1. Introduction
Porous carbonaceous materials have been known about since earliest times, e.g., the Egyptians and Sumerians used wood chars in medicine [
1], water purification and the manufacture of bronze [
2]. Nowadays, an enormous variety of activated carbons (ACs) are available in grains, extrudates, monolith, fibers, cloths, etc. [
2,
3,
4]. Commercial ACs are widely used in various adsorption technologies because they are produced at a large scale and have a high adsorption capacity. In particular, ACs were proposed for adsorptive heat transformation and storage (AHTS) [
5,
6,
7,
8], which is an emerging sustainable technology to utilize low-temperature heat. This heat is readily available from renewable sources, such as solar energy, and heat wasted from industry, transport, dwellings, etc. A novel cycle “Heat from Cold” (HeCol) was proposed for amplification of low temperature ambient heat in cold countries [
9].
The HeCol cycle uses the heat of a natural non-frozen water basin (e.g., river, lake, groundwater, sea, etc.) with the temperature
TM = 0–20 °C as a heat source for evaporation and the adsorbent regeneration. The ambient air with a low temperature
TL from −50 to −20 °C serves as a sink for condensation heat. The useful heat is generated at the temperature
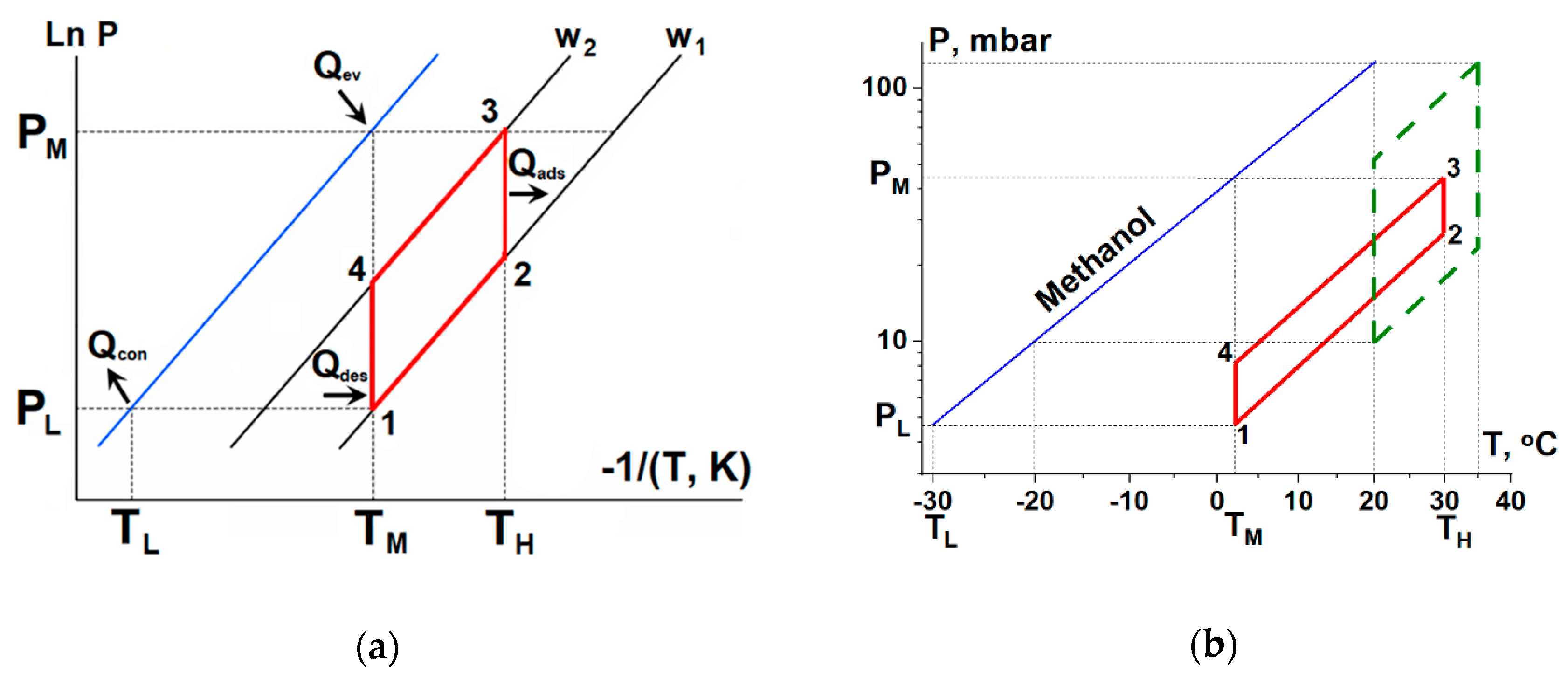
TH > 30–35 °C, sufficient, e.g., for floor heating in dwellings. The 3T working cycles consists of two isosteres (1–2 and 3–4 in
Figure 1) and two isotherms (2–3 and 4–1). The HeCol cycle is realized as follows: the adsorbent with the minimum uptake
w1 is isosterically heated from
TM to
TH (line 1–2), then the adsorber is connected to the evaporator maintained at
TM. A jump in adsorptive pressure over the adsorbent initiates the isothermal adsorption process (2–3). The released adsorption heat
Qads is transferred to a consumer at
TH, while the heat
Qev for evaporation is consumed for free from the ambient heat source at
TM. When the adsorbent is saturated by adsorptive to the maximum uptake
w2, the adsorber is disconnected from the evaporator and isosterically cooled down to the medium temperature
