The Modulation of Gut Microbiota Composition in the Pathophysiology of Gestational Diabetes Mellitus: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.3. Data Extraction and Synthesis
2.4. Quality Assessment and Risk of Bias
3. Results
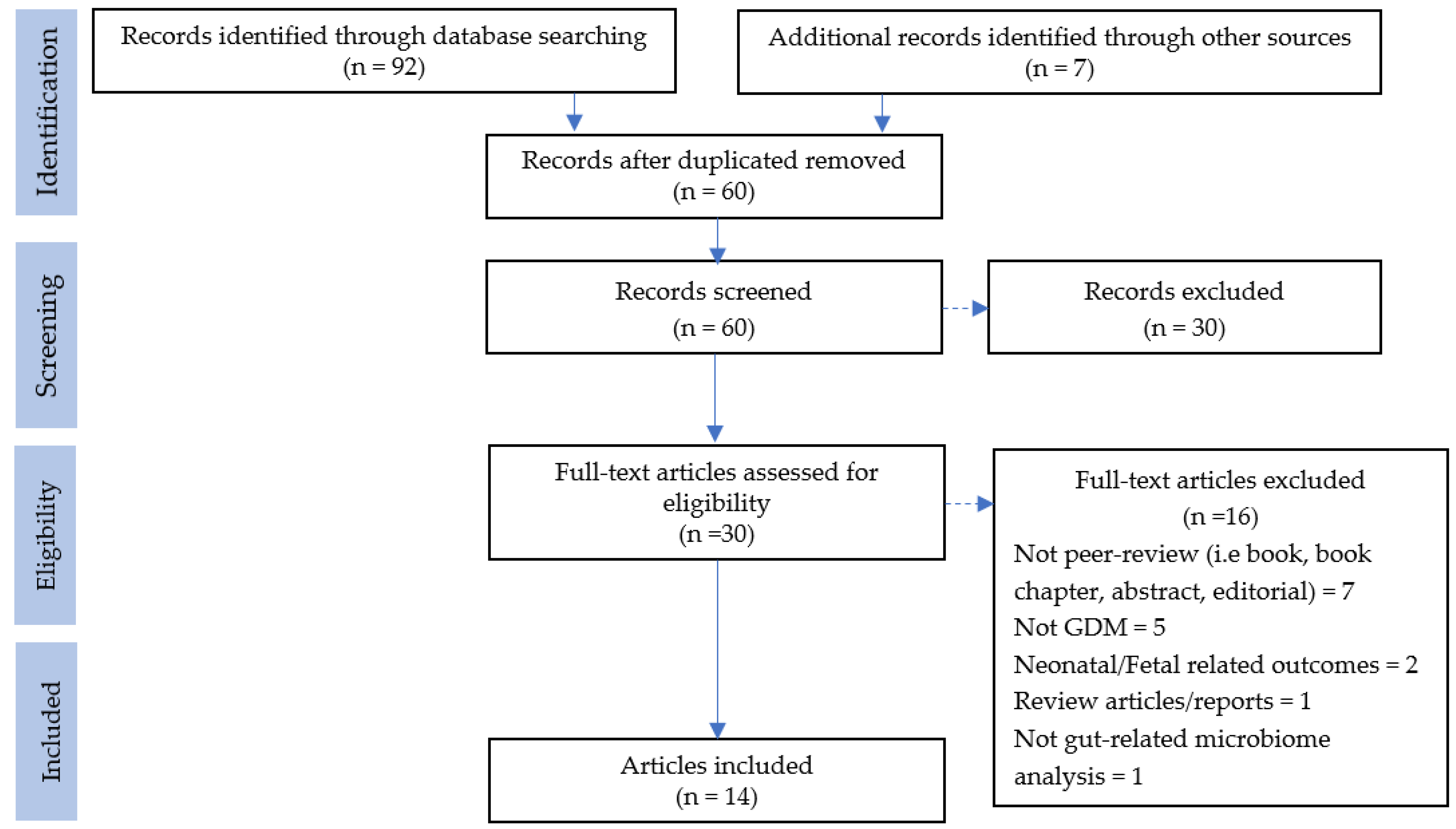
3.1. Study Selection
3.2. Study Characteristics
3.3. Study Quality
3.4. Modulation of Gut Microbiota in Pregnancy and Its Association with GDM
3.4.1. Distribution of Gut Microbiota during Pregnancy
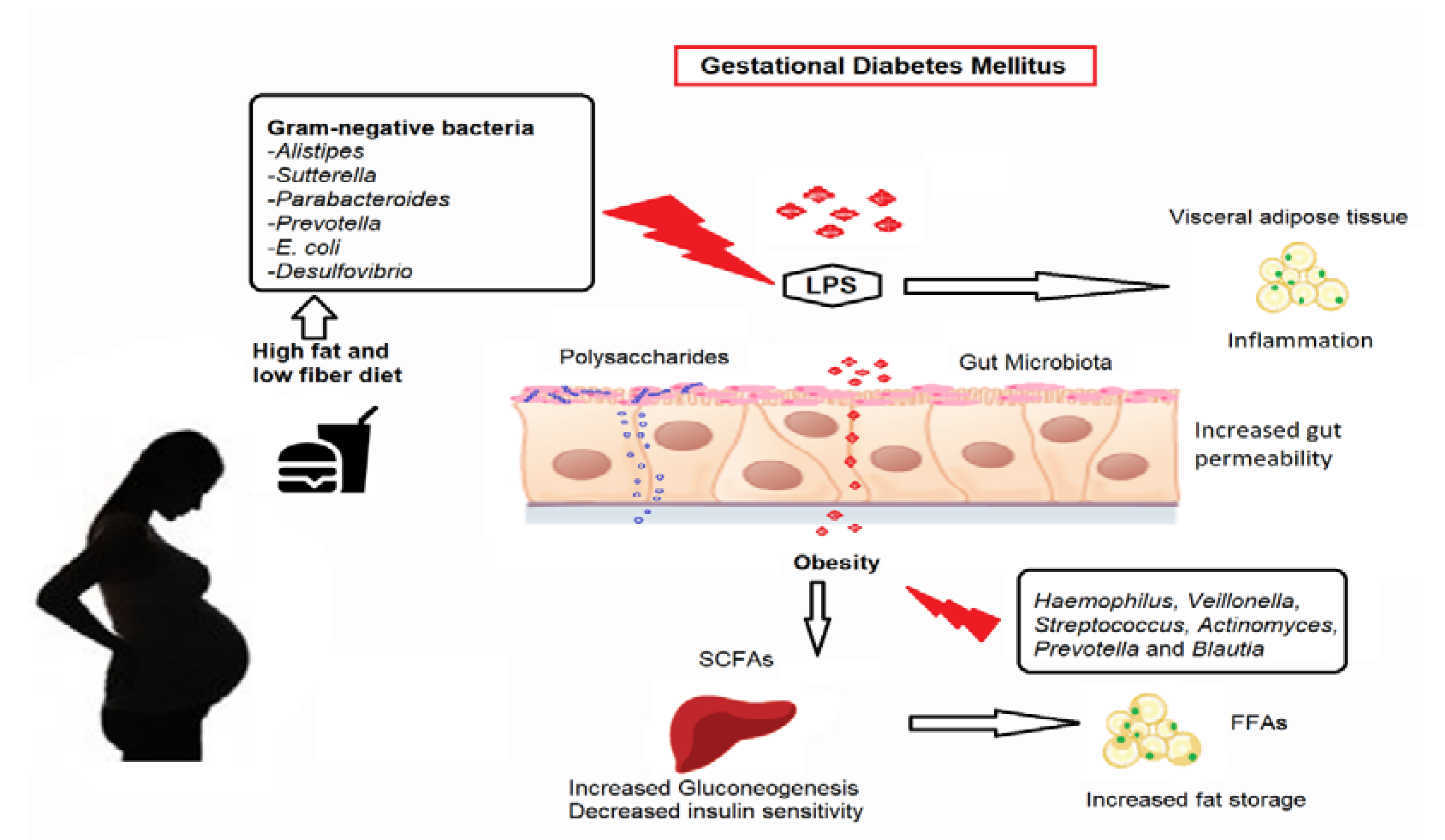
3.4.2. Influences of Gut Microbiota in Obese GDM Women
3.4.3. Association of Gut Microbiota in Glucose Response, Lipid Metabolism, and Inflammation in GDM
3.4.4. Gut Microbiota Pattern in Post-Pregnancy Women with a History of GDM
4. Discussion
4.1. Microbiota Impact on Metabolism in Women with GDM: Potential Pathways
4.1.1. Modulation of Inflammation
4.1.2. Glucose Metabolism
4.1.3. Fatty Acid Oxidation, Synthesis, and Energy Expenditure
4.2. Challenges in Gut Microbiota Research
4.2.1. Study Design
4.2.2. Sample Size
4.2.3. Enrollment Criteria
4.2.4. Time of Sample Collection
4.2.5. Methods of Sampling and Sequencing Tool
4.3. Future Recommendation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koh, A.; Molinaro, A.; Ståhlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell 2018, 175, 947–961.e917. [Google Scholar] [CrossRef] [Green Version]
- Prattichizzo, F.; Giuliani, A.; Mensà, E.; Sabbatinelli, J.; De Nigris, V.; Rippo, M.R.; La Sala, L.; Procopio, A.D.; Olivieri, F.; Ceriello, A. Pleiotropic effects of metformin: Shaping the microbiome to manage type 2 diabetes and postpone ageing. Ageing Res. Rev. 2018, 48, 87–98. [Google Scholar] [CrossRef]
- Brunkwall, L.; Orho-Melander, M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: From current human evidence to future possibilities. Diabetologia 2017, 60, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [Green Version]
- DiBaise, J.K.; Zhang, H.; Crowell, M.D.; Krajmalnik-Brown, R.; Decker, G.A.; Rittmann, B.E. Gut microbiota and its possible relationship with obesity. Mayo Clin. Proc. 2008, 83, 460–469. [Google Scholar] [CrossRef] [Green Version]
