The Biology of Autoimmune Response in the Scurfy Mice that Lack the CD4+Foxp3+ Regulatory T-Cells
Abstract
:1. Introduction
2. Genetic Control of MOI in Sf Mice
2.1. Lymphocyte Requirement
2.2. T-Cell Repertoire Requirement
2.3. Sf.Cd4−/− and Sf.β2m−/− Mice
2.4. Sf.Cd28−/− Mice
2.5. K/BxN.Foxp3sf Mice
2.6. NOD.Foxp3sf Mice
2.7. Sf.Aire−/− Mice
2.8. Sf.Il2−/− Mice
2.9. Sf.Itgae−/− Mice
2.10. Sf.Faslpr/lpr Mice
3. Participation of Th Subsets in Sf MOI
3.1. How IL-2 Controls Skin and Lung Inflammation?
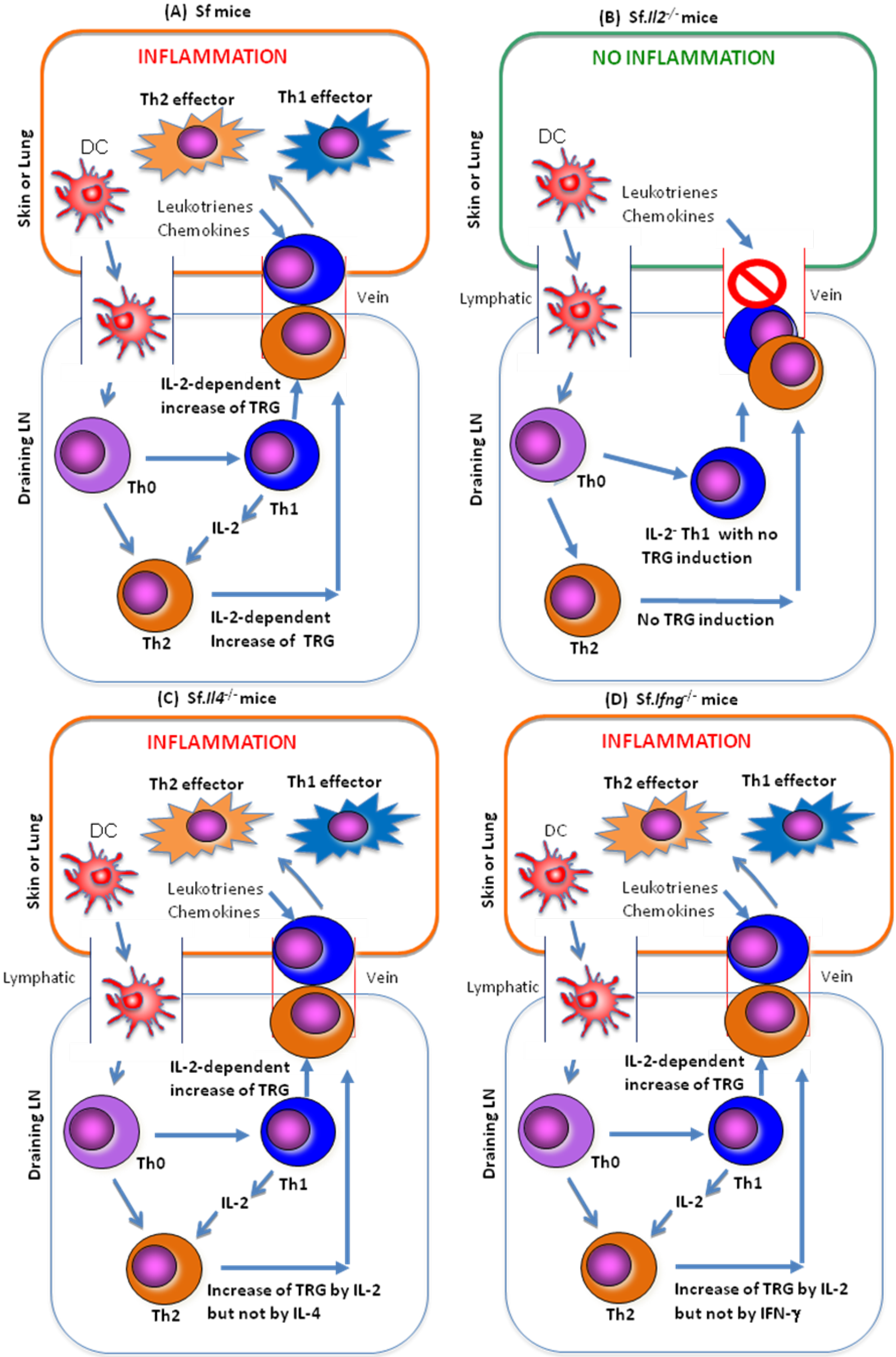
3.2. Genome-Wide Microarray Comparison among CD4+ T-Cells of Sf and Sf.Il2−/− Mice
3.3. IL-2 Regulates many TRG in the CD4+ T-Cells of Sf Mice
3.4. Sf CD4+ T-Cells that Displayed Differential Gene Expression Selectively Transferred Skin and Lung Inflammation
3.5. IL-2 Regulates Expression of Inflammatory Cytokines
3.6. Restoration of TRG Expression by rIL-2
3.7. Th1 Response is Dominant and Controlling in Skin and Lung Inflammation in Sf Mice
3.8. MOI in Sf.Ifng−/− Mice
3.9. Sf.II4−/− and Sf.Stat6−/− Mice Develop Inflammation in the Skin and Lungs
4. Comparison of Cytokine-Producing Profiles of CD4+ T-Cells
4.1. Cytokine-Producing CD4+ T-Cells upon ex vivo Activation
4.2. Serum Levels of Cytokines and IgE do not Always Reflect Inflammation Status in the Skin and Lungs of Sf and Sf Double Mutants
5. Th Cytokines Regulate TRG: Mechanism and Specificity
5.1. IL-2 but not IL-4 or IFN-γ Regulates TRG for Skin and Lung Inflammtion
5.2. Study of Sf.Ltb4r1−/−, Sf.Alox5−/−, Sf.Cx3cr1gfp/gfp and Sf.Il10−/− Mice
6. Environmental and Age Effects on MOI
6.1. Skin Inflammation
6.2. Inflammation in Salivary and Lacrimal Glands
6.3. Lung Inflammation
6.4. Gastritis and Small Intestine Inflammation
6.5. Liver Inflammation and Cholangitis
6.6. Pancreatitis
6.7. Colitis
6.8. Myositis
6.9. Inflammation in the Accessory Reproductive Organs
7. Conclusions
| Gene examined | Change in lymphocytes | Change in MOI | Lifespan |
|---|---|---|---|
| Sf [21] | Th1 and Th2 subset expansion | MOI in skin, lungs and liver | 3–4 wk |
| Sf.TgTCR.Rag−/− [13] | TCR Tg T-cells only | No MOI | >20 wk |
| Sf.Cd4−/− [21] | No CD4+ T-cells | Delayed 1 wk | 6 wk |
| Sf.β2m−/− [21] | No CD8+ T-cells | Not delayed | 4 wk |
| Sf.TCR Tg [13] | T-cells reduced, dual-TCR T-cells expanded | Delayed 2–3 wk | 7 wk |
| Sf.NOD [26] | N.D. * | More severe than B6.Foxp3sf mice | N.D. |
| Sf.BDC2.5 Tg TCR in NOD [27] | Lympho-proliferation was ameliorated | Rapid development of insulitis and diabetes, MOI was not addressed. | N.D. ** |
| Sf.Aire−/− [28] | N.D. | MOI fastened but did not extend to endocrine organs. | 2–3 wk |
| Sf.Cd28−/− [22] | Inhibit T-cell activation and cytokine production | Inhibited | 50% lived >30 wk |
| Sf.Stat6−/− mice (Balb/c) [53] | Inhibit IgE and Th2 cytokine production | Inhibited eosinophilia and lung Goblet cell metaplasia | 5 wk |