TM (line 3–4). Then, the adsorber is connected to the condenser at the low temperature
TL. The pressure drops to
PL, triggering desorption at
TM. The heat for desorption
Qdes is supplied from the natural water basin at
TM and the condensation heat
Qcon is dissipated to the ambient air at
TL.
Thus, the heat of non-frozen water basin at TM is transformed into the useful heat Qus at TH, and the rest is refused to the ambient at TL. Since the heat sink with low temperature is a prerequisite for the cycle operation, it was called “Heat from Cold”.
For the first theoretical [
10,
11] and experimental [
12,
13,
14,
15] studies of the HeCol cycle, methanol was used as an adsorptive because of its low freezing temperature (−97.6 °C). It always remains liquid under any conditions of the HeCol cycle. A commercial carbon ACM-35.4 was tested as an adsorbent, as it had been successfully used in AHTS processes [
4,
16]. In addition, it is available and cheap. These studies demonstrated that the HeCol cycle is feasible, and the temperature level of the useful heat can be sufficient for floor heating in low-energy buildings. A quite large specific power obtained in the first HeCol prototype (8.6 kW/kg-carbon) indicated quite fast methanol adsorption on this carbon. However, the specific useful heat
Qus reachable by using the working pair “methanol—AMC-35.4” does not exceed 235 J/g-carbon (for the cycle with boundary temperatures
TL/
TM/
TH = −20/20/35 °C). This could be mostly due to the relatively small specific mass of methanol exchanged in the cycle (Δ
w = 0.24 g/g-carbon). This mass corresponds well to the equilibrium methanol exchange for the studied carbon [
12]. At the lower temperature of the heat source for desorption (
TL/
TM/
TH = −30/2/30 °C), the useful heat dramatically drops to 70 J/g-carbon. This is due to reducing the amount of methanol exchanged to Δ
w = 0.08 g/g-carbon [
12]. Such a modest useful heat leads to the enhanced size of HeCol units, which, in its turn, increases the inert thermal mass of the unit and further reduces the
Qus-value. Therefore, carbons with advanced methanol exchange in the HeCol cycle are highly desirable.
