokine Responses in U937 Monocytes: The Modulator Role of HCQ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAP | capsaicin |
| CAS3 | caspase -3 |
| CAS9 | caspase -9 |
| cCa2+ | cytosolic free Ca2+ |
| CPZ | capsazepine |
| cROS | cytosolic oxygen free radical species |
| HCQ | hydroxychloroquine |
| INF | inflammation |
| LPS | lipopolysaccharide |
| mROS | mitochondrial oxygen free radical products |
| MS | multiple sclerosis |
| NO | nitric oxide |
| OS | oxidative stress |
| SLE | lupus erythematosus |
| TRP | transient receptor potential |
| TRPV1 | Transient receptor potential 1 |
| ΔΨm | mitochondria membrane potential |
References
- Webb, R.; Hughes, M.G.; Thomas, A.W.; Morris, K. The Ability of Exercise-Associated Oxidative Stress to Trigger Redox-Sensitive Signalling Responses. Antioxidants 2017, 6, 63. [Google Scholar] [CrossRef]
- Köse, S.A.; Nazıroğlu, M. N-acetyl cysteine reduces oxidative toxicity, apoptosis, and calcium entry through TRPV1 channels in the neutrophils of patients with polycystic ovary syndrome. Free Radic Res. 2015, 49, 338–346. [Google Scholar] [CrossRef]
- El Ali, Z.; Ollivier, A.; Manin, S.; Rivard, M.; Motterlini, R.; Foresti, R. Therapeutic effects of CO-releaser/Nrf2 activator hybrids (HYCOs) in the treatment of skin wound, psoriasis and multiple sclerosis. Redox Biol. 2020, 34, 101521. [Google Scholar] [CrossRef]
- Yin, N.; Gao, Q.; Tao, W.; Chen, J.; Bi, J.; Ding, F.; Wang, Z. Paeoniflorin relieves LPS-induced inflammatory pain in mice by inhibiting NLRP3 inflammasome activation via transient receptor potential vanilloid 1. J. Leukoc. Biol. 2020, 108, 229–241. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y. The Role of Nociceptive Neurons in the Pathogenesis of Psoriasis. Front. Immunol. 2020, 11, 1984. [Google Scholar] [CrossRef]
- Stampanoni Bassi, M.; Gentile, A.; Iezzi, E.; Zagaglia, S.; Musella, A.; Simonelli, I.; Gilio, L.; Furlan, R.; Finardi, A.; Marfia, G.A.; et al. Transient Receptor Potential Vanilloid 1 Modulates Central Inflammation in Multiple Sclerosis. Front. Neurol. 2019, 10, 30. [Google Scholar] [CrossRef]
- Kim, Y.; Eom, J.-I.; Jeung, H.-K.; Jang, J.E.; Kim, J.S.; Cheong, J.-W.; Kim, Y.S.; Min, Y.H. Induction of cytosine arabinoside-resistant human myeloid leukemia cell death through autophagy regulation by hydroxychloroquine. Biomed. Pharmacother. 2015, 73, 87–96. [Google Scholar] [CrossRef]
- Chen, D.; Xie, J.; Fiskesund, R.; Dong, W.; Liang, X.; Lv, J.; Jin, X.; Liu, J.; Mo, S.; Zhang, T.; et al. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat. Commun. 2018, 9, 873. [Google Scholar] [CrossRef]
- Neuman, M.G.; Maor, Y.; Nanau, R.M.; Melzer, E.; Mell, H.; Opris, M.; Cohen, L.; Malnick, S. Alcoholic Liver Disease: Role of Cytokines. Biomolecules 2015, 5, 2023–2034. [Google Scholar] [CrossRef]
- Ferro, D.; Baratta, F.; Pastori, D.; Cocomello, N.; Colantoni, A.; Angelico, F.; Del Ben, M. New Insights into the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Gut-Derived Lipopolysaccharides and Oxidative Stress. Nutrients 2020, 12, 2762. [Google Scholar] [CrossRef]
- Fock, E.; Parnova, R. Protective Effect of Mitochondria-Targeted Antioxidants against Inflammatory Response to Lipopolysaccharide Challenge: A Review. Pharmaceutics 2021, 13, 144. [Google Scholar] [CrossRef]
