Bioactive and Physicochemical Characteristics of Natural Food: Palmyra Palm (Borassus flabellifer Linn.) Syrup
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
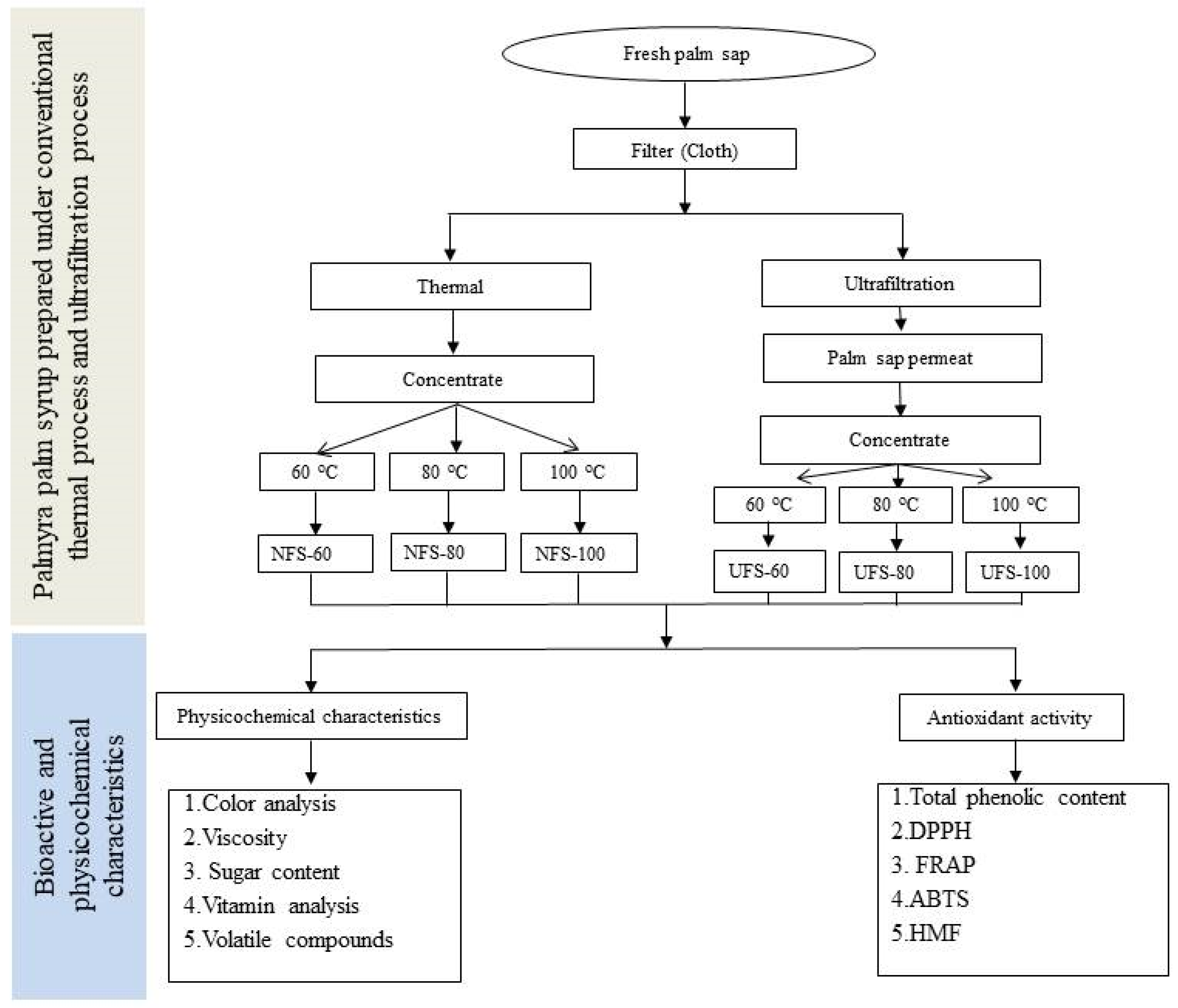
2.1. Preparation of Palmyra Palm Syrup
2.2. Physicochemical Analysis
2.3. Vitamin Analysis
2.4. Volatile Compounds Analysis
2.5. Odor Description
2.6. Determination of Total Phenolic Content
2.7. Determination of DPPH Radical Scavenging Activity
2.8. Determination of Ferric Reducing Antioxidant Power (FRAP)
2.9. ABTS Radical Cation Decolorization Assay
2.10. Determination of HMF Content
2.11. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Characteristics
3.2. Vitamin Composition
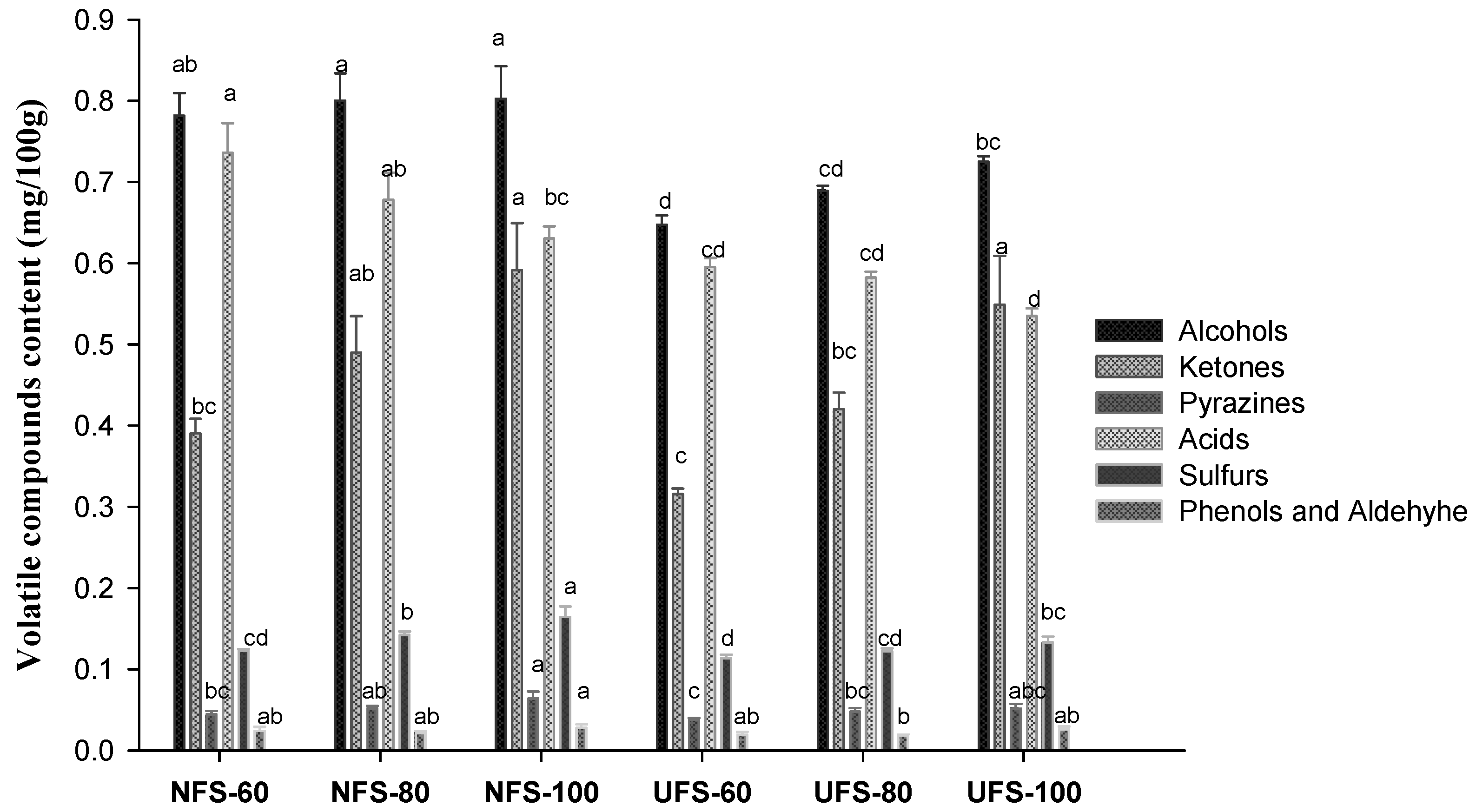
3.3. Volatile Compounds
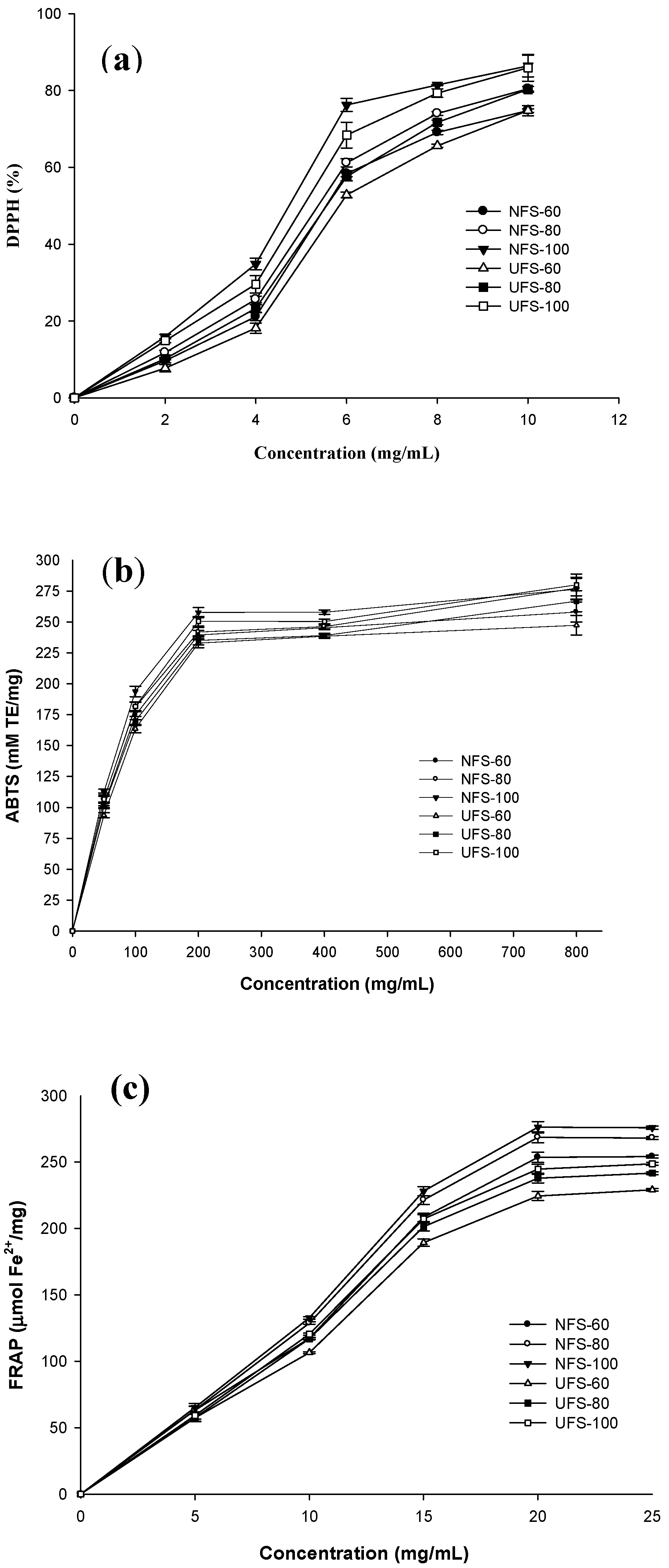
3.4. Antioxidant Acitivities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, T.K. Edible Medicinal and Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Hebbar, K.; Manivannan, A.; Ramarathinam, M.; Mathew, A.; Thamban, C.; Thomas, G.; Chowdappa, P. Coconut inflorescence sap and its value addition as sugar—Collection techniques, yield, properties and market perspective. Curr. Sci. 2015, 109, 1411–1417. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.; Verma, A.K.; Haq, S.I.U.; Mounika, N. Evaluation and determination of antifungal potentials of sap of Borassus flabellifer. J. Pharm. Sci. Biosci. Res. 2017, 7, 111–113. Available online: http://www.jpsbr.org/volume_7/JPSBR_Vol_7_Issue_1_htm_files/JPSBR17RS1014.pdf (accessed on 1 September 2021).