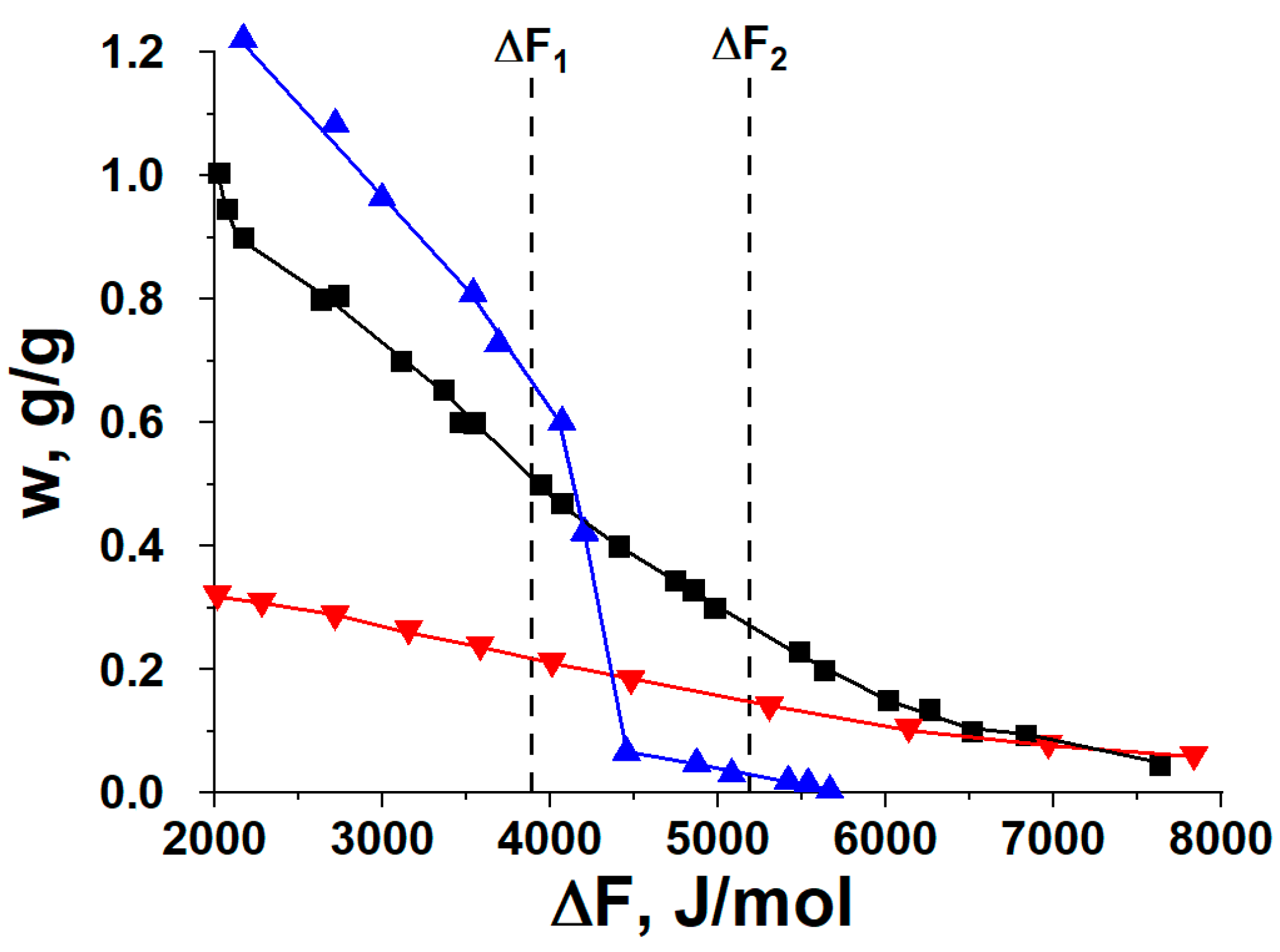
In this work, we make a comparative thermodynamic analysis of various commercial carbons as methanol adsorbents, keeping in mind the application in the HeCol cycles (
Section 3). An innovative composite “LiCl confined to multi-wall carbon nanotubes” (LiCl/MWCNT) [
17] is also briefly considered and compared with pure carbons because of its high methanol sorption capacity. This sorbent appears to be very promising for many AHTS applications in various climates [
18]. Carbon Maxsorb III [
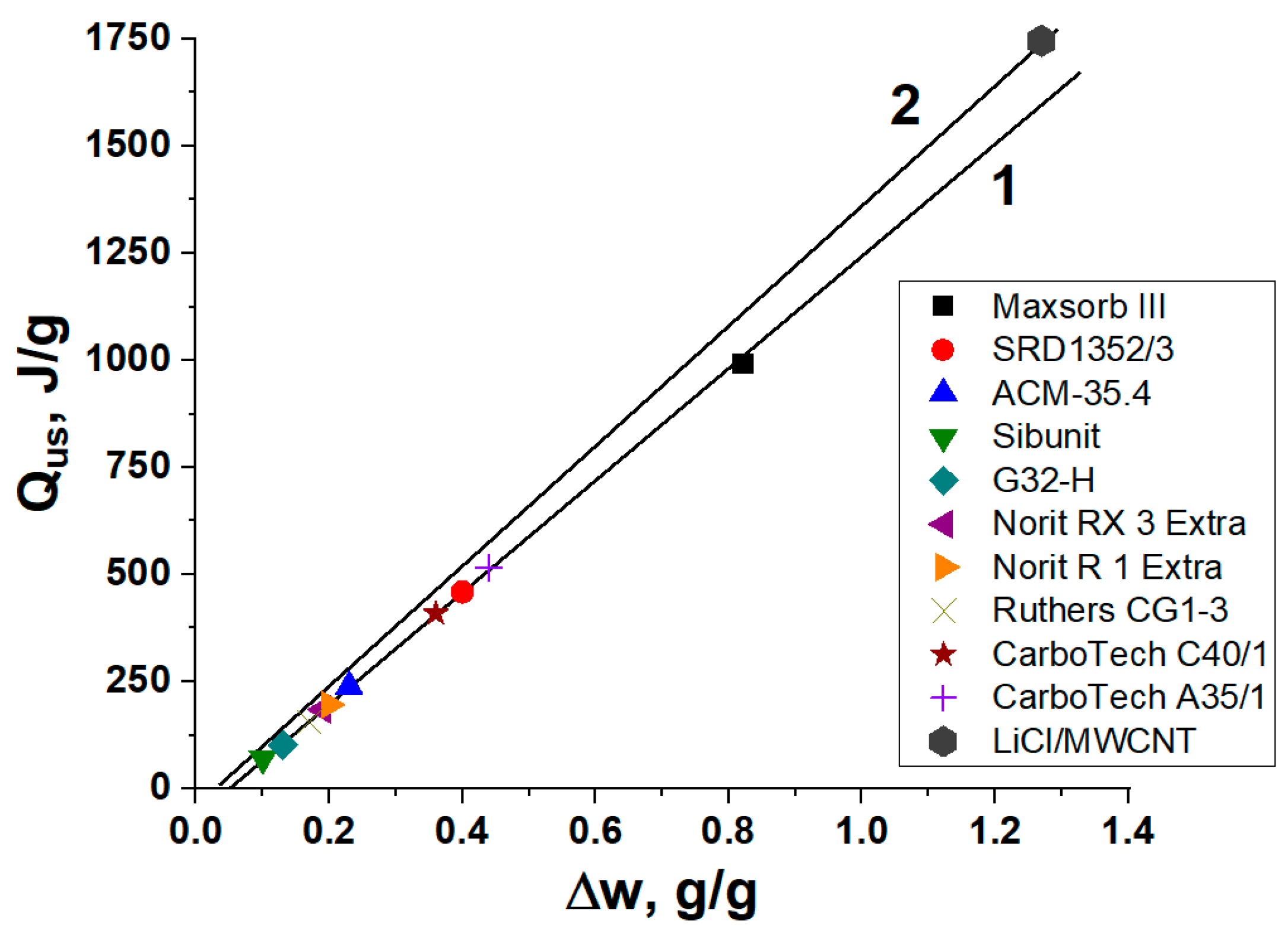
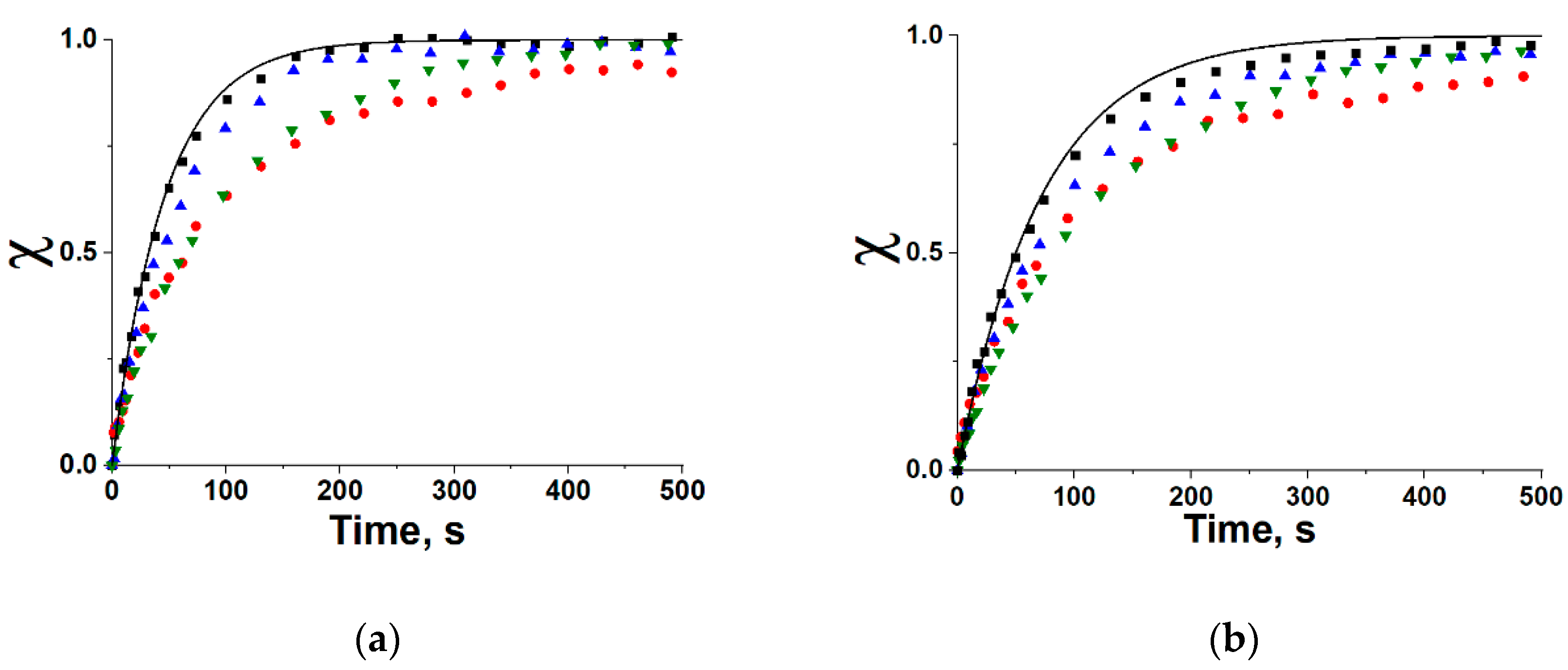
19] is found to possess the largest methanol uptake variation under conditions of the HeCol cycle among the ACs studied. As it ensures the best performance of the cycle, it is chosen for studying methanol adsorption dynamics (
Section 4). Its isothermal dynamics is compared with that on ACM-35.4 and LiCl/MWCNT for both adsorption and desorption stages of the selected HeCol cycle. The commercial Maxsorb III is available in the form of fine powder with the particle size < 10 μm, which could not be directly used for dynamic testing. Therefore, the powder is shaped to larger particles by either using a binder (polyvinyl alcohol, PVA) or pressing (