- Udayappan, S.D.; Hartstra, A.V.; Dallinga-Thie, G.M.; Nieuwdorp, M. Intestinal microbiota and faecal transplantation as treatment modality for insulin resistance and type 2 diabetes mellitus. Clin. Exp. Immunol. 2014, 177, 24–29. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; McIntyre, H.D.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunasegaran, T.; Balasubramaniam, V.; Arasoo, V.J.T.; Palanisamy, U.D.; Ramadas, A. Gestational Diabetes Mellitus in Southeast Asia: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 1272. [Google Scholar] [CrossRef] [PubMed]
- Damm, P.; Houshmand-Oeregaard, A.; Kelstrup, L.; Lauenborg, J.; Mathiesen, E.R.; Clausen, T.D. Gestational diabetes mellitus and long-term consequences for mother and offspring: A view from Denmark. Diabetologia 2016, 59, 1396–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girgis, C.M.; Gunton, J.E.; Cheung, N.W. The influence of ethnicity on the development of type 2 diabetes mellitus in women with gestational diabetes: A prospective study and review of the literature. ISRN Endocrinol. 2012, 2012, 341638. [Google Scholar] [CrossRef]
- Kim, C.; Kim, S.Y.; Sappenfield, W.; Wilson, H.G.; Salihu, H.M.; Sharma, A.J. Are gestational diabetes mellitus and preconception diabetes mellitus less common in non-Hispanic black women than in non-Hispanic white women? Matern. Child Health J. 2014, 18, 698–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, G.; Zhang, L.; Wang, M.; Chen, Y.; Gu, S.; Wang, K.; Leng, J.; Gu, Y.; Xie, X. The Gut Microbiota in Women Suffering from Gestational Diabetes Mellitus with the Failure of Glycemic Control by Lifestyle Modification. J. Diabetes Res. 2019, 2019, 6081248. [Google Scholar] [CrossRef] [PubMed]
- Cortez, R.V.; Taddei, C.R.; Sparvoli, L.G.; Ângelo, A.G.S.; Padilha, M.; Mattar, R.; Daher, S. Microbiome and its relation to gestational diabetes. Endocrine 2019, 64, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, J.; Shi, W.; Du, N.; Xu, X.; Zhang, Y.; Ji, P.; Zhang, F.; Jia, Z.; Wang, Y.; et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 2018, 67, 1614. [Google Scholar] [CrossRef]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 2018, 6, 89. [Google Scholar] [CrossRef]
- Kuang, Y.S.; Lu, J.H.; Li, S.H.; Li, J.H.; Yuan, M.Y.; He, J.R.; Chen, N.N.; Xiao, W.Q.; Shen, S.Y.; Qiu, L.; et al. Connections between the human gut microbiome and gestational diabetes mellitus. Gigascience 2017, 6, 1–12. [Google Scholar] [CrossRef]
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.A.; Wong, R.J.; Shaw, G.; et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065. [Google Scholar] [CrossRef] [Green Version]