- Yoshikawa, M.; Xu, F.; Morikawa, T.; Pongpiriyadacha, Y.; Nakamura, S.; Asao, Y.; Matsuda, H. Medicinal flowers. XII.1) Naew spirostane-type steroid saponins with antidiabetogenic activity from Borassus flabellifer. Chem. Pharm. Bull. 2007, 55, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Paschapur, M.S.; Patil, M.B.; Kumar, R.; Patil, S.R. Evaluation of anti-inflammatory activity of ethanolic extract of Borassus flabellifer L. male flowers (inflorescences) in experimental animals. J. Med. Plant Res. 2009, 3, 49–54. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.875.7726&rep=rep1&type=pdf (accessed on 1 September 2021).
- Paschapur, M.S.; Patil, S.; Patil, S.R.; Kumar, R.; Patil, M.B. Evaluation of the anagesic and antipyretic activities of ethanolic extract of male folwers (inflorescences) of Borassus flabellifer L. (Arecaceae). Int. J. Pharm. Pharm. Sci. 2009, 1, 98–106. Available online: https://pdfs.semanticscholar.org/8423/5cac7f7bd64de1b7a54a53159a7310f649e5.pdf (accessed on 1 September 2021).
- Saputro, A.D.; Van de Walle, D.; Dewettinck, K. Palm sap sugar: A review. Sugar Tech 2019, 21, 862–867. [Google Scholar] [CrossRef]
- Khongsak, S.; Janya, S.; Wirot, L. Productions and functional properties of palm sugars. Walailak J. Sci. Technol. 2018, 21, 897–907. Available online: http://wjst.wu.ac.th/index.php/wjst/article/view/5323 (accessed on 1 September 2021).
- Naknean, P.; Meenune, M.; Roudaut, G. Changes in properties of palm sugar syrup produced by an open pan and a vacuum evaporator during storage. Int. Food Res. J. 2013, 20, 2323–2334. Available online: http://ifrj.upm.edu.my/20%20(05)%202013/38%20IFRJ%2020%20(05)%202013%20Meeune%20214.pdf (accessed on 1 September 2021).
- Naknean, P.; Meenune, M. Impact of clarification of palm sap and processing method on the quality of palm sugar syrup (Borassus flabellifer Linn.). Sugar Tech 2015, 17, 195–203. [Google Scholar] [CrossRef]
- Reshma, M.V.; Jacob, J.; Syamnath, V.L.; Habeeba, V.P.; Dileep Kumar, B.S.; Lankalapalli, R.S. First report on isolation of 2,3,4-trihydroxy-5-methylacetophenone from palmyra palm (Borassus flabellifer Linn.) syrup, its antioxidant and antimicrobial properties. Food Chem. 2017, 228, 491–496. [Google Scholar] [CrossRef]
- Luis, G.; Rubio, C.; Gutiérrez, A.; Hernandez, C.; González-Weller, D.; Revert, C.; Hardisson, A. Palm tree syrup: Nutritional composition of a natural edulcorant. Nutr. Hosp. 2012, 27, 548–552. [Google Scholar] [CrossRef]
- Girard, B.; Fukumoto, L.R. Apple juice clarification using microfiltration and ultrafiltration polymeric membranes. LWT Food Sci. Technol. 1999, 32, 290–298. [Google Scholar] [CrossRef]
- Youn, K.S.; Hong, J.H.; Bae, D.H.; Kim, S.J.; Kim, S.D. Effective clarifying process of reconstituted apple juice using membrane filtration with filter-aid pretreatment. J. Membr. Sci. 2004, 228, 179–186. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Ng, C.Y.; Lim, Y.P.; Ng, G.H. Ultrafiltration in food processing industry: Review on application, membrane fouling, and fouling control. Food Bioprocess Technol. 2012, 5, 1143–1156. [Google Scholar] [CrossRef]
- Makhlouf-Gafsi, I.; Baklouti, S.; Mokni, A.; Danthine, S.; Attia, H.; Blecker, C.; Masmoudi, M. Effect of ultrafiltration process on physico-chemical, rheological, microstructure and thermal properties of syrups from male and female date palm saps. Food Chem. 2016, 203, 175–182. [Google Scholar] [CrossRef]
- Naknean, P.; Meenune, M. Characteristics and antioxidant activity of palm sugar syrup produced in Songkhla Province, Southern Thailand. Asian J. Food Agro-Ind. 2011, 4, 204–212. Available online: http://www.ajofai.info/Abstract/Characteristics%20and%20antioxidant%20activity%20of%20palm%20sugar%20syrup%20produced%20in%20songkhla%20province,%20southern%20thailand.pdf (accessed on 1 September 2021).
- Nielsen, S.S. (Ed.) Total carbohydrate by phenol-sulfuric acid method. In Food Analysis Laboratory Manual; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Rizzolo, A.; Polesello, S. Chromatographic determination of vitamins in foods. J. Chromatogr. A 1992, 624, 103–152. [Google Scholar] [CrossRef]
- Antakli, S.S.N.; Sarraf, T. Determination of water-soluble vitamins B1, B2, B3, B6, B9, B12 and C on C18 column with particle size 3 µM in some manufactured food products b HPLC with UV-DAD/FLD detection. Int. J. Pharm. Pharm. Sci. 2015, 7, 219–224. Available online: https://innovareacademics.in/journals/index.php/ijpps/article/view/4465 (accessed on 1 September 2021).