- SanGiovanni, E.; Di Lorenzo, C.; Piazza, S.; Manzoni, Y.; Brunelli, C.; Fumagalli, M.; Magnavacca, A.; Martinelli, G.; Colombo, F.; Casiraghi, A.; et al. Leaf Extract Inhibits In Vitro Mediators of Inflammation and Oxidative Stress Involved in Inflammatory-Based Skin Diseases. Antioxidants 2019, 8, 134. [Google Scholar] [CrossRef]
- Conde, C.; Escribano, B.; Luque, E.; Aguilar-Luque, M.; Feijóo, M.; Ochoa, J.; Latorre, M.; Giraldo, A.; Lillo, R.; Aguera-Morales, E.; et al. The protective effect of extra-virgin olive oil in the experimental model of multiple sclerosis in the rat. Nutr. Neurosci. 2018, 23, 37–48. [Google Scholar] [CrossRef]
- Liu, S.T.; Wang, C.R.; Yin, G.D.; Liu, M.F.; Lee, G.L.; Chen, M.Y.; Chuang, C.Y.; Chen, C.Y. Hydroxychloroquine sulphate inhibits in vitro apoptosis of circulating lymphocytes in patients with systemic lupus erythematosus. Asian Pac. J. Allergy Immunol. 2001, 19, 29–35. [Google Scholar] [CrossRef]
- Rainsford, K.D.; Parke, A.L.; Clifford-Rashotte, M.; Kean, W.F. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 2015, 23, 231–269. [Google Scholar] [CrossRef]
- Boechat, N.; Carvalho, R.C.; Ferreira, M.D.L.G.; Coutinho, J.P.; Sa, P.M.; Seito, L.N.; Rosas, E.C.; Krettli, A.U.; Bastos, M.M.; Pinheiro, L.C. Antimalarial and anti-inflammatory activities of new chloroquine and primaquine hybrids: Targeting the blockade of malaria parasite transmission. Bioorganic. Med. Chem. 2020, 28, 115832. [Google Scholar] [CrossRef]
- Legssyer, R.; Josse, C.; Piette, J.; Ward, R.J.; Crichton, R.R. Changes in function of iron-loaded alveolar macrophages after in vivo administration of desferrioxamine and/or chloroquine. J. Inorg. Biochem. 2003, 94, 36–42. [Google Scholar] [CrossRef]
- Paraje, M.G.; Barnes, A.I.; Albesa, I. An Enterobacter cloacae toxin able to generate oxidative stress and to provoke dose-dependent lysis of leukocytes. Int. J. Med. Microbiol. 2005, 295, 109–116. [Google Scholar] [CrossRef]
- Chen, T.-H.; Chang, P.-C.; Chang, M.-C.; Lin, Y.-F.; Lee, H.-M. Chloroquine induces the expression of inducible nitric oxide synthase in C6 glioma cells. Pharmacol. Res. 2005, 51, 329–336. [Google Scholar] [CrossRef]
- Kang, J.M.; Lee, H.-S.; Kim, J.; Yang, D.H.; Jeong, H.Y.; Lee, Y.H.; Kim, D.-J.; Park, S.H.; Sung, M.; Kim, J.; et al. Beneficial Effect of Chloroquine and Amodiaquine on Type 1 Diabetic Tubulopathy by Attenuating Mitochondrial Nox4 and Endoplasmic Reticulum Stress. J. Korean Med. Sci. 2020, 35, e305. [Google Scholar] [CrossRef]
- Dai, C.; Xiao, X.; Li, D.; Tun, S.; Wang, Y.; Velkov, T.; Tang, S. Chloroquine ameliorates carbon tetrachloride-induced acute liver injury in mice via the concomitant inhibition of inflammation and induction of apoptosis. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Wong, S.K. Repurposing New Use for Old Drug Chloroquine against Metabolic Syndrome: A Review on Animal and Human Evidence. Int. J. Med. Sci. 2021, 18, 2673–2688. [Google Scholar] [CrossRef]
- Uslusoy, F.; Nazıroğlu, M.; Çiğ, B. Inhibition of the TRPM2 and TRPV1 Channels through Hypericum perforatum in Sciatic Nerve Injury-induced Rats Demonstrates their Key Role in Apoptosis and Mitochondrial Oxidative Stress of Sciatic Nerve and Dorsal Root Ganglion. Front. Physiol. 2017, 8, 335. [Google Scholar] [CrossRef]