| Sf.Faslpr/lpr [12,29] | Slight increase in lymphocytes in LN | Not delayed but lifespan prolonged, developed inflammation in accessory reproductive organs and colitis | 6–18 wk |
| Sf.Itgαε−/− [31] | Lymphocyte number decreased by ~40% | Delayed 2–3 wk, developed colitis | 6–7 wk |
| Sf.Il2−/− [29] | Lymphocytes in LN increased 100%. CD103 and trafficking receptors inhibited | Delayed 3–5 wk. No skin inflammation, greatly reduced lung inflammation, liver inflammation remained, developed colitis | 6–10 wk |
| Sf.Il4−/− [18] | IL-4, IL-5, and IL-13 CD4+ T-cells were inhibited. TRG controlled by IL-4 were inhibited. IgE expression was inhibited | Skin and lung inflammation were not inhibited | 4 wk |
| Sf.Stat6−/− (B6) [18] | Reduced IL-4, IL-5 and IL-13 CD4+ T-cell expression. TRG controlled by Stat6 were inhibited. IgE expression was inhibited. TRG controlled by IL-2 were not affected | Skin and lung inflammation were not inhibited | 4 wk |
| Sf.Ifng−/− [18] | Lymphocyte expansion delayed but fully restored later. IL-2-producing Th1 cells were normal. IL-2-regulated TRG were not affected | MOI was delayed for 1–3 wk but fully developed later with skin and lung inflammation | 5–8 wk |
| Sf.Ltb4r1−/− [18] | Expanded Th1 and Th2 responses | No effect on MOI Inflammation in skin, lung and liver was similar to Sf mice | 4–5 wk |
| Sf.Alox5−/− [18] | Th1 response remained high and Th2 response was further enhanced | No effect on MOI | 3 wk |
| Sf.Il10−/− [18] | Th1 and Th2 remained high | No effect on MOI | 3–4 wk |
| Sf.Cx3cr1gfp/gfp [18] | Th1 and Th2 remained high | No effect on MOI | 3–4 wk |
Competing Interests
Acknowledgements
References
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Shevach, E.M.; DiPaolo, R.A.; Andersson, J.; Zhao, D.M.; Stephens, G.L.; Thornton, A.M. The lifestyle of naturally occurring CD4+CD25+Foxp3+ regulatory T cells. Immunol. Rev. 2006, 212, 60–73. [Google Scholar] [CrossRef]
- Zheng, Y.; Rudensky, A.Y. Foxp3 in control of the regulatory T cell lineage. Nat. Immunol. 2007, 8, 457–462. [Google Scholar] [CrossRef]
- Brunkow, M.E.; Jeffery, E.W.; Hjerrild, K.A.; Paeper, B.; Clark, L.B.; Yasayko, S.A.; Wilkinson, J.E.; Galas, D.; Ziegler, S.F.; Ramsdell, F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001, 27, 68–73. [Google Scholar]
- Wildin, R.S.; Ramsdell, F.; Peake, J.; Faravelli, F.; Casanova, J.; Buist, N.; Levy-Lahad, E.; Mazzella, M.; Goulet, O.; Perroni, L.; et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001, 27, 18–20. [Google Scholar] [CrossRef]
- Wildin, R.S.; Smyk-Pearson, S.; Filipovich, A.H. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. J. Med. Genet. 2002, 39, 537–545. [Google Scholar] [CrossRef]
- Godfrey, V.L.; Wilkinson, J.E.; Rinchik, E.M.; Russell, L.B. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a Sf thymic environment: Potential model for thymic education. Proc. Natl. Acad. Sci. USA 1991, 88, 5528–5532. [Google Scholar] [CrossRef]