- Huynh Thi Le, D.; Lu, W.C.; Li, P.H. Sustainable processes and chemical characterization of natural food additives: Palmyra palm (Borassus flabellifer Linn.) granulated sugar. Sustainability 2020, 12, 2650. Available online: https://www.mdpi.com/2071-1050/12/7/2650 (accessed on 1 September 2021). [CrossRef] [Green Version]
- Asikin, Y.; Hirose, N.; Tamaki, H.; Ito, S.; Oku, H.; Wada, K. Effects of different drying–solidification processes on physical properties, volatile fraction, and antioxidant activity of non-centrifugal cane brown sugar. LWT Food Sci. Technol. 2016, 66, 340–347. [Google Scholar] [CrossRef]
- Lee, J.S.; Ramalingam, S.; Jo, I.G.; Kwon, Y.S.; Bahuguna, A.; Oh, Y.S.; Kim, M. Comparative study of the physicochemical, nutritional, and antioxidant properties of some commercial refined and non-centrifugal sugars. Food Res. Int. 2018, 109, 614–625. [Google Scholar] [CrossRef]
- Phillips, K.M.; Carlsen, M.H.; Blomhoff, R. Total antioxidant content of alternatives to refined sugar. J. Am. Diet. Assoc. 2009, 109, 64–71. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C. In vitro antioxidant activities and polyphenol contents of seven commercially available fruits. Pharmacogn. Res. 2016, 8, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Payet, B.; Shum Cheong Sing, A.; Smadja, J. Assessment of antioxidant activity of cane brown sugars by ABTS and DPPH radical scavenging assays: Determination of their polyphenolic and volatile constituent. J. Agric. Food Chem. 2005, 53, 10074–10079. [Google Scholar] [CrossRef]
- de Bruijn, J.P.F.; Venegas, A.; Martínez, J.A.; Bórquez, R. Ultrafiltration performance of Carbosep membranes for the clarification of apple juice. LWT Food Sci. Technol. 2003, 36, 397–406. [Google Scholar] [CrossRef]
- Cliff, M.A.; Fukumoto, L.R.; King, M.C.; Edwards, B.J.; Girard, B. Sensory and physio-chemical properties of membrance filtered apple juices. J. Food Qual. 2000, 23, 171–184. [Google Scholar] [CrossRef]
- Phetrit, R.; Chaijan, M.; Sorapukdee, S.; Panpipat, W. Characterization of nipa palm’s (Nypa fruticans Wurmb.) sap and syrup as functional food Ingredients. Sugar Tech. 2020, 22, 191–201. [Google Scholar] [CrossRef]
- Makhlouf-Gafsi, I.; Krichen, F.; Mansour, R.B.; Mokni, A.; Sila, A.; Bougatef, A.; Besbes, S. Ultrafiltration and thermal processing effects on Maillard reaction products and biological properties of date palm sap syrups (Phoenix dactylifera L.). Food Chem. 2018, 256, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Abbès, F.; Besbes, S.; Brahim, B.; Kchaou, W.; Attia, H.; Blecker, C. Effect of concentration temperature on some bioactive compounds and antioxidant proprieties of date syrup. Food Sci. Technol. Int. 2013, 19, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Sakač, M.J.P.; Petrović, J.; Pezo, L.; Fišteš, A.; Lončarević, I.; Pajin, B. Hydroxymethylfurfural content and colour parameters of cookies with defatted wheat germ. Czech J. Food Sci. 2019, 37, 285–291. [Google Scholar] [CrossRef]
- Nie, S.; Huang, J.; Hu, J.; Zhang, Y.; Wang, S.; Li, C.; Xie, M. Effect of pH, temperature and heating time on the formation of furan in sugar–glycine model systems. Food Sci. Hum. Wellness 2013, 2, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Billaud, C.; Brun-Mérimée, S.; Louarme, L.C.; Nicolas, J. Effect of glutathione and Maillard reaction products prepared from glucose or fructose with glutathione on polyphenoloxidase from apple—I: Enzymatic browning and enzyme activity inhibition. Food Chem. 2004, 84, 223–233. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Soria, A.C.; Sanz, M.L.; Martínez-Castro, I. Characterization of traditional Spanish edible plant syrups based on carbohydrate GC–MS analysis. J. Food Compos. Anal. 2010, 23, 260–263. [Google Scholar] [CrossRef]
- de Carvalho, L.M.J.; de Castro, I.M.; da Silva, C.A.B. A study of retention of sugars in the process of clarification of pineapple juice (Ananas comosus, L. Merril) by micro- and ultra-filtration. J. Food Eng. 2008, 87, 447–454. [Google Scholar] [CrossRef]
- Wu, S.; Hu, J.; Wei, L.; Du, Y.; Shi, X.; Zhang, L. Antioxidant and antimicrobial activity of Maillard reaction products from xylan with chitosan/chitooligomer/glucosamine hydrochloride/taurine model systems. Food Chem. 2014, 148, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Z.; Gong, P.F.; Lu, R.R.; Zhang, B.; Morata, A.; Han, S.Y. Effect of different clarification treatments on the volatile composition and aromatic attributes of ‘Italian Riesling’ icewine. Molecules 2020, 25, 2657. Available online: https://www.mdpi.com/1420-3049/25/11/2657 (accessed on 1 September 2021). [CrossRef]
- Valder, R.; Nooralabettu, K.P. Physico-chemical changes in Palmyra Palm (Borassus flabellifer) sap at different temperature. Int. J. Sci. Eng. Res. 2018, 9, 761–766. Available online: https://www.ijser.org/onlineResearchPaperViewer.aspx?Physico-chemical-Changes-in-Palmyra-Palm-Borassus-flabellifer-sap-at-different-temperature.pdf (accessed on 1 September 2021).
