A Time-Efficient Dip Coating Technique for the Deposition of Microgels onto the Optical Fiber Tip
Abstract
1. Introduction
2. Materials and Methods
2.1. Microgel Synthesis and Characterization
2.2. LOF Probe Fabrication
2.3. Bare Optical Fiber Probe Silanization
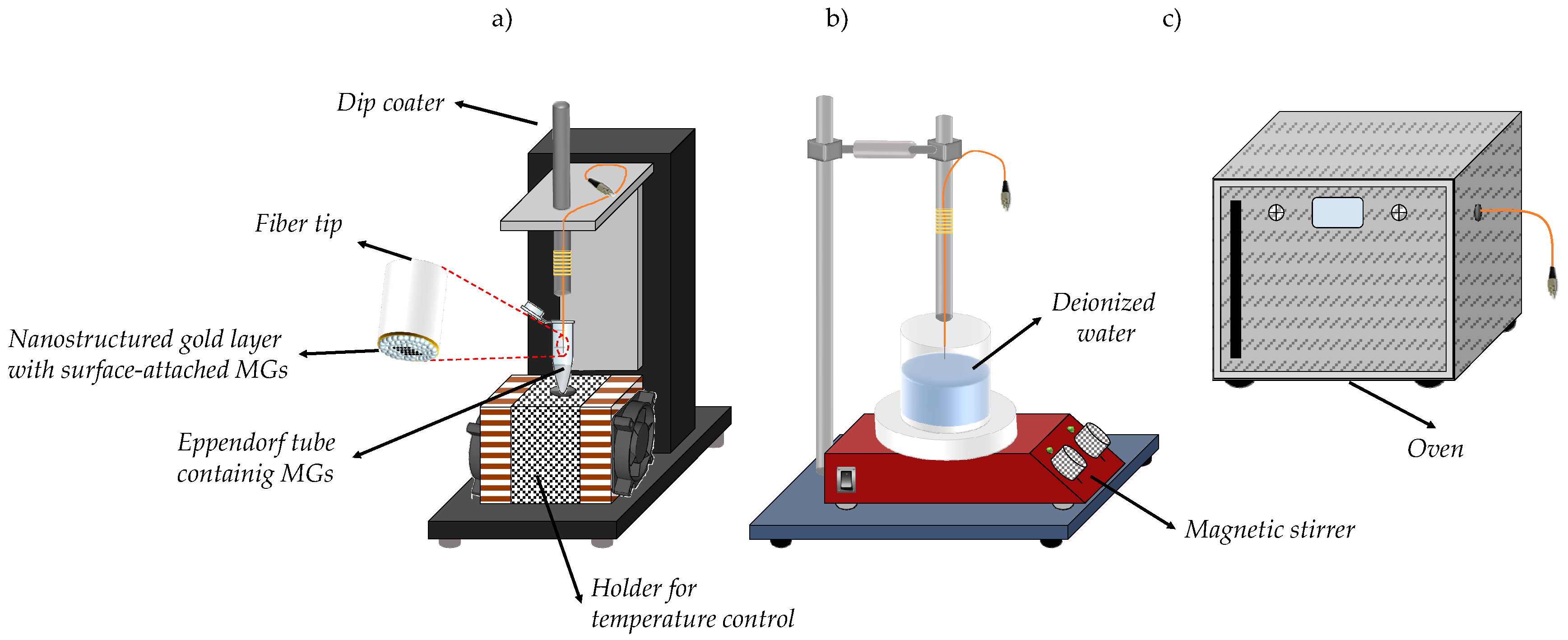
2.4. The Dip Coating Technique
2.5. Optical Characterization
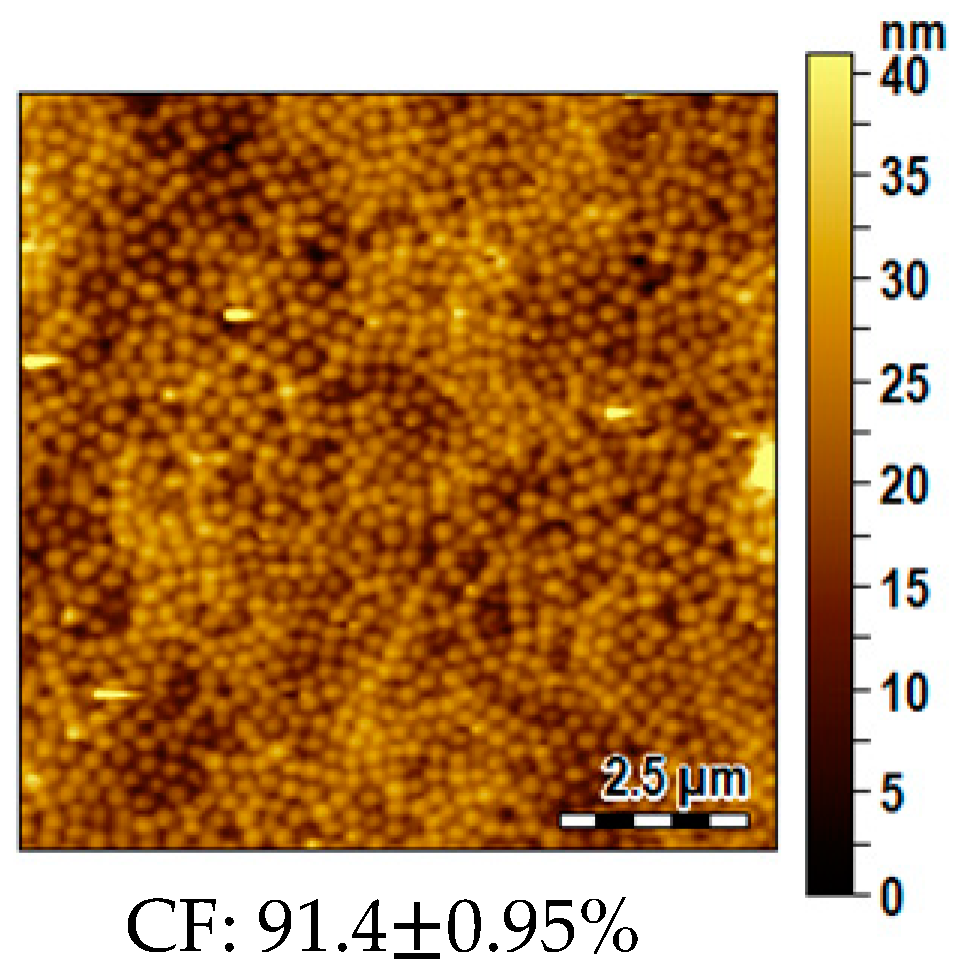
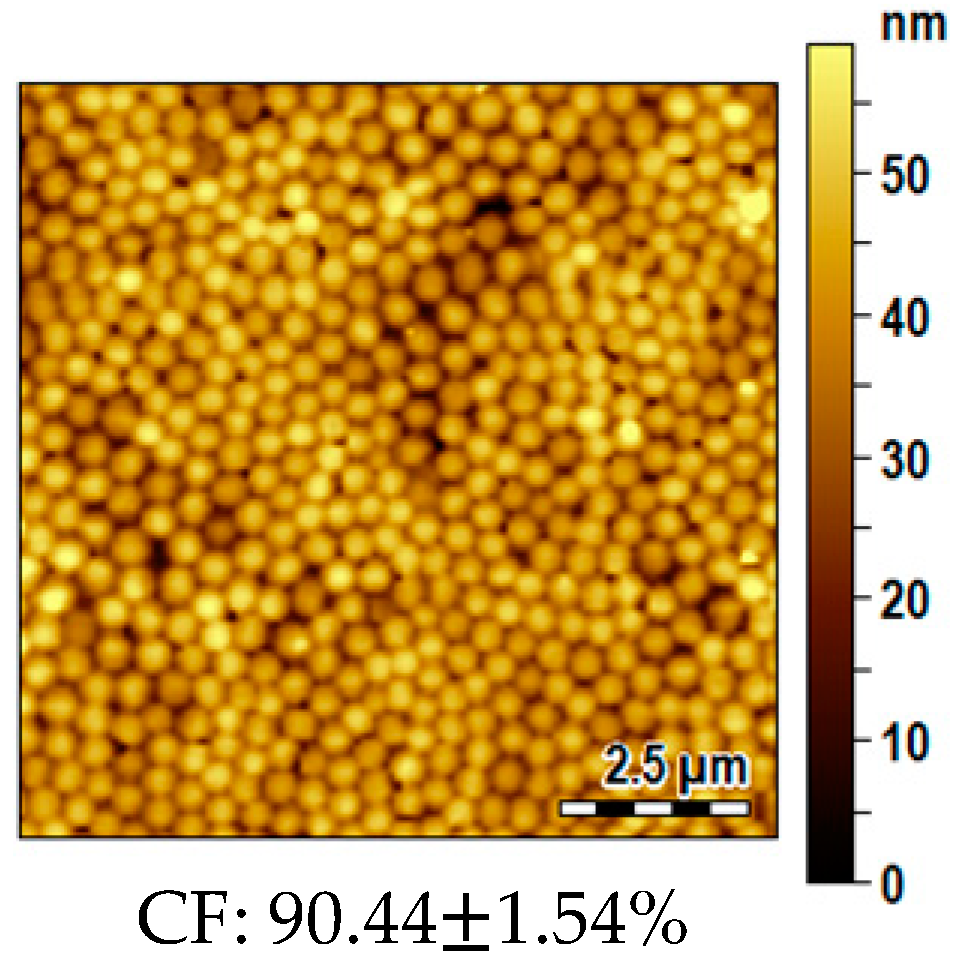
2.6. Morphological Analysis
3. Results and Discussion
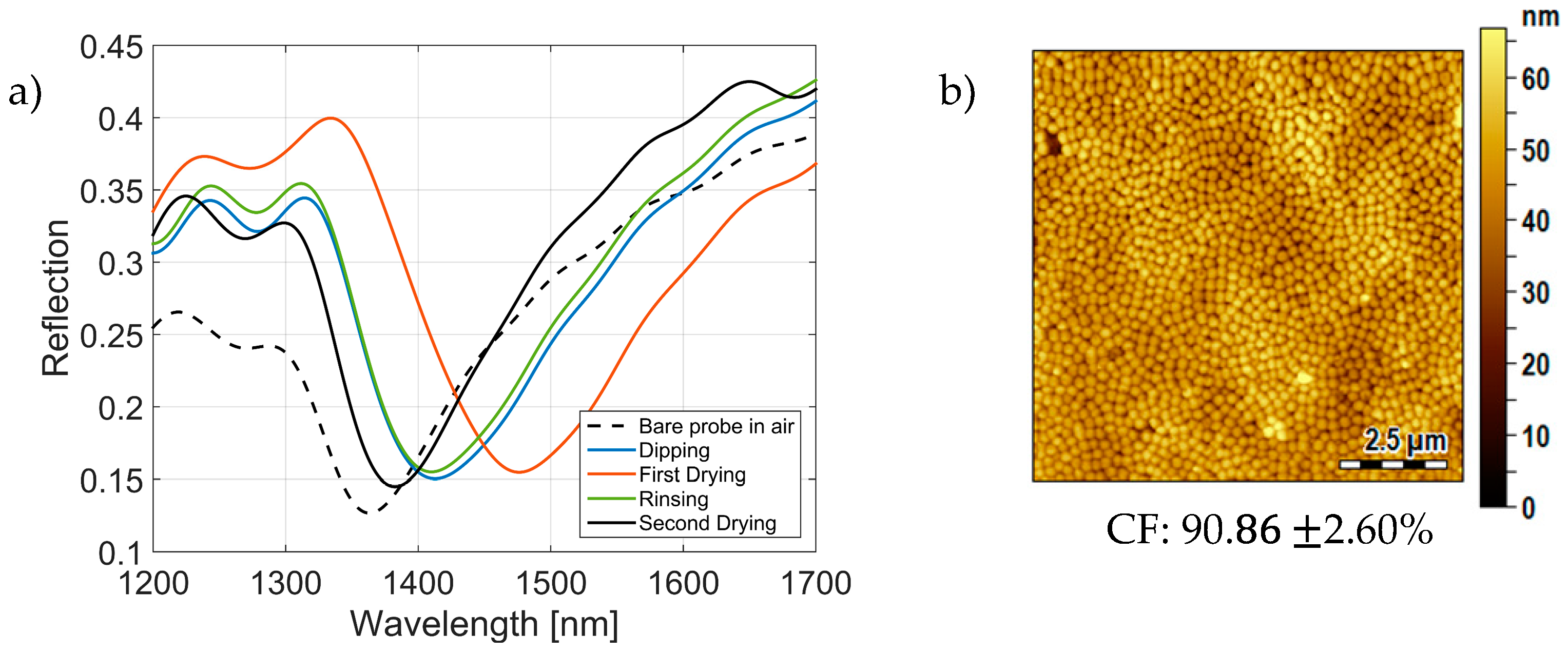
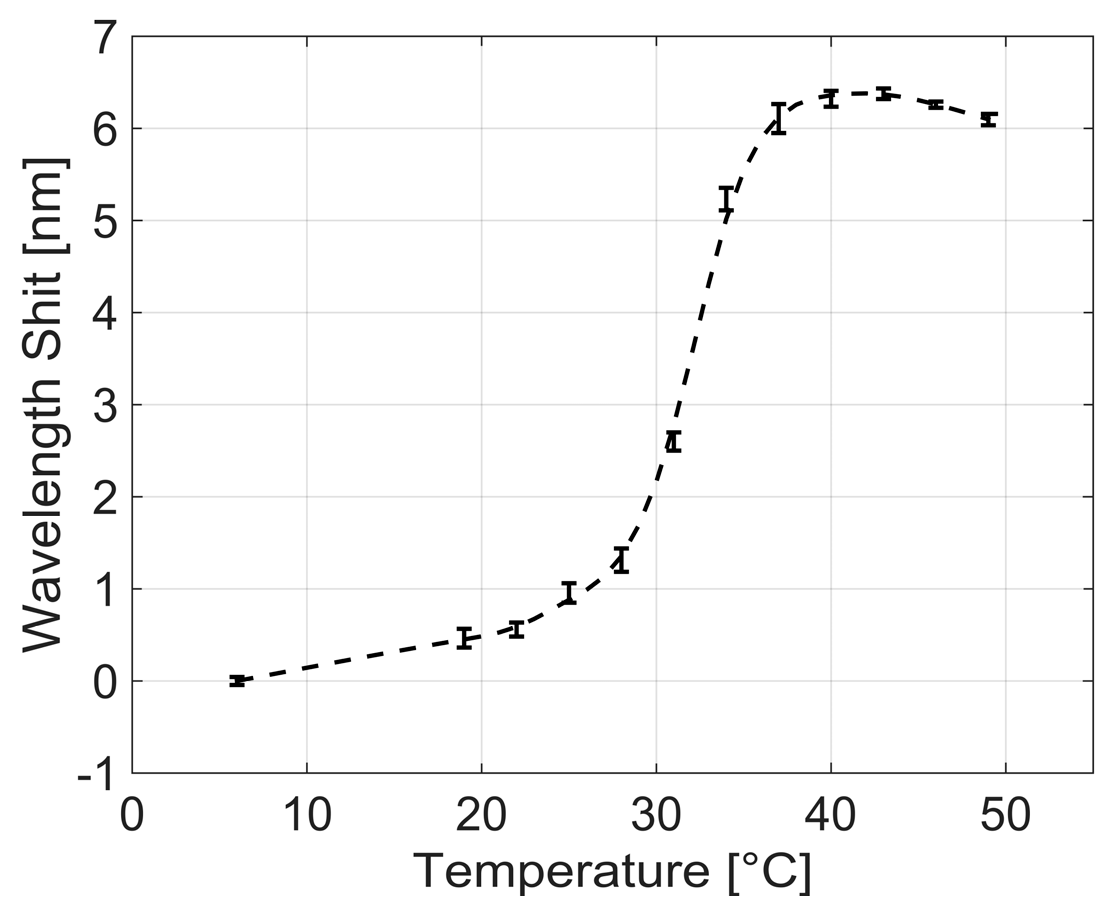
3.1. Optical Monitoring of the Fabrication Process
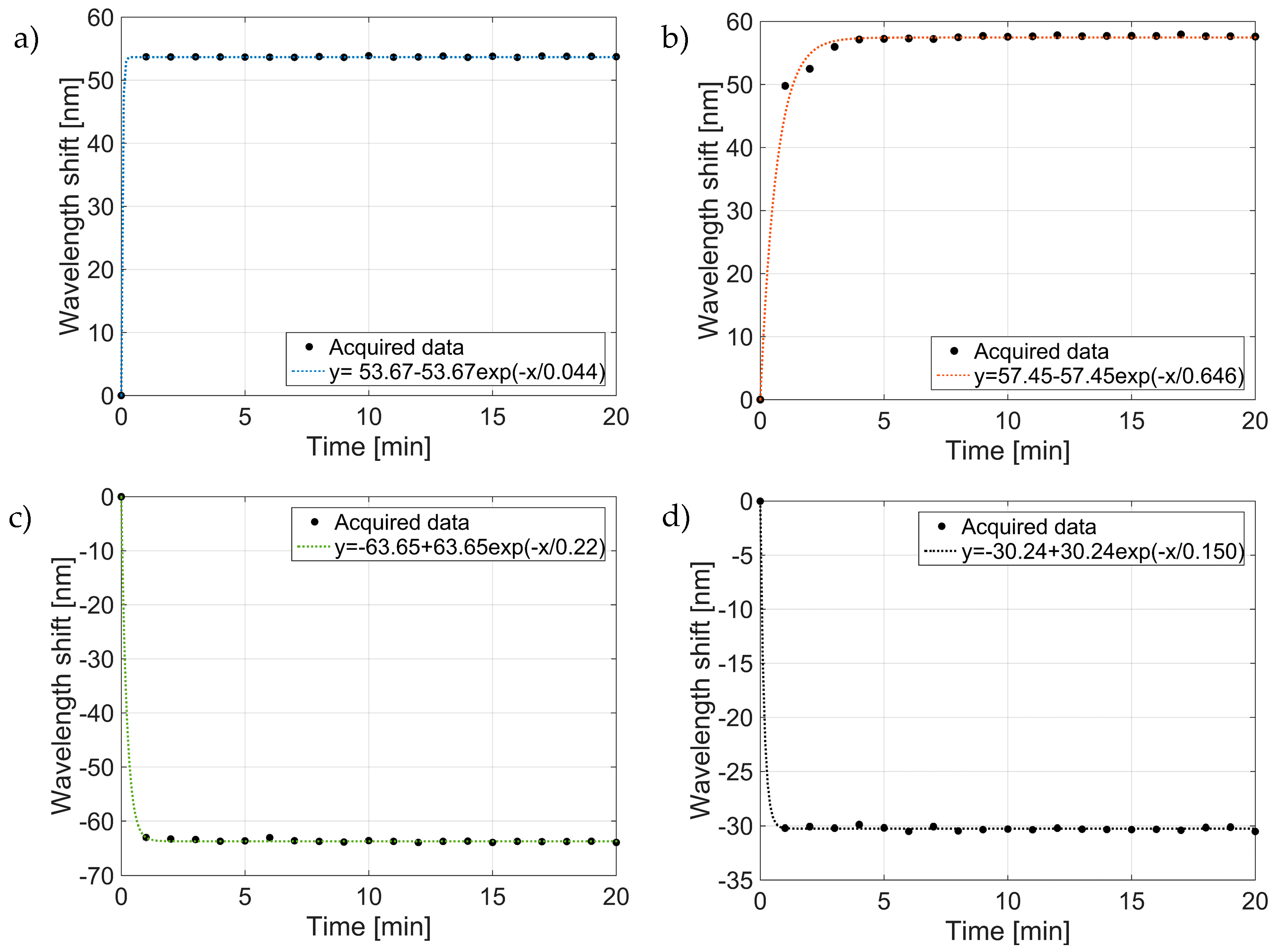
3.2. Study on the Dipping Step
3.3. Study on the Drying Steps
3.4. Study on the Rinsing Step
3.5. Remarks on the Optrode Performances
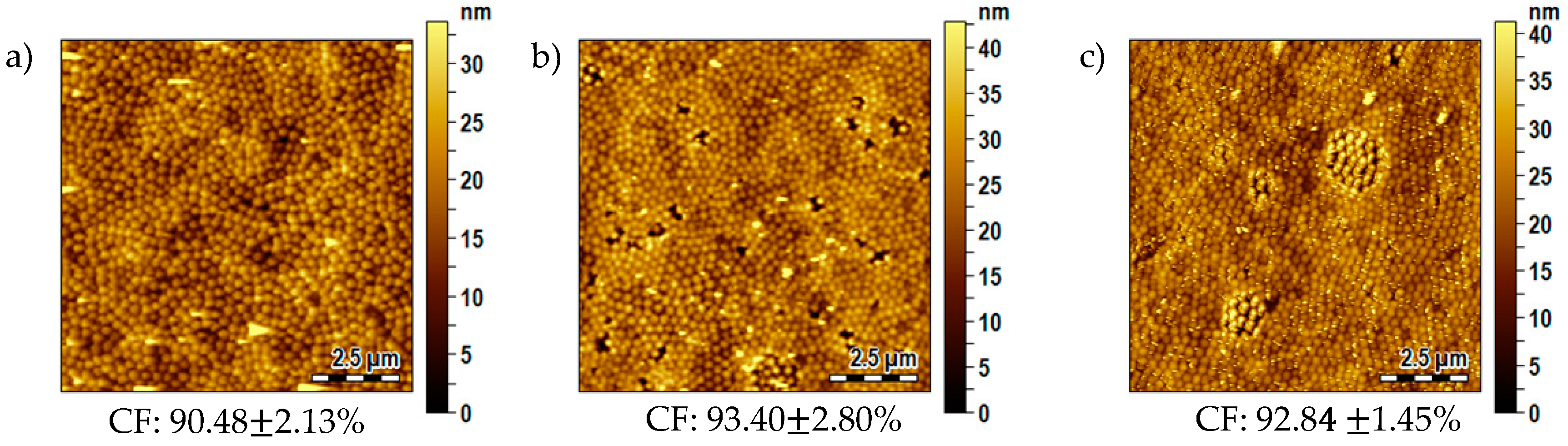
3.6. Influence of the Substrate Typology
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cusano, A.; Consales, M.; Crescitelli, A.; Ricciardi, A. Lab-on-Fiber Technology; Springer: New York, NY, USA, 2015; Volume 56. [Google Scholar]