- Godfrey, V.L.; Rouse, B.T.; Wilkinson, J.E. Transplantation of T cell-mediated, lymphoreticular disease from the scurfy (sf) mouse. Am. J. Pathol. 1994, 145, 281–286. [Google Scholar]
- Chang, X.; Gao, J.X.; Jiang, Q.; Wen, J.; Seifers, N.; Su, L.; Godfrey, V.L.; Zuo, T.; Zheng, P.; Liu, Y. The scurfy mutation of foxp3 in the thymus stroma leads to defective thymopoiesis. J. Exp. Med. 2005, 202, 1141–1151. [Google Scholar] [CrossRef]
- Means, G.D.; Toy, D.Y.; Baum, P.R.; Derry, J.M. A transcript map of a 2-Mb BAC contig in the proximal portion of the mouse x chromosome and regional mapping of the scurfy mutation. Genomics 2000, 65, 213–223. [Google Scholar] [CrossRef]
- Sharma, R.; Sung, S.-S.J.; Fu, S.M.; Ju, S.-T. Regulation of multi-organ inflammation in the regulatory T cell-deficient scurfy mice. J. Biomed. Sci. 2009, 16. [Google Scholar] [CrossRef]
- Sharma, R.; Deshmukh, U.S.; Zheng, L.; Fu, S.M.; Ju, S.-T. X-linked Foxp3 (scurfy) mutation dominantly inhibits submandibular gland development and inflammation respectively through adaptive and innate immune mechanisms. J. Immunol. 2009, 183, 3212–3218. [Google Scholar] [CrossRef]
- Zahorsky-Reeves, J.L.; Wilkinson, J.E. The murine mutation scurfy (sf) results in an antigen-dependent lymphoproliferative disease with altered T cell sensitivity. Eur. J. Immunol. 2001, 31, 196–204. [Google Scholar] [CrossRef]
- Heath, W.R.; Carbone, F.R.; Bertolino, P.; Kelly, J.; Cose, S.; Miller, J.F.A.P. Expression of two T cell receptor α chains on the surface of normal murine T cells. Eur. J. Immunol. 1995, 25, 1617–1623. [Google Scholar] [CrossRef]
- Padovan, E.; Giachino, C.; Cella, M.; Valitutti, S.; Acuto, O.; Lanzavecchia, A. Normal T lymphocytes can express two different T cell receptor β chains: Implications for the mechanism of allelic exclusion. J. Exp. Med. 1995, 181, 1587–1591. [Google Scholar] [CrossRef]
- Sharma, R.; Ju, A.C.-Y.; Kung, J.T.; Fu, S.M.; Ju, S.-T. Rapid and selective expansion of nonclonotypic T cells in regulatory T cell-deficient, foreign antigen-specific TCR-transgenic scurfy mice: Antigen-dependent expansion and TCR analysis. J. Immunol. 2008, 181, 6934–6941. [Google Scholar]
- Sharma, R.; Sharma, P.R.; Kim, Y.C.; Leitinger, N.; Lee, J.K.; Fu, S.M.; Ju, S.-T. Il-2-controlled expression of multiple -T cell trafficking genes and th2 cytokines in the regulatory T cell-deficient scurfy mice: Implication to multiorgan inflammation and control of skin and lung inflammation. J. Immunol. 2011, 186, 1268–1278. [Google Scholar] [CrossRef]
- Sharma, R.; Sung, S.-S.J.; Gaskin, F.; Fu, S.M.; Ju, S.-T. A novel function of IL-2: Chemokine/chemoattractant/retention receptor genes induction in Th subsets for skin and lung inflammation. J. Autoimmun. 2012. [Google Scholar] [CrossRef]