- Ilame, S.A.; Singh, S.V. Physico-chemical properties of ultrafiltered kinnow (mandarin) fruit juice. J. Food Sci. Technol. 2018, 55, 2189–2196. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Saxena, V.K.; Dutta, S. Analysis of fouling and juice quality in crossflow ultrafiltration of watermelon juice. Food Sci. Technol. 2018, 38, 71–76. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0101-20612018000500071&nrm=iso (accessed on 1 September 2021). [CrossRef] [Green Version]
- Morton, J. Notes on distribution, propagation, and products of Borassus Palms (Arecaceae). Econ. Bot. 1988, 42, 420–441. [Google Scholar] [CrossRef]
- Borse, B.B.; Rao, L.J.M.; Ramalakshmi, K.; Raghavan, B. Chemical composition of volatiles from coconut sap (neera) and effect of processing. Food Chem. 2007, 101, 877–880. [Google Scholar] [CrossRef]
- Johnson, J.R.; Braddock, R.J.; Chen, C.S. Flavor Losses in Orange Juice during Ultrafiltration and Subsequent Evaporation. J. Food Sci. 1996, 61, 540–543. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, H.; Wang, W.; Jiao, W.; Chen, W.; Zhong, Q.; Chen, W. Characterization of Volatile Profiles and Marker Substances by HS-SPME/GC-MS during the Concentration of Coconut Jam. Food 2020, 9, 347. Available online: https://www.mdpi.com/2304-8158/9/3/347 (accessed on 1 September 2021). [CrossRef] [Green Version]
- Ho, C.W.; Aida, W.M.W.; Maskat, M.Y.; Osman, H. Changes in volatile compounds of palm sap (Arenga pinnata) during the heating process for production of palm sugar. Food Chem. 2007, 102, 1156–1162. [Google Scholar] [CrossRef]
- Wu, X.; Kassie, F.; Mersch-Sundermann, V. Induction of apoptosis in tumor cells by naturally occurring sulfur-containing compounds. Mutat. Res. 2005, 589, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, F.; Vainio, H. Isothiocyanates in cancer prevention. Drug Metab. Rev. 2004, 36, 655–667. [Google Scholar] [CrossRef]
- Xiao, H.; Parkin, K.L. Antioxidant functions of selected allium thiosulfinates and S-alk(en)yl-l-cysteine sulfoxides. J. Agric. Food Chem. 2002, 50, 2488–2493. [Google Scholar] [CrossRef]
- Iranshahi, M. A review of volatile sulfur-containing compounds from terrestrial plants: Biosynthesis, distribution and analytical methods. J. Essent. Oil Res. 2012, 24, 393–434. [Google Scholar] [CrossRef]
- Maga, J.A.; Sizer, C.E. Pyrazines in foods. A Review. J. Agric. Food Chem. 1973, 21, 22–30. [Google Scholar] [CrossRef]
- Czerny, M.; Grosch, W. Potent odorants of raw Arabica coffee, their changes during roasting. J. Agric. Food Chem. 2000, 48, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Wan Aida, W.; Ho, C.; Maskat, M.; Osman, H. Relating descriptive sensory analysis to gas chromatography/mass spectrometry of palm sugars using partial least squares regression. ASEAN Food J. 2008, 15, 35–45. Available online: http://www.ifrj.upm.edu.my/afjv15(1)2008/35-45.pdf (accessed on 1 September 2021).
- Naknaen, P.; Meenune, M. Quality profiles of pasteurized palm sap (Borassus flabellifer Linn.) collected from different regions in Thailand. Walailak J. Sci. Technol. 2016, 13, 165–176. Available online: https://www.scopus.com/inward/record.uri?eid=2s2.084957552237&partnerID=40&md5=6019a49a9fd47c07786177005fbcc931 (accessed on 1 September 2021).
- Abbès, F.; Kchaou, W.; Blecker, C.; Ongena, M.; Lognay, G.; Attia, H.; Besbes, S. Effect of processing conditions on phenolic compounds and antioxidant properties of date syrup. Ind. Crops Prod. 2013, 44, 634–642. [Google Scholar] [CrossRef]
- Grabek-Lejko, D.; Tomczyk-Ulanowska, K. Phenolic content, antioxidant and antibacterial activity of selected natural sweeteners available on the Polish market. J. Environ. Sci. Health B 2013, 48, 1089–1096. [Google Scholar] [CrossRef]
- Kongkaew, S.; Chaijan, M.; Riebroy, S. Some characteristics and antioxidant activity of commercial sugars produced in Thailand. Curr. Appl. Sci. Technol. 2014, 14, 1–9. Available online: https://li01.tci-thaijo.org/index.php/cast/article/view/135496 (accessed on 1 September 2021).