- Ferrocino, I.; Ponzo, V.; Gambino, R.; Zarovska, A.; Leone, F.; Monzeglio, C.; Goitre, I.; Rosato, R.; Romano, A.; Grassi, G.; et al. Changes in the gut microbiota composition during pregnancy in patients with gestational diabetes mellitus (GDM). Sci. Rep. 2018, 8, 12216. [Google Scholar] [CrossRef]
- Allalou, A.; Nalla, A.; Prentice, K.J.; Liu, Y.; Zhang, M.; Dai, F.F.; Ning, X.; Osborne, L.R.; Cox, B.J.; Gunderson, E.P.; et al. A Predictive Metabolic Signature for the Transition From Gestational Diabetes Mellitus to Type 2 Diabetes. Diabetes 2016, 65, 2529–2539. [Google Scholar] [CrossRef] [Green Version]
- Eades, C.E.; Styles, M.; Leese, G.P.; Cheyne, H.; Evans, J.M. Progression from gestational diabetes to type 2 diabetes in one region of Scotland: An observational follow-up study. BMC Pregnancy Childbirth 2015, 15, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health. Study Quality Assessment Tools. Available online: http://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 1 September 2021).
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; You, Y.; Huang, L.; Long, S.; Zhang, J.; Guo, C.; Zhang, N.; Wu, X.; Xiao, Y.; Tan, H. Alterations in Gut Microbiota of Gestational Diabetes Patients During the First Trimester of Pregnancy. Front. Cell. Infect. Microbiol. 2020, 10, 58. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Zhong, M.; Shen, Q.; Wu, Y.; Cao, M.; Ju, S.; Chen, L. Gut microbiota in early pregnancy among women with Hyperglycaemia vs. Normal blood glucose. BMC Pregnancy Childbirth 2020, 20, 284. [Google Scholar] [CrossRef]
- Fugmann, M.; Breier, M.; Rottenkolber, M.; Banning, F.; Ferrari, U.; Sacco, V.; Grallert, H.; Parhofer, K.G.; Seissler, J.; Clavel, T.; et al. The stool microbiota of insulin resistant women with recent gestational diabetes, a high risk group for type 2 diabetes. Sci. Rep. 2015, 5, 13212. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M. Connections Between the Gut Microbiome and Metabolic Hormones in Early Pregnancy in Overweight and Obese Women. Diabetes 2016, 65, 2214–2223. [Google Scholar] [CrossRef] [Green Version]
- Mokkala, K.; Houttu, N.; Vahlberg, T.; Munukka, E.; Rönnemaa, T.; Laitinen, K. Gut microbiota aberrations precede diagnosis of gestational diabetes mellitus. Acta Diabetol. 2017, 54, 1147–1149. [Google Scholar] [CrossRef]
