Preparation and Characterization of Polymeric-Hybrid PES/TiO2 Hollow Fiber Membranes for Potential Applications in Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dope Viscosity Measurements
2.2. Polymeric Dope Preparation, Hollow Fiber Spinning, and Post-Treatment
2.3. Fiber Morphology Characterization
2.4. Fiber Porosity
2.5. Pure Water Permeability Tests
2.6. Molecular Weight Cut-Off
2.7. Stability Tests of Membranes under UV-A Irradiation
2.8. Qualitative Methylene Blue (MB) Degradation Test
3. Results and Discussion
3.1. Polymeric Dope Viscosity