- Akpınar, H.; Nazıroğlu, M.; Övey, I.S.; Çiğ, B.; Akpınar, O. The neuroprotective action of dexmedetomidine on apoptosis, calcium entry and oxidative stress in cerebral ischemia-induced rats: Contribution of TRPM2 and TRPV1 channels. Sci. Rep. 2016, 6, 37196. [Google Scholar] [CrossRef]
- Köse, S.A.; Nazıroğlu, M. Selenium Reduces Oxidative Stress and Calcium Entry Through TRPV1 Channels in the Neutrophils of Patients with Polycystic Ovary Syndrome. Biol. Trace Element Res. 2014, 158, 136–142. [Google Scholar] [CrossRef]
- Walker, J.; Ley, J.P.; Schwerzler, J.; Lieder, B.; Beltran, L.; Ziemba, P.M.; Hatt, H.; Hans, J.; Widder, S.; Krammer, G.E.; et al. Nonivamide, a capsaicin analogue, exhibits anti-inflammatory properties in peripheral blood mononuclear cells and U-937 macrophages. Mol. Nutr. Food Res. 2017, 61, 1600474. [Google Scholar] [CrossRef]
- Nazıroglu, M. Psychiatric Disorders and TRP Channels: Focus on Psychotropic Drugs. Curr. Neuropharmacol. 2015, 13, 248–257. [Google Scholar] [CrossRef]
- Naziroğlu, M. Molecular role of catalase on oxidative stress-induced Ca2+ signaling and TRP cation channel activation in nervous system. J. Recept. Signal Transduct. 2012, 32, 134–141. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Hohmann, M.S.; Borghi, S.M.; Zarpelon, A.C.; Guazelli, C.F.; Manchope, M.F.; Casagrande, R.; Verri, W.A. Protective effects of the flavonoid hesperidin methyl chalcone in inflammation and pain in mice: Role of TRPV1, oxidative stress, cytokines and NF-κB. Chem. Interact. 2015, 228, 88–99. [Google Scholar] [CrossRef]
- Jian, T.; Chen, J.; Ding, X.; Lv, H.; Li, J.; Wu, Y.; Ren, B.; Tong, B.; Zuo, Y.; Su, K.; et al. Flavonoids isolated from loquat (Eriobotrya japonica) leaves inhibit oxidative stress and inflammation induced by cigarette smoke in COPD mice: The role of TRPV1 signaling pathways. Food Funct. 2020, 11, 3516–3526. [Google Scholar] [CrossRef]
- Bok, E.; Chung, Y.C.; Kim, K.-S.; Baik, H.H.; Shin, W.-H.; Jin, B.K. Modulation of M1/M2 polarization by capsaicin contributes to the survival of dopaminergic neurons in the lipopolysaccharide-lesioned substantia nigra in vivo. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Omari, S.A.; Adams, M.J.; Kunde, D.A.; Geraghty, D.P. Capsaicin-Induced Death of Human Haematological Malignant Cell Lines Is Independent of TRPV1 Activation. Pharmacology 2016, 98, 79–86. [Google Scholar] [CrossRef]
- Kunde, D.A.; Yingchoncharoen, J.; Jurković, S.; Geraghty, D.P. TRPV1 mediates capsaicin-stimulated metabolic activity but not cell death or inhibition of interleukin-1β release in human THP-1 monocytes. Toxicol. Appl. Pharmacol. 2018, 360, 9–17. [Google Scholar] [CrossRef]
- Wehrhahn, J.; Kraft, R.; Harteneck, C.; Hauschildt, S. Transient receptor potential melastatin 2 is required for lipopolysaccharide-induced cytokine production in human monocytes. J. Immunol. 2010, 184, 2386–2393. [Google Scholar] [CrossRef]
- Chai, Y.; Cao, Z.; Yu, R.; Liu, Y.; Yuan, D.; Lei, L. Dexmedetomidine Attenuates LPS-Induced Monocyte-Endothelial Adherence via Inhibiting Cx43/PKC-α/NOX2/ROS Signaling Pathway in Monocytes. Oxidative Med. Cell. Longev. 2020, 2020, 2930463. [Google Scholar] [CrossRef]
- Güzel, M.; Nazıroğlu, M.; Akpınar, O.; Çınar, R. Interferon Gamma-Mediated Oxidative Stress Induces Apoptosis, Neuroinflammation, Zinc Ion Influx, and TRPM2 Channel Activation in Neuronal Cell Line: Modulator Role of Curcumin. Inflammation 2021, 1–17. [Google Scholar] [CrossRef]