- Liu, H.; Pan, L.-L.; Lv, S.; Yang, Q.; Zhang, H.; Chen, W.; Lv, Z.; Sun, J. Alterations of Gut Microbiota and Blood Lipidome in Gestational Diabetes Mellitus With Hyperlipidemia. Front. Physiol. 2019, 10, 1015. [Google Scholar] [CrossRef] [Green Version]
- Mokkala, K.; Paulin, N.; Houttu, N.; Koivuniemi, E.; Pellonperä, O.; Khan, S.; Pietilä, S.; Tertti, K.; Elo, L.L.; Laitinen, K. Metagenomics analysis of gut microbiota in response to diet intervention and gestational diabetes in overweight and obese women: A randomised, double-blind, placebo-controlled clinical trial. Gut 2021, 70, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD-what role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J. Atheroscler. Thromb. 2017, 24, 660–672. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Narasimhan, S.; Marchesi, J.R.; Benson, A.; Wong, F.S.; Wen, L. Long term effect of gut microbiota transfer on diabetes development. J. Autoimmun. 2014, 53, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kameyama, K.; Itoh, K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egshatyan, L.; Kashtanova, D.; Popenko, A.; Tkacheva, O.; Tyakht, A.; Alexeev, D.; Karamnova, N.; Kostryukova, E.; Babenko, V.; Vakhitova, M.; et al. Gut microbiota and diet in patients with different glucose tolerance. Endocr. Connect. 2016, 5, 1–9. [Google Scholar] [CrossRef]
- Ottosson, F.; Brunkwall, L.; Ericson, U.; Nilsson, P.M.; Almgren, P.; Fernandez, C.; Melander, O.; Orho-Melander, M. Connection Between BMI-Related Plasma Metabolite Profile and Gut Microbiota. J. Clin. Endocrinol. Metab. 2018, 103, 1491–1501. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, T.A.; Xiang, A.; Kjos, S.L.; Watanabe, R. What is gestational diabetes? Diabetes Care 2007, 30 (Suppl. 2), S105–S111. [Google Scholar] [CrossRef] [Green Version]
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular Mechanisms for Insulin Resistance in Normal Pregnancy and Gestational Diabetes. Diabetes Care 2007, 30, S112. [Google Scholar] [CrossRef] [Green Version]