- Lahl, K.; Mayer, C.T.; Bopp, T.; Huehn, J.; Loddenkemper, C.; Eberl, G.; Wirnsberger, G.; Dornmair, K.; Geffers, R.; Schmitt, E.; et al. Nonfunctional regulatory T cells and defective control of Th2 cytokine production in natural scurfy mutant mice. J. Immunol. 2009, 183, 5662–5672. [Google Scholar] [CrossRef]
- Kuczma, M.; Podolsky, R.; Garge, N.; Daniely, D.; Pacholczyk, R.; Ignatowicz, L.; Kraj, P. Foxp3-deficient regulatory T cells do not revert into conventional effector CD4+ T cells but constitute a unique cell subset. J. Immunol. 2009, 183, 3731–3741. [Google Scholar] [CrossRef]
- Blair, P.J.; Bultman, S.J.; Haas, J.C.; Rouse, B.T.; Wilkinson, J.E.; Godfrey, V.L. CD4+CD8− T cells are the effector cells in disease pathogenesis in the scurfy (sf) mouse. J. Immunol. 1994, 153, 3764–3774. [Google Scholar]
- Singh, N.; Chandler, P.R.; Seki, Y.; Baban, B.; Takezaki, M.; Kahler, D.J.; Munn, D.H.; Larsen, C.P.; Mellor, A.L.; Iwashima, M. Role of CD28 in fatal autoimmune disorder in scurfy mice. Blood 2007, 110, 1199–1206. [Google Scholar] [CrossRef]
- Kouskoff, V.; Korganow, A.S.; Duchatelle, V.; Degott, C.; Benoist, C.; Mathis, D. Organ-specific disease provoked by systemic autoimmunity. Cell 1996, 87, 811–822. [Google Scholar] [CrossRef]
- Matsumoto, I.; Staub, A.; Benoist, C.; Mathis, D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science 1999, 286, 1732–1735. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Jacobs, J.; Mathis, D.; Benoist, C. Where Foxp3-dependent regulatory T cells impinge on the development of inflammatory arthritis. Arthritis Rheum. 2007, 56, 509–520. [Google Scholar] [CrossRef]
- Chen, Z.; Benoist, C.; Mathis, D. How defects in central tolerance impinge on a deficiency in regulatory T cells. Proc. Natl. Acad. Sci. USA 2005, 102, 14735–14740. [Google Scholar] [CrossRef]
- Chen, Z.; Herman, A.E.; Matos, M.; Mathis, D.; Benoist, C. Where CD4+CD25+ treg cells impinge on autoimmune diabetes. J. Exp. Med. 2005, 202, 1387–1397. [Google Scholar] [CrossRef]
- Villaseñor, J.; Benoist, C.; Mathis, D. AIRE and APECED: Molecular insights into an autoimmune disease. Immunol. Rev. 2005, 204, 156–164. [Google Scholar] [CrossRef]
- Zheng, L.; Sharma, R.; Gaskin, F.; Fu, S.M.; Ju, S.-T. A novel role of IL-2 in organ-specific autoimmune inflammation beyond regulatory T cell check-point: Both IL-2 knockout and fas mutation prolong lifespan of scurfy mice but by different mechanisms. J. Immunol. 2007, 179, 8035–8041. [Google Scholar]
- Vang, K.B.; Yang, J.; Mahmud, S.A.; Burchill, M.A.; Vegoe, A.L.; Farrar, M.A. IL-2, -7 and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J. Immunol. 2008, 181, 3285–3290. [Google Scholar]
- Sharma, R.; Sung, S.-S.J.; Abaya, C.E.; Ju, A.C.-Y.; Fu, S.M.; Ju, S.-T. IL-2 regulates CD103 expression on CD4+ T cells in scurfy mice that display both CD103-dependent and independent inflammation. J. Immunol. 2009, 183, 1065–1073. [Google Scholar] [CrossRef]