- Wiriyaphan, C.; Xiao, H.; Decker, E.A.; Yongsawatdigul, J. Chemical and cellular antioxidative properties of threadfin bream (Nemipterus spp.) surimi byproduct hydrolysates fractionated by ultrafiltration. Food Chem. 2015, 167, 7–15. [Google Scholar] [CrossRef] [PubMed]
| Parameter | NFS-60 | NFS-80 | NFS-100 | UFS-60 | UFS-80 | UFS-100 |
|---|---|---|---|---|---|---|
| L* value | 88.14 ± 0.49 b | 85.25 ± 0.49 cd | 83.85 ± 0.40 e | 92.13 ± 0.22 a | 86.41 ± 0.61 c | 84.48 ± 0.34 de |
| a* value | −2.41 ± 0.38 c | −1.31 ± 0.04 b | −0.58 ± 0.05 a | −5.46 ± 0.36 e | −3.62 ± 0.15 d | −0.80 ± 0.06 ab |
| b* value | 8.31 ± 0.32 b | 6.60 ± 0.40 c | 2.26 ± 0.19 d | 14.32 ± 0.37 a | 7.62 ± 0.40 b | 3.04 ± 0.09 d |
| Viscosity (cP) | 1144.00 ± 45.13 a | 1158.33 ± 34.02 a | 1153.00 ± 37.32 a | 1012.33 ± 15.01 b | 1050.00 ± 16.09 b | 1058.67 ± 13.65 b |
| Total sugar (%) | 73.17 ± 0.89 a | 73.11 ± 0.82 a | 73.02 ± 0.94 a | 73.23 ± 1.07 a | 73.23 ± 0.88 a | 73.13 ± 1.03 a |
| RS (%) | 17.38 ± 0.23 f | 18.31 ± 0.18 e | 18.92 ± 0.13 d | 25.24 ± 0.10 c | 28.27 ± 0.10 b | 30.08 ± 0.07 a |
| Protein (%) | 1.48 ± 0.08 a | 1.30 ± 0.09 ab | 1.13 ± 0.04 b | 0.91 ± 0.06 c | 0.88 ± 0.04 c | 0.77 ± 0.10 c |
| 5-HMF (mg/100 g) | 0.12 ± 0.05 e | 2.15 ± 0.07 c | 14.95 ± 0.11 a | 0.02 ± 0.01 e | 1.13 ± 0.06 d | 6.48 ± 0.24 b |
| TPC (mg GAE/g) | 2.11 ± 0.09 d | 4.44 ± 0.11 b | 5.15 ± 0.12 a | 1.78 ± 0.04 d | 2.84 ± 0.08 c | 4.28 ± 0.57 b |
| Vitamin (per 100 g) | NFS-60 | NFS-80 | NFS-100 | UFS-60 | UFS-80 | UFS-100 |
|---|---|---|---|---|---|---|
| A (mg) | 1.65 ± 0.07 a | 1.64 ± 0.06 a | 1.57 ± 0.06 a | 1.31 ± 0.03 b | 1.28 ± 0.02 b | 1.21 ± 0.04 b |
| B1 (mg) | 0.98 ± 0.04 a | 0.79 ± 0.02 b | 0.60 ± 0.06 d | 0.82 ± 0.04 b | 0.69 ± 0.03 c | 0.54 ± 0.03 e |
| B2 (mg) | 0.11 ± 0.02 a | 0.09 ± 0.01 b | 0.08 ± 0.01 c | 0.06 ± 0.01 d | 0.05 ± 0.01 de | 0.04 ± 0.01 e |
| B3 (mg) | 1.46 ± 0.03 a | 1.39 ± 0.02 b | 1.36 ± 0.03 b | 1.20 ± 0.03 c | 1.18 ± 0.03 cd | 1.13 ± 0.03 d |
| B5 (mg) | 0.43 ± 0.03 a | 0.36 ± 0.04 ab | 0.27 ± 0.02 c | 0.32 ± 0.03 bc | 0.25 ± 0.04 cd | 0.17 ± 0.02 d |
| B6 (mg) | 0.11 ± 0.01 a | 0.11 ± 0.01 a | 0.10 ± 0.01 a | 0.06 ± 0.01 b | 0.06 ± 0.01 b | 0.05 ± 0.01 b |
| Folic acid (μg) | 2.08 ± 0.06 a | 1.76 ± 0.02 b | 1.49 ± 0.07 c | 1.81 ± 0.06 b | 1.50 ± 0.04 c | 1.22 ± 0.06 d |
| C (mg) | 2.85 ± 0.04 a | 2.17 ± 0.02 b | 1.77 ± 0.09 c | 1.88 ± 0.05 c | 1.26 ± 0.05 d | 1.06 ± 0.13 e |
| D2 (mg) | 1.24 ± 0.03 a | 1.11 ± 0.03 ab | 1.04 ± 0.06 bc | 1.01 ± 0.08 bc | 0.95 ± 0.03 c | 0.92 ± 0.03 c |
| E (mg) | 46.87 ± 0.33 a | 46.19 ± 0.06 a | 46.05 ± 0.13 a | 44.06 ± 0.82 b | 44.11 ± 0.14 b | 43.87 ± 0.24 b |
| No. | RI | Compound | Content (mg/100 g) | Odor Description | |||||
|---|---|---|---|---|---|---|---|---|---|
| NFS-60 | NFS-80 | NFS-100 | UFS-60 | UFS-80 | UFS-100 | ||||
| 1 | 931 | Ethanol | 0.184 ± 0.004 ab | 0.200 ± 0.009 a | 0.195 ± 0.011 a | 0.162 ± 0.003 c | 0.167 ± 0.002 bc | 0.171 ± 0.002 bc | Alcoholic, solvent |
| 2 | 1540 | R-(R′,R′)-2,3-butanediol | 0.158 ± 0.002 b | 0.183 ± 0.010 a | 0.176 ± 0.006 a | 0.148 ± 0.004 b | 0.151 ± 0.002 b | 0.147 ± 0.003 b | Sweet, grassy, fruity |
| 3 | 1579 | S-(R′,R′)-2,3-butanediol | 0.342 ± 0.014 bc | 0.386 ± 0.012 a | 0.364 ± 0.020 ab | 0.313 ± 0.005 c | 0.313 ±0.002 c | 0.317 ± 0.005 c | Sweet, flowery, rancid |
| 4 | 1656 | 2-Furanmethanol | 0.083 ± 0.008 a | 0.024 ± 0.003 c | 0.057 ± 0.008 b | 0.019 ± 0.002 c | 0.049 ± 0.007 b | 0.077 ± 0.006 a | Roasted, nutty, fruity |
| 5 | 1720 | 5-Methyl-2-furanmethanol | 0.013 ± 0.002 a | 0.006 ± 0.001 de | 0.009 ± 0.001 bc | 0.005 ± 0.001 e | 0.008 ± 0.001 cd | 0.012 ± 0.002 ab | Sweet, fruity, minty |