- Serino, M.; Fernández-Real, J.M.; García-Fuentes, E.; Queipo-Ortuño, M.; Moreno-Navarrete, J.M.; Sánchez, A.; Burcelin, R.; Tinahones, F. The gut microbiota profile is associated with insulin action in humans. Acta Diabetol. 2013, 50, 753–761. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; García-Fuentes, E.; Cardona, F.; Queipo-Ortuño, M.I.; Tinahones, F.J. Insulin resistance is associated with specific gut microbiota in appendix samples from morbidly obese patients. Am. J. Transl. Res. 2016, 8, 5672–5684. [Google Scholar] [PubMed]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Vaarala, O.; Atkinson, M.A.; Neu, J. The “perfect storm” for type 1 diabetes: The complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 2008, 57, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Manco, M.; Putignani, L.; Bottazzo, G.F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr. Rev. 2010, 31, 817–844. [Google Scholar] [CrossRef] [Green Version]
- Abreu, M.T. Toll-like receptor signalling in the intestinal epithelium: How bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 2010, 10, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M.; Matthews, P.; Poston, L. Maternal metabolism and obesity: Modifiable determinants of pregnancy outcome. Hum. Reprod. Update 2010, 16, 255–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Stability of the maternal gut microbiota during late pregnancy and early lactation. Curr. Microbiol. 2014, 68, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Greenhill, C. Gut microbiota: Firmicutes and Bacteroidetes involved in insulin resistance by mediating levels of glucagon-like peptide 1. Nat. Rev. Endocrinol. 2015, 11, 254. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef] [Green Version]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Jovel, J.; Patterson, J.; Wang, W.; Hotte, N.; O’Keefe, S.; Mitchel, T.; Perry, T.; Kao, D.; Mason, A.L.; Madsen, K.L.; et al. Characterization of the Gut Microbiome Using 16S or Shotgun Metagenomics. Front. Microbiol. 2016, 7, 459. [Google Scholar] [CrossRef] [Green Version]
- Ranjan, R.; Rani, A.; Metwally, A.; McGee, H.S.; Perkins, D.L. Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem. Biophys. Res. Commun. 2016, 469, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.R.; Shulzhenko, N.; Morgun, A. Transkingdom Networks: A Systems Biology Approach to Identify Causal Members of Host-Microbiota Interactions. Methods Mol. Biol. 2018, 1849, 227–242. [Google Scholar] [CrossRef]
- Burgess, S.; Foley, C.N.; Allara, E.; Staley, J.R.; Howson, J.M.M. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat. Commun. 2020, 11, 376. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | n (%) | |