- Yamane, H.; Zhu, J.; Paul, W.E. Independent roles for IL-2 and GATA-3 in stimulating naïve CD4+ T cells to generate a Th2-inducing cytokine environment. J. Exp. Med. 2005, 202, 793–804. [Google Scholar] [CrossRef]
- Cote-Sierra, J.; Foucras, G.; Guo, L.; Chiodetti, L.; Young, H.A.; Hu-Li, J.; Zhu, J.; Paul, W.E. Interleukin 2 plays a central role in Th2 differentiation. Proc. Natl. Acad. Sci. USA 2004, 101, 3880–3885. [Google Scholar]
- Hansen, G.; Berry, G.; DeKruyff, R.H.; Umetsu, D.T. Allergen-specific Th1 cells fail to counterbalance Th2 Cell-induced airway hyperreactivity but cause severe airway inflammation. J. Clin. Invest. 1999, 103, 175–183. [Google Scholar] [CrossRef]
- Randolph, D.A.; Stephens, R.; Carruthers, C.J.; Chaplin, D.D. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J. Clin. Invest. 1999, 104, 1021–1029. [Google Scholar] [CrossRef]
- Medoff, B.D.; Thomas, S.Y.; Luster, A.D. T cell trafficking in allergic asthma: The ins and outs. Annu. Rev. Immunol. 2008, 26, 205–232. [Google Scholar] [CrossRef]
- Schaerli, P.; Ebert, L.; Willimann, K.; Blaser, A.; Roos, R.S.; Loetscher, P.; Moser, B. A skin-selective homing mechanism for human immune surveillance T cells. J. Exp. Med. 2004, 199, 1265–1275. [Google Scholar] [CrossRef]
- Huter, E.N.; Natarajan, K.; Torgerson, T.R.; Glass, D.D.; Shevach, E.M. Autoantibodies in scurfy mice and IPEX patients recognize keratin 14. J. Invest. Dermatol. 2010, 130, 1391–1399. [Google Scholar] [CrossRef]
- Zhang, W.; Sharma, R.; Ju, S.-T.; He, X.S.; Tao, Y.; Tsuneyama, K.; Tian, Z.; Lian, Z.X.; Fu, S.M.; Gershwin, M.E. Deficiency in regulatory T cells results in development of antimitochondrial antibodies and autoimmune cholangitis. Hepatology 2009, 49, 545–552. [Google Scholar] [CrossRef]
- Szabo, S.J.; Sullivan, B.M.; Stemmann, C.; Satoskar, A.R.; Sleckman, B.P.; Glimcher, L.H. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science 2002, 295, 338–342. [Google Scholar] [CrossRef]
- Huang, S.; Hendriks, W.; Althage, A.; Hemmi, S.; Bluethmann, H.; Kamijo, R.; Vilcek, J.; Zinkernagel, R.M.; Aguet, M. Immune response in mice that lack the interferon-gamma receptor. Science 1993, 259, 1742–1745. [Google Scholar]
- Mach, F.; Sauty, A.; Iarossi, A.S.; Sukhova, G.K.; Neote, K.; Libby, P.; Luster, A.D. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J. Clin. Invest. 1999, 104, 1041–1050. [Google Scholar] [CrossRef]
- Cole, K.E.; Strick, C.A.; Paradis, T.J.; Ogborne, K.T.; Loetscher, M.; Gladue, R.P.; Lin, W.; Boyd, J.G.; Moser, B.; Wood, D.E.; et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): A novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J. Exp. Med. 1998, 187, 2009–2021. [Google Scholar] [CrossRef]