| 6 | 2069 | 5-Methyl-2-pyrazinylmethanol | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | Acidic, sweat-like, sweet |
| Total alcohols | 0.782 | 0.800 | 0.802 | 0.647 | 0.689 | 0.725 | |||
| 7 | 1256 | 4,5-Dihydro-2-methyl-3(2 H)-furanone | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 b | 0.002 ± 0.000 b | 0.002 ± 0.000 b | 0.002 ± 0.000 b | Toasted, buttery |
| 8 | 1278 | 3-Hydroxy-2-butanone | 0.005 ± 0.000 a | 0.004 ± 0.001 ab | 0.004 ± 0.001 ab | 0.004 ± 0.000 ab | 0.004 ± 0.001 ab | 0.003 ± 0.001 b | Sweet, nutty, dairy-like |
| 9 | 1292 | 1-Hydroxy-2-propanone | 0.051 ± 0.005 ab | 0.061 ± 0.005 a | 0.056 ± 0.005 a | 0.041 ± 0.004 b | 0.052 ± 0.002 a | 0.052 ± 0.004 a | Sweet, grassy, coffee-like |
| 10 | 1614 | Butyrolactone | 0.007 ± 0.001 a | 0.007 ± 0.001 a | 0.008 ± 0.002 a | 0.006 ± 0.001 a | 0.006 ± 0.001 a | 0.007 ± 0.002 a | Cooked, sweet |
| 11 | 1746 | 2(5 H)-Furanone | 0.002 ± 0.000 c | 0.017 ± 0.002 b | 0.039 ± 0.005 a | 0.002 ± 0.000 c | 0.015 ± 0.002 b | 0.036 ± 0.004 a | Pungent, cheesy |
| 12 | 1826 | 3-Methyl-1,2-cyclopentanedione | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | Sweet, maple-like |
| 13 | 1966 | 2-Acetyl pyrrole | 0.013 ± 0.003 cd | 0.023 ± 0.005 b | 0.037 ± 0.003 a | 0.010 ± 0.002 d | 0.020 ± 0.003 bc | 0.035 ± 0.003 a | Herbaceous, metallic |
| 14 | 2027 | Pantolactone | 0.028 ± 0.005 ab | 0.033 ± 0.006 a | 0.038 ± 0.001 a | 0.023 ± 0.004 b | 0.029 ± 0.004 ab | 0.035 ± 0.002 a | Sweet, caramel |
| 15 | 2035 | 2,5-Dimethyl-4-hydroxy-3(2 H)-furanone | 0.004 ± 0.000 c | 0.013 ± 0.003 b | 0.026 ± 0.002 a | 0.003 ± 0.000 c | 0.011 ± 0.002 b | 0.024 ± 0.002 a | Sweet, cotton candy-like |
| 16 | 2044 | 2-Pyrrolidinone | 0.001 ± 0.000 bc | 0.004 ± 0.001 b | 0.008 ± 0.002 a | 0.001 ± 0.000 c | 0.003 ± 0.001 bc | 0.007 ± 0.002 a | Sweet, cotton candy-like |
| 17 | 2268 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4 H-pyran-4-one | 0.231 ± 0.002 ab | 0.283 ± 0.023 ab | 0.329 ± 0.065 a | 0.187 ± 0.005 b | 0.243 ± 0.010 ab | 0.306 ± 0.065 a | Sweet, maple-like |
| 18 | 2467 | 2,5-Pyrrolidinedione | 0.045 ± 0.007 a | 0.040 ± 0.003 ab | 0.044 ± 0.003 ab | 0.036 ± 0.004 ab | 0.034 ± 0.003 b | 0.041 ± 0.002 ab | Sweet, cotton candy-like |
| Total ketones | 0.390 | 0.490 | 0.591 | 0.316 | 0.420 | 0.549 | |||
| 19 | 1262 | 2-Methyl-pyrazine | 0.003 ± 0.001 a | 0.003 ± 0.001 a | 0.003 ± 0.001 a | 0.003 ± 0.001 a | 0.002 ± 0.001 a | 0.003 ± 0.001 a | Sweet, grassy, acidic |
| 20 | 1321 | 2,5-Dimethyl-pyrazine | 0.023 ± 0.004 bc | 0.030 ± 0.002 ab | 0.039 ± 0.005 a | 0.019 ± 0.003 c | 0.028 ± 0.003 bc | 0.032 ± 0.004 ab | Nutty, earthy, roasted |
| 21 | 1327 | 2,6-Dimethyl-pyrazine | 0.007 ± 0.001 a | 0.009 ± 0.002 a | 0.010 ± 0.002 a | 0.006 ± 0.001 a | 0.008 ± 0.002 a | 0.008 ± 0.002 a | Nutty, sweet |
| 22 | 1345 | 2,3-Dimethyl-pyrazine | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | Nutty, roasted coffee-like |
| 23 | 1407 | 2,3,5-Trimethyl-pyrazine | 0.007 ± 0.000 a | 0.006 ± 0.000 ab | 0.007 ± 0.001 a | 0.006 ± 0.000 ab | 0.006 ± 0.000 ab | 0.006 ± 0.001 b | Nutty, earthy, roasted |
| 24 | 1458 | 2-Ethyl-3,6-dimethyl-pyrazine | 0.003 ± 0.000 a | 0.003 ± 0.000 b | 0.003 ±0.000 a | 0.003 ± 0.000 b | 0.003 ± 0.000 b | 0.003 ± 0.000 b | Nutty, earthy, coffee-like |
| Total pyrazines | 0.044 | 0.052 | 0.064 | 0.038 | 0.048 | 0.052 | |||
| 25 | 1528 | Propanoic acid | 0.048 ± 0.003 a | 0.038 ± 0.003 b | 0.047 ± 0.003 a | 0.039 ± 0.003 b | 0.033 ± 0.001 b | 0.033 ± 0.002 b | Rancid, acidic |
| 26 | 1560 | 2-Methyl-propanoic acid | 0.026 ± 0.005 cd | 0.036 ± 0.003 b | 0.049 ± 0.004 c | 0.021 ± 0.004 d | 0.031 ± 0.002 bcd | 0.035 ± 0.004 bc | ---- |