|---|---|---|
| Study design | Cross-sectional | 8 (57.1) |
| Prospective cohort | 3 (21.4) | |
| Case-control | 2 (14.2) | |
| Intervention | 1 (7.4) | |
| Country | China | 6 (42.9) |
| Finland | 3 (21.4) | |
| Germany | 1 (7.4) | |
| Australia | 1 (7.4) | |
| Denmark | 1 (7.4) | |
| Brazil | 1 (7.4) | |
| Italy | 1 (7.4) | |
| Sample size | <50 | 2 (14.2) |
| 50–100 | 7 (50.0) | |
| >100 | 5 (35.8) | |
| Gestational age (trimester) | First | 3 (21.4) |
| Second | 3 (21.4) | |
| Third | 3 (21.4) | |
| Post-partum | 1 (7.4) | |
| Multiple | 4 (26.6) | |
| Body weight | Overweight/obese | 7 (58.3) |
| Normal weight | 5 (41.7) |
| Gut Microbiome | Metabolic Outcome | References | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BG | Insulin | HbA1c | BMI | Adiposity | HDL | Leptin | TG | Adipokine | TC | PG | LPEt | LdMePE | LPS | CRP | GI | PC | ||
| Proteobacteria | ↑ | ↑ | ↑ | ↑ | ↑ | [25,31] | ||||||||||||
| Actinobacteria | ↑ | ↑ | ↑ | [25] | ||||||||||||||
| Faecalibacterium | ↓↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↓ | [16,20,25,31] | ||||||||||
| Firmicutes | ↑ | ↓ | ↓ | ↓ | [15,28] | |||||||||||||
| Bacteroidaceae | ↑ | ↓ | ↓ | ↓ | ↑ | ↑ | [20,27,28] | |||||||||||
| Prevotellaceae | ↑ | ↑ | ↑ | ↑ | ↑ | [17,20,28] | ||||||||||||
| Ruminococcaceae | ↑ | ↑ | [29,30] | |||||||||||||||
| Lachnospiraceae | ↑ | ↑ | [18,29] | |||||||||||||||
| Collinsella | ↑ | [20,29] | ||||||||||||||||
| Holdemania filiformis | ↑ | [33] | ||||||||||||||||
| Eisenbergiella | ↑ | [26] | ||||||||||||||||
| Tyzzerella 4ycer | ↑ | [26] | ||||||||||||||||
| Haemophilus | ↑ | [31] | ||||||||||||||||
| Veillonella | ↑ | [31] | ||||||||||||||||
| Actinomyces | ↑ | [31] | ||||||||||||||||
| Streptococcus | ↑ | [31] | ||||||||||||||||
| Christensenella | ↑ | [17] | ||||||||||||||||
| Akkermansia | ↓ | [17] | ||||||||||||||||
| Blautia | ↑ | ↑ | [14,17,20] | |||||||||||||||
| Sutterella | ↑ | ↑ | [20] | |||||||||||||||
| Alistipes | ↑ | [20] | ||||||||||||||||
| Allofustis seminis | ↑ | [18] | ||||||||||||||||
| Megamonas spp. | ↑ | [18] | ||||||||||||||||
| Eggerthella spp. | ↑ | [18] | ||||||||||||||||
| E. rectale | ↑ | [18] | ||||||||||||||||
| K. variicola | ↑ | [18] | ||||||||||||||||
| P. distasonis | ↑ | [18] | ||||||||||||||||
| Coprococcus | ↑ | [29] | ||||||||||||||||