- Metwali, A.; Blum, A.; Elliott, D.E.; Weinstock, J.V. Interleukin-4 receptor α chain and STAT6 signaling inhibit gamma interferon but not Th2 cytokine expression within schistosome granulomas. Infect. Immun. 2002, 70, 5651–5658. [Google Scholar] [CrossRef]
- Huber, S.; Gagliani, N.; Esplugues, E.; O’Connor, W., Jr.; Huber, F.J.; Chaudhry, A.; Kamanaka, M.; Kobayashi, Y.; Booth, C.J.; Rudensky, A.Y.; et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3- and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 2011, 34, 554–565. [Google Scholar] [CrossRef]
- Duroudier, N.P.; Tulah, A.S.; Sayers, I. Leukotriene pathway genetics and pharmacogenetics in allergy. Allergy 2009, 64, 823–839. [Google Scholar] [CrossRef]
- Suri-Payer, E.; Kehn, P.J.; Cheever, A.W.; Shevach, E.M. Pathogenesis of post-thymectomy autoimmune gastritis. Identification of Anti-H/K adenosine triphosphatase-reactive T cells. J. Immunol. 1996, 157, 1799–1805. [Google Scholar]
- Fukuma, K.; Sakaguchi, S.; Kuribayashi, K.; Chen, W.-L.; Morishita, R.; Sekita, K.; Uchino, H.; Masuda, T. Immunologic and clinical studies on murine experimental autoimmune gastritis induced by neonatal thymectomy. Gastroenterology 1988, 94, 274–283. [Google Scholar]
- Setiady, Y.Y.; Ohno, K.; Samy, E.T.; Bagavant, H.; Qiao, H.; Sharp, C.; She, J.X.; Tung, K.S.K. Physiologic self antigens rapidly capacitate autoimmune disease-specific polyclonal CD4+CD25+ regulatory T cells. Blood 2006, 107, 1056–1062. [Google Scholar]
- Alard, P.; Thompson, C.; Agersborg, S.S.; Thatte, J.; Setiady, Y.; Samy, E.; Tung, K.S.K. Endogenous öocyte antigens are required for rapid induction and progression of autoimmune ovarian disease following day-3 thymectomy. J. Immunol. 2001, 166, 4363–4369. [Google Scholar]
- Wen, L.; Ley, R.E.; Volchkov, P.Y.; Stranges, P.B.; Avanesyan, L.; Stonebraker, A.C.; Hu, C.; Wong, F.S.; Szot, G.L.; Bluestone, J.A.; Gordon, J.I.; Chervonsky, A.V. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature 2008, 455, 1109–1113. [Google Scholar] [CrossRef]
- Sharma, R.; Jarjour, W.N.; Zheng, L.; Gaskin, F.; Fu, S.M.; Ju, S.-T. Large functional repertoire of regulatory T-cell suppressible autoimmune T cells in scurfy mice. J. Autoimmun. 2007, 29, 10–19. [Google Scholar] [CrossRef]
- Lin, W.; Truong, N.; Grossman, W.J.; Haribhai, D.; Williams, C.B.; Wang, J.; Martín, M.G.; Chatila, T.A. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J. Allergy Clin. Immunol. 2005, 116, 1106–1115. [Google Scholar] [CrossRef]
- Sharma, R.; Zheng, L.; Guo, X.; Fu, S.M.; Ju, S.-T.; Jarjour, W.N. Novel animal models for Sjögren’s syndrome: Expression and transfer of salivary gland dysfunction from regulatory T cell-deficient mice. J. Autoimmun. 2006, 27, 289–296. [Google Scholar] [CrossRef]
- Sawada, K.; Noumura, T. Effects of castration and sex steroids on sexually dimorphic development of the mouse submandibular gland. Acta Anat. (Basel) 1991, 140, 97–103. [Google Scholar] [CrossRef]