Section 2). Their textural characteristics were studied by a low-temperature nitrogen adsorption.
5. Summary
Porous carbonaceous materials have been well known since ancient times, when wood chars were used in medicine, water purification and the manufacture of bronze. In this day, many commercial activated carbons (ACs) are widely used in various adsorption technologies, including adsorptive heat transformation and storage (AHTS). This work addresses the applicability of commercial ACs and an innovative carbonaceous composite “LiCl in multi-wall carbon nanotubes” (LiCl/MWCNT) in a new cycle “Heat from Cold” (HeCol). This cycle proposed for the amplification of low-temperature ambient heat in cold countries was analyzed with methanol as an adsorptive and a commercial carbon ACM-35.4 as an adsorbent. These studies demonstrated that the HeCol cycle is feasible; however, carbons with a larger mass of methanol exchanged in the HeCol cycle are welcome.
The useful heat generated per cycle is the main performance indicator of HeCol cycles. It can reach 990 and 1750 J/g by using the carbon Maxsorb III and the composite, respectively, which is much larger than for ACM-35.4. For these materials, methanol adsorption dynamics under typical conditions of HeCol cycles is experimentally studied by the large pressure jump method. Before making this analysis, the fine carbon powder is consolidated by using a binder (polyvinyl alcohol) or pressing to obtain larger particles (ca. 2 mm). The methanol desorption at
T = 2 °C from the consolidated samples of Maxsorb III is faster than for LiCl/MWCNT, and the maximum (initial) useful power reaches (2.5–4.0) kW/kg-carbon. It is promising for designing compact HeCol units utilizing this carbon. It is worth mentioning that Maxsorb III is also very capable of adsorbing ammonia and hydrofluorocarbons (R32, R134, R152), which can be considered as alternative adsorptives for HeCol cycles [
33].
This study emphasizes the promise of activated carbons for application in the new adsorptive cycle “Heat from Cold” and highlights that the carbon Maxsorb III is the most promising from both thermodynamic and kinetic points of view.