| Eubacterium_hallii | ↑ | [14] | ||||||||||||||||
| Enterobacteriaceae | ↑ | [27] | ||||||||||||||||
| Fusobacteriaceae | ↑ | [27] | ||||||||||||||||
| Characteristics | n (%) | |
|---|---|---|
| Sample | Fecal | 14 (100.0) |
| Immediate storage temperature | 4 °C | 1 (8.3) |
| −20 °C | 6 (50.0) | |
| −80 °C | 5 (41.7) | |
| DNA isolation methods | QIAamp DNA Stool Mini Kit | 8 (61.5) |
| PSP Spin Stool DNA Plus Kit | 1 (7.7) | |
| NucleoSpin Soil kit (Macherey-Nagel | 1 (7.7) | |
| RNeasy Power Microbiome KIT | 1 (7.7) | |
| PowerMax (stool/soil) DNA isolation kit | 1 (7.7) | |
| GTX stool extraction kit | 1 (7.7) | |
| Sequencing | Amplicon | 12 (85.7) |
| Metagenomic | 2 (14.3) | |
| Variable region amplified | V1–V2 | 2 (18.1) |
| V3–V4 | 5 (45.5) | |
| V4 | 3 (27.3) | |
| V6–V8 | 1 (9.1) | |
| Platform | Illumina HiSeq | 5 (45.5) |
| Illumina MiSeq | 8 (61.5) | |
| Unknown | 1 (9.1) | |
| Bioinformatics pipeline | RDP classifier | 1 (7.14) |
| QIIME | 8 (57.1) | |
| MOCAT | 1 (7.14) | |
| Multiple | 4 (28.6) | |
| Reference database | Silva | 6 (50.0) |
| Greengenes | 4 (33.0) | |
| Vsearch | 1 (8.3) | |
| EzTaxon | 1 (8.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunasegaran, T.; Balasubramaniam, V.R.M.T.; Arasoo, V.J.T.; Palanisamy, U.D.; Ramadas, A. The Modulation of Gut Microbiota Composition in the Pathophysiology of Gestational Diabetes Mellitus: A Systematic Review. Biology 2021, 10, 1027. https://doi.org/10.3390/biology10101027
Kunasegaran T, Balasubramaniam VRMT, Arasoo VJT, Palanisamy UD, Ramadas A. The Modulation of Gut Microbiota Composition in the Pathophysiology of Gestational Diabetes Mellitus: A Systematic Review. Biology. 2021; 10(10):1027. https://doi.org/10.3390/biology10101027
Chicago/Turabian StyleKunasegaran, Thubasni, Vinod R. M. T. Balasubramaniam, Valliammai Jayanthi T. Arasoo, Uma Devi Palanisamy, and Amutha Ramadas. 2021. "The Modulation of Gut Microbiota Composition in the Pathophysiology of Gestational Diabetes Mellitus: A Systematic Review" Biology 10, no. 10: 1027. https://doi.org/10.3390/biology10101027
APA StyleKunasegaran, T., Balasubramaniam, V. R. M. T., Arasoo, V. J. T., Palanisamy, U. D., & Ramadas, A. (2021). The Modulation of Gut Microbiota Composition in the Pathophysiology of Gestational Diabetes Mellitus: A Systematic Review. Biology, 10(10), 1027. https://doi.org/10.3390/biology10101027