- Consales, M.; Ricciardi, A.; Crescitelli, A.; Esposito, E.; Cutolo, A.; Cusano, A. Lab-on-Fiber Technology: Toward Multifunctional Optical Nanoprobes. ACS Nano 2012, 6, 3163–3170. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.; Consoles, M.; Quero, G.; Crescitelli, A.; Esposito, E.; Cusano, A. Versatile Optical Fiber Nanoprobes: From Plasmonic Biosensors to Polarization-Sensitive Devices. ACS Photonics 2014, 1, 69–78. [Google Scholar] [CrossRef]
- Ricciardi, A.; Crescitelli, A.; Vaiano, P.; Quero, G.; Consales, M.; Pisco, M.; Esposito, E.; Cusano, A. Lab-on-fiber technology: A new vision for chemical and biological sensing. Analyst 2015, 140, 8068–8079. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, P.; Carotenuto, B.; Pisco, M.; Ricciardi, A.; Quero, G.; Consales, M.; Crescitelli, A.; Esposito, E.; Cusano, A. Lab on Fiber Technology for biological sensing applications. Laser Photonics Rev. 2016, 10, 922–961. [Google Scholar] [CrossRef]
- Kostovski, G.; Stoddart, P.R.; Mitchell, A. The Optical Fiber Tip: An Inherently Light-Coupled Microscopic Platform for Micro- and Nanotechnologies. Adv. Mater. 2014, 26, 3798–3820. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, B.; Micco, A.; Ricciardi, A.; Amorizzo, E.; Mercieri, M.; Cutolo, A.; Cusano, A. Optical Guidance Systems for Epidural Space Identification. IEEE J. Sel. Top. Quantum Electron. 2016, 23. [Google Scholar] [CrossRef]
- Aliberti, A.; Ricciardi, A.; Giaquinto, M.; Micco, A.; Bobeico, E.; La Ferrara, V.; Ruvo, M.; Cutolo, A.; Cusano, A. Microgel assisted Lab-on-Fiber Optrode. Sci. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, M.; Micco, A.; Aliberti, A.; Ricciardi, A.; Ruvo, M.; Cutolo, A.; Cusano, A. Microgel Photonics and Lab on Fiber Technology for Advanced Label Free Fiber Optic Nanoprobes. Proc. SPIE 2016. [Google Scholar] [CrossRef]
- Ricciardi, A.; Aliberti, A.; Giaquinto, M.; Micco, A.; Cusano, A. Microgel Photonics: A Breathing Cavity onto OPTICAL FIBRE TIP. In Proceedings of the 24th International Conference on Optical Fibre Sensors, Curitiba, Brazil, 28 September–2 October 2015; SPIE: Bellingham, WA, USA, 2015. [Google Scholar]