- Rubtsov, Y.P.; Rasmussen, J.P.; Chi, E.Y.; Fontenot, J.; Castelli, L.; Ye, X.; Treuting, P.; Siewe, L.; Roers, A.; Henderson, W.R., Jr.; et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 2008, 28, 546–558. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Rasmussen, J.P.; Gavin, M.A.; Rudensky, A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005, 6, 1142–1151. [Google Scholar] [CrossRef]
- Sharma, R.; Zheng, L.; Deshmukh, U.S.; Jarjour, W.N.; Sung, S.-S.J.; Fu, S.M.; Ju, S.-T. Cutting edge: A regulatory T cell-dependent novel function of CD25 (IL-2Rα) controlling memory CD8+ T cell homeostasis. J. Immunol. 2007, 178, 1251–1255. [Google Scholar]
- Hsu, W.; Zhang, W.; Tsuneyama, K.; Moritoki, Y.; Ridgway, W.M.; Ansari, A.A.; Coppel, R.L.; Lian, Z.X.; Mackay, I.; Gershwin, M.E. Differential mechanisms in the pathogenesis of autoimmune cholangitis versus inflammatory bowel disease in interleukin-2Ralpha(−/−) mice. Hepatology 2009, 49, 133–140. [Google Scholar] [CrossRef]
- Bennett, C.L.; Christie, J.; Ramsdell, F.; Brunkow, M.E.; Ferguson, P.J.; Whitesell, L.; Kelly, T.E.; Saulsbury, F.T.; Chance, P.F.; Ochs, H.D. The immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001, 27, 20–21. [Google Scholar] [CrossRef]
- Hsieh, C.S.; Liang, Y.; Tyznik, A.J.; Self, S.G.; Liggitt, D.; Rudensky, A.Y. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity 2004, 21, 267–277. [Google Scholar] [CrossRef]
- Pacholczyk, R.; Kern, J.; Singh, N.; Iwashima, M.; Kraj, P.; Ignatowicz, L. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity 2007, 27, 493–504. [Google Scholar] [CrossRef]
- Annacker, O.; Coombes, J.L.; Malmsgtrom, V.; Uhlig, H.H.; Bourne, T.; Johansson-Lindbom, B.; Agace, W.W.; Parker, C.M.; Powrie, F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J. Exp. Med. 2005, 202, 1051–1061. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ju, S.-T.; Sharma, R.; Gaskin, F.; Kung, J.T.; Fu, S.M. The Biology of Autoimmune Response in the Scurfy Mice that Lack the CD4+Foxp3+ Regulatory T-Cells. Biology 2012, 1, 18-42. https://doi.org/10.3390/biology1010018
Ju S-T, Sharma R, Gaskin F, Kung JT, Fu SM. The Biology of Autoimmune Response in the Scurfy Mice that Lack the CD4+Foxp3+ Regulatory T-Cells. Biology. 2012; 1(1):18-42. https://doi.org/10.3390/biology1010018
Chicago/Turabian StyleJu, Shyr-Te, Rahul Sharma, Felicia Gaskin, John T. Kung, and Shu Man Fu. 2012. "The Biology of Autoimmune Response in the Scurfy Mice that Lack the CD4+Foxp3+ Regulatory T-Cells" Biology 1, no. 1: 18-42. https://doi.org/10.3390/biology1010018
APA StyleJu, S.-T., Sharma, R., Gaskin, F., Kung, J. T., & Fu, S. M. (2012). The Biology of Autoimmune Response in the Scurfy Mice that Lack the CD4+Foxp3+ Regulatory T-Cells. Biology, 1(1), 18-42. https://doi.org/10.3390/biology1010018