- Akpınar, O.; Özşimşek, A.; Güzel, M.; Nazıroğlu, M. Clostridium botulinum neurotoxin A induces apoptosis and mitochondrial oxidative stress via activation of TRPM2 channel signaling pathway in neuroblastoma and glioblastoma tumor cells. J. Recept. Signal Transduct. 2020, 40, 620–632. [Google Scholar] [CrossRef]
- Joshi, D.; Bakowska, J.C. Determination of Mitochondrial Membrane Potential and Reactive Oxygen Species in Live Rat Cortical Neurons. J. Vis. Exp. 2011, 51, e2704. [Google Scholar] [CrossRef]
- Ertilav, K. Pregabalin protected cisplatin-induced oxidative neurotoxicity in neuronal cell line. J. Cell. Neurosci. Oxidative Stress 2019, 11, 815–824. [Google Scholar] [CrossRef]
- Woo, H.J.; Jun, D.Y.; Lee, J.Y.; Woo, M.H.; Yang, C.H.; Kim, Y.H. Apoptogenic activity of 2α,3α-dihydroxyurs-12-ene-28-oic acid from Prunella vulgaris var. lilacina is mediated via mitochondria-dependent activation of caspase cascade regulated by Bcl-2 in human acute leukemia Jurkat T cells. J. Ethnopharmacol. 2011, 135, 626–635. [Google Scholar] [CrossRef]
- Priya, A.M.; Jayachandran, S. Induction of apoptosis and cell cycle arrest by Bis (2-ethylhexyl) phthalate produced by marine Bacillus pumilus MB 40. Chem. Interact. 2012, 195, 133–143. [Google Scholar] [CrossRef]
- Nesuashvili, L.; Hadley, S.H.; Bahia, P.K.; Taylor-Clark, T.E. Sensory nerve terminal mitochondrial dysfunction activates airway sensory nerves via transient receptor potential (TRP) channels. Mol. Pharmacol. 2013, 83, 1007-19. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Xu, M.; Zhang, H.; Chen, Y.; Chung, K.F.; Adcock, I.M.; Li, F. Roles of TRPA1 and TRPV1 in cigarette smoke -induced airway epithelial cell injury model. Free Radic Biol. Med. 2019, 134, 229–238. [Google Scholar] [CrossRef]
- Jing, Z.; Sui, X.; Yao, J.; Xie, J.; Jiang, L.; Zhou, Y.; Pan, H.; Han, W. SKF-96365 activates cytoprotective autophagy to delay apoptosis in colorectal cancer cells through inhibition of the calcium/CaMKIIγ/AKT-mediated pathway. Cancer Lett. 2016, 372, 226–238. [Google Scholar] [CrossRef]
- Nazıroğlu, M.; Çiğ, B.; Ozgül, C. Neuroprotection induced by N-acetylcysteine against cytosolic glutathione depletion-induced Ca2+ influx in dorsal root ganglion neurons of mice: Role of TRPV1 channels. Neuroscience 2013, 242, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Övey, I.; Naziroğlu, M. Homocysteine and cytosolic GSH depletion induce apoptosis and oxidative toxicity through cytosolic calcium overload in the hippocampus of aged mice: Involvement of TRPM2 and TRPV1 channels. Neuroscience 2015, 284, 225–233. [Google Scholar] [CrossRef]
- Cui, J.; Li, Z.; Zhuang, S.; Qi, S.; Li, L.; Zhou, J.; Zhang, W.; Zhao, Y. Melatonin alleviates inflammation-induced apoptosis in human umbilical vein endothelial cells via suppression of Ca2+-XO-ROS-Drp1-mitochondrial fission axis by activation of AMPK/SERCA2a pathway. Cell Stress Chaperon 2017, 23, 281–293. [Google Scholar] [CrossRef]
- Suzuki, T.; Kobayashi, M.; Isatsu, K.; Nishihara, T.; Aiuchi, T.; Nakaya, K.; Hasegawa, K. Mechanisms Involved in Apoptosis of Human Macrophages Induced by Lipopolysaccharide from Actinobacillus actinomycetemcomitans in the Presence of Cycloheximide. Infect. Immun. 2004, 72, 1856–1865. [Google Scholar] [CrossRef][Green Version]