- Giaquinto, M.; Micco, A.; Aliberti, A.; Bobeico, E.; La Ferrara, V.; Menotti, R.; Ricciardi, A.; Cusano, A. Optimization Strategies for Responsivity Control of Microgel Assisted Lab-On-Fiber Optrodes. Sensors 2018, 18, 1119. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, M.; Ricciardi, A.; Aliberti, A.; Micco, A.; Bobeico, E.; Ruvo, M.; Cusano, A. Light-microgel interaction in resonant nanostructures. Sci. Rep. 2018, 8, 9331. [Google Scholar] [CrossRef] [PubMed]
- Pelton, R.; Hoare, T. Microgels and their synthesis: An introduction. In Microgel Suspensions: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2011; pp. 1–32. [Google Scholar]
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloids Interface Sci. 2000, 85, 1–33. [Google Scholar] [CrossRef]
- Plamper, F.A.; Richtering, W. Functional Microgels and Microgel Systems. Accounts Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.L.; Gao, Y.F.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Islam, M.R.; Ahiabu, A.; Li, X.; Serpe, M.J. Poly-(N-isopropylacrylamide) Microgel-Based Optical Devices for Sensing and Biosensing. Sensors 2014, 14, 8984–8995. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Irvine, J.; Serpe, M.J. Photothermally induced optical property changes of poly-(N-isopropylacrylamide) microgel-based etalons. ACS Appl. Mater. Interfaces 2015, 7, 24370–24376. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, S.; Bahadur, D.; Kundu, G.C.; Srivastava, R. Maleic acid incorporated poly-(N-isopropylacrylamide) polymer nanogels for dual-responsive delivery of doxorubicin hydrochloride. Eur. Polym. J. 2013, 49, 22–32. [Google Scholar] [CrossRef]
- Micco, A.; Ricciardi, A.; Pisco, M.; La Ferrara, V.; Cusano, A. Optical fiber tip templating using direct focused ion beam milling. Sci. Rep. 2015, 5, 15935. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Hellweg, T.; von Klitzing, R. Packing density control in P (NIPAM-co-AAc) microgel monolayers: Effect of surface charge, pH, and preparation technique. Langmuir 2008, 24, 12595–12602. [Google Scholar] [CrossRef] [PubMed]