| 27 | 1618 | Butanoic acid | 0.013 ± 0.003 a | 0.009 ± 0.002 a | 0.014 ± 0.004 a | 0.010 ± 0.002 a | 0.008 ± 0.002 a | 0.013 ± 0.004 a | Cheesy, yogurt-like |
| 28 | 1622 | 2-Propenoic acid | 0.341 ± 0.006 a | 0.328 ± 0.021 a | 0.294 ± 0.006 b | 0.276 ± 0.006 bc | 0.282 ± 0.009 bc | 0.263 ± 0.008 c | Baked, vinegar-like |
| 29 | 1664 | 3-Methyl-butanoic acid | 0.024 ± 0.004 a | 0.018 ± 0.001 a | 0.022 ± 0.005 a | 0.019 ± 0.003 a | 0.016 ± 0.002 a | 0.020 ± 0.004 a | Cheesy, foul smell |
| 30 | 1735 | Pentanoic acid | 0.002 ± 0.000 a | 0.002 ± 0.000 a | 0.002 ± 0.000 b | 0.002 ± 0.000 b | 0.002 ± 0.000 b | 0.002 ± 0.000 b | Rancid, buttery |
| 31 | 2176 | 2-Hydroxy-propanoic acid | 0.088 ± 0.007 a | 0.071 ± 0.002 b | 0.055 ± 0.003 cd | 0.071 ± 0.004 b | 0.061 ± 0.004 bc | 0.048 ± 0.006 d | Grassy, sweet-like |
| 32 | 2417 | Benzoic acid | 0.150 ± 0.012 a | 0.140 ± 0.007 ab | 0.114 ± 0.005 c | 0.121 ± 0.006 bc | 0.120 ± 0.004 c | 0.105 ± 0.003 c | Sweet, caramel |
| 33 | 2482 | Dodecanoic acid | 0.043 ± 0.005 a | 0.035 ± 0.005 ab | 0.035 ± 0.006 ab | 0.035 ± 0.003 ab | 0.030 ± 0.003 b | 0.016 ± 0.004 c | Dairy-like, caramel |
| Total acids | 0.736 | 0.678 | 0.631 | 0.595 | 0.583 | 0.535 | |||
| 34 | 1581 | Dimethyl sulfoxide | 0.115 ± 0.002 c | 0.134 ± 0.005 ab | 0.148 ± 0.012 a | 0.106 ± 0.003 c | 0.115 ± 0.003 c | 0.120 ± 0.007 bc | Rancid, pungent, metallic |
| 35 | 1895 | Dimethyl sulfone | 0.008 ± 0.001 b | 0.008 ± 0.005 b | 0.016 ± 0.002 a | 0.007 ± 0.002 b | 0.007 ± 0.001 b | 0.013 ± 0.001 a | Sweet, waxy, sulfuric |
| Total sulfurs | 0.122 | 0.142 | 0.165 | 0.114 | 0.122 | 0.133 | |||
| 36 | 1852 | 2-Methoxy-phenol | 0.014 ± 0.003 ab | 0.014 ± 0.001 ab | 0.017 ± 0.002 a | 0.011 ± 0.003 b | 0.012 ± 0.001 ab | 0.016 ± 0.001 ab | Sweet, medicinal |
| 37 | 2263 | 2,6-Dimethoxy-phenol | 0.007 ± 0.001 a | 0.004 ± 0.001 b | 0.006 ± 0.002 ab | 0.006 ± 0.001 ab | 0.003 ± 0.001 b | 0.006 ± 0.002 ab | Sweet, maple-like |
| 38 | 2549 | Vanillin | 0.003 ± 0.001 a | 0.004 ± 0.001 a | 0.005 ± 0.002 a | 0.003 ± 0.001 a | 0.003 ± 0.001 a | 0.004 ± 0.002 a | Sweet, cotton candy-like |
| Total phenols and aldehyde | 0.024 | 0.021 | 0.028 | 0.019 | 0.018 | 0.026 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thi Le, D.H.; Chiu, C.-S.; Chan, Y.-J.; Wang, C.-C.R.; Liang, Z.-C.; Hsieh, C.-W.; Lu, W.-C.; Mulio, A.T.; Wang, Y.-J.; Li, P.-H. Bioactive and Physicochemical Characteristics of Natural Food: Palmyra Palm (Borassus flabellifer Linn.) Syrup. Biology 2021, 10, 1028. https://doi.org/10.3390/biology10101028
Thi Le DH, Chiu C-S, Chan Y-J, Wang C-CR, Liang Z-C, Hsieh C-W, Lu W-C, Mulio AT, Wang Y-J, Li P-H. Bioactive and Physicochemical Characteristics of Natural Food: Palmyra Palm (Borassus flabellifer Linn.) Syrup. Biology. 2021; 10(10):1028. https://doi.org/10.3390/biology10101028
Chicago/Turabian StyleThi Le, Dung Huynh, Chien-Shan Chiu, Yung-Jia Chan, Chiun-Chuan R. Wang, Zeng-Chin Liang, Chang-Wei Hsieh, Wen-Chien Lu, Amanda Tresiliana Mulio, Yin-Jun Wang, and Po-Hsien Li. 2021. "Bioactive and Physicochemical Characteristics of Natural Food: Palmyra Palm (Borassus flabellifer Linn.) Syrup" Biology 10, no. 10: 1028. https://doi.org/10.3390/biology10101028
APA StyleThi Le, D. H., Chiu, C.-S., Chan, Y.-J., Wang, C.-C. R., Liang, Z.-C., Hsieh, C.-W., Lu, W.-C., Mulio, A. T., Wang, Y.-J., & Li, P.-H. (2021). Bioactive and Physicochemical Characteristics of Natural Food: Palmyra Palm (Borassus flabellifer Linn.) Syrup. Biology, 10(10), 1028. https://doi.org/10.3390/biology10101028