- Zheng, H.; Zhang, Y.; He, J.; Yang, Z.; Zhang, R.; Li, L.; Luo, Z.; Ye, Y.; Sun, Q. Hydroxychloroquine Inhibits Macrophage Activation and Attenuates Renal Fibrosis After Ischemia-Reperfusion Injury. Front. Immunol. 2021, 12, 645100. [Google Scholar] [CrossRef]
- Li, M.; Xu, Y.; Lu, W.; Li, Y.; Tan, S.; Lin, H.; Wu, T.; Li, Y.; Wang, S.; Zhao, Y. Chloroquine potentiates the anticancer effect of sunitinib on renal cell carcinoma by inhibiting autophagy and inducing apoptosis. Oncol. Lett. 2017, 15, 2839–2846. [Google Scholar] [CrossRef]
- Guo, R.; Zhou, F.-M.; Su, C.-J.; Liu, T.-T.; Zhou, Y.; Fan, L.; Wang, Z.-H.; Liu, X.; Huang, Y.; Liu, T.; et al. Epigallocatechin-3-gallate attenuates acute and chronic psoriatic itch in mice: Involvement of antioxidant, anti-inflammatory effects and suppression of ERK and Akt signaling pathways. Biochem. Biophys. Res. Commun. 2018, 496, 1062–1068. [Google Scholar] [CrossRef]
- Wu, Y.; You, H.; Ma, P.; Li, L.; Yuan, Y.; Li, J.; Ye, X.; Liu, X.; Yao, H.; Chen, R.; et al. Role of Transient Receptor Potential Ion Channels and Evoked Levels of Neuropeptides in a Formaldehyde-Induced Model of Asthma in Balb/c Mice. PLoS ONE 2013, 8, e62827. [Google Scholar] [CrossRef]
- Griffiths, H.R.; Gao, D.; Pararasa, C. Redox regulation in metabolic programming and inflammation. Redox Biol. 2017, 12, 50–57. [Google Scholar] [CrossRef]
- Hou, X.; Xiao, H.; Zhang, Y.; Zeng, X.; Huang, M.; Chen, X.; Birnbaumer, L.; Liao, Y. Transient receptor potential channel 6 knockdown prevents apoptosis of renal tubular epithelial cells upon oxidative stress via autophagy activation. Cell Death Dis. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Salimi, A.; Pirhadi, R.; Jamali, Z.; Ramazani, M.; Yousefsani, B.S.; Pourahmad, J. Mitochondrial and lysosomal protective agents ameliorate cytotoxicity and oxidative stress induced by cyclophosphamide and methotrexate in human blood lymphocytes. Hum. Exp. Toxicol. 2019, 38, 1266–1274. [Google Scholar] [CrossRef]
- Than, J.; Li, L.; Hasan, R.; Zhang, X. Excitation and Modulation of TRPA1, TRPV1, and TRPM8 Channel-expressing Sensory Neurons by the Pruritogen Chloroquine. J. Biol. Chem. 2013, 288, 12818–12827. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güzel, M.; Akpınar, O. Hydroxychloroquine Attenuates Acute Inflammation (LPS)-Induced Apoptosis via Inhibiting TRPV1 Channel/ROS Signaling Pathways in Human Monocytes. Biology 2021, 10, 967. https://doi.org/10.3390/biology10100967
Güzel M, Akpınar O. Hydroxychloroquine Attenuates Acute Inflammation (LPS)-Induced Apoptosis via Inhibiting TRPV1 Channel/ROS Signaling Pathways in Human Monocytes. Biology. 2021; 10(10):967. https://doi.org/10.3390/biology10100967
Chicago/Turabian StyleGüzel, Mustafa, and Orhan Akpınar. 2021. "Hydroxychloroquine Attenuates Acute Inflammation (LPS)-Induced Apoptosis via Inhibiting TRPV1 Channel/ROS Signaling Pathways in Human Monocytes" Biology 10, no. 10: 967. https://doi.org/10.3390/biology10100967
APA StyleGüzel, M., & Akpınar, O. (2021). Hydroxychloroquine Attenuates Acute Inflammation (LPS)-Induced Apoptosis via Inhibiting TRPV1 Channel/ROS Signaling Pathways in Human Monocytes. Biology, 10(10), 967. https://doi.org/10.3390/biology10100967