- Nerapusri, V.; Keddie, J.L.; Vincent, B.; Bushnak, I.A. Swelling and deswelling of adsorbed microgel monolayers triggered by changes in temperature, pH, and electrolyte concentration. Langmuir 2006, 22, 5036–5041. [Google Scholar] [CrossRef] [PubMed]
- Spackova, B.; Wrobel, P.; Bockova, M.; Homola, J. Optical Biosensors Based on Plasmonic Nanostructures: A Review. Proc. IEEE 2016, 104, 2380–2408. [Google Scholar] [CrossRef]
- Serpe, M.J.; Jones, C.D.; Lyon, L.A. Layer-by-layer deposition of thermoresponsive microgel thin films. Langmuir 2003, 19, 8759–8764. [Google Scholar] [CrossRef]
- Sorrell, C.D.; Carter, M.C.; Serpe, M.J. A “paint-on” protocol for the facile assembly of uniform microgel coatings for color tunable etalon fabrication. ACS Appl. Mater. Interfaces 2011, 3, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Drechsler, M. Charge-induced self-assembly of 2-dimensional thermosensitive microgel particle patterns. Langmuir 2009, 25, 13100–13105. [Google Scholar] [CrossRef] [PubMed]
- Iori, F.; Corni, S.; Di Felice, R. Unraveling the interaction between histidine side chain and the Au(111) surface: A DFT study. J. Phys. Chem. C 2008, 112, 13540–13545. [Google Scholar] [CrossRef]
- Burmistrova, A.; Steitz, R.; von Klitzing, R. Temperature Response of PNIPAM Derivatives at Planar Surfaces: Comparison between Polyelectrolyte Multilayers and Adsorbed Microgels. Chemphyschem 2010, 11, 3571–3579. [Google Scholar] [CrossRef] [PubMed]

| Deposition Step | Time | Temperature |
|---|---|---|
| Dipping | 1 h 1 | 10 °C |
| First drying | 1 h | 45 °C |
| Rinsing | 12 h | room |
| Second Drying | 2 h | 30 °C |
| Deposition Step | Time | Temperature |
|---|---|---|
| Dipping | 1, 0.05, 0.025 min 1 | 10 °C |
| First drying | 1 h | 45 °C |
| Rinsing | 12 h | room |
| Second Drying | 2 h | 30 °C |
| Deposition Step | Time | Temperature |
| Dipping | 1 min 1 | 10 °C |
| First drying | 10 min | room |
| Rinsing | 12 h | room |
| Second Drying | 10 min | room |
| Deposition Step | Time | Temperature |
|---|---|---|
| Dipping | 1 min 1 | 10 °C |
| First drying | 10 min | room |
| Rinsing | 10 min | room |
| Second Drying | 10 min | room |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherino, L.; Giaquinto, M.; Micco, A.; Aliberti, A.; Bobeico, E.; La Ferrara, V.; Ruvo, M.; Ricciardi, A.; Cusano, A. A Time-Efficient Dip Coating Technique for the Deposition of Microgels onto the Optical Fiber Tip. Fibers 2018, 6, 72. https://doi.org/10.3390/fib6040072
Scherino L, Giaquinto M, Micco A, Aliberti A, Bobeico E, La Ferrara V, Ruvo M, Ricciardi A, Cusano A. A Time-Efficient Dip Coating Technique for the Deposition of Microgels onto the Optical Fiber Tip. Fibers. 2018; 6(4):72. https://doi.org/10.3390/fib6040072
Chicago/Turabian StyleScherino, Lorenzo, Martino Giaquinto, Alberto Micco, Anna Aliberti, Eugenia Bobeico, Vera La Ferrara, Menotti Ruvo, Armando Ricciardi, and Andrea Cusano. 2018. "A Time-Efficient Dip Coating Technique for the Deposition of Microgels onto the Optical Fiber Tip" Fibers 6, no. 4: 72. https://doi.org/10.3390/fib6040072
APA StyleScherino, L., Giaquinto, M., Micco, A., Aliberti, A., Bobeico, E., La Ferrara, V., Ruvo, M., Ricciardi, A., & Cusano, A. (2018). A Time-Efficient Dip Coating Technique for the Deposition of Microgels onto the Optical Fiber Tip. Fibers, 6(4), 72. https://doi.org/10.3390/fib6040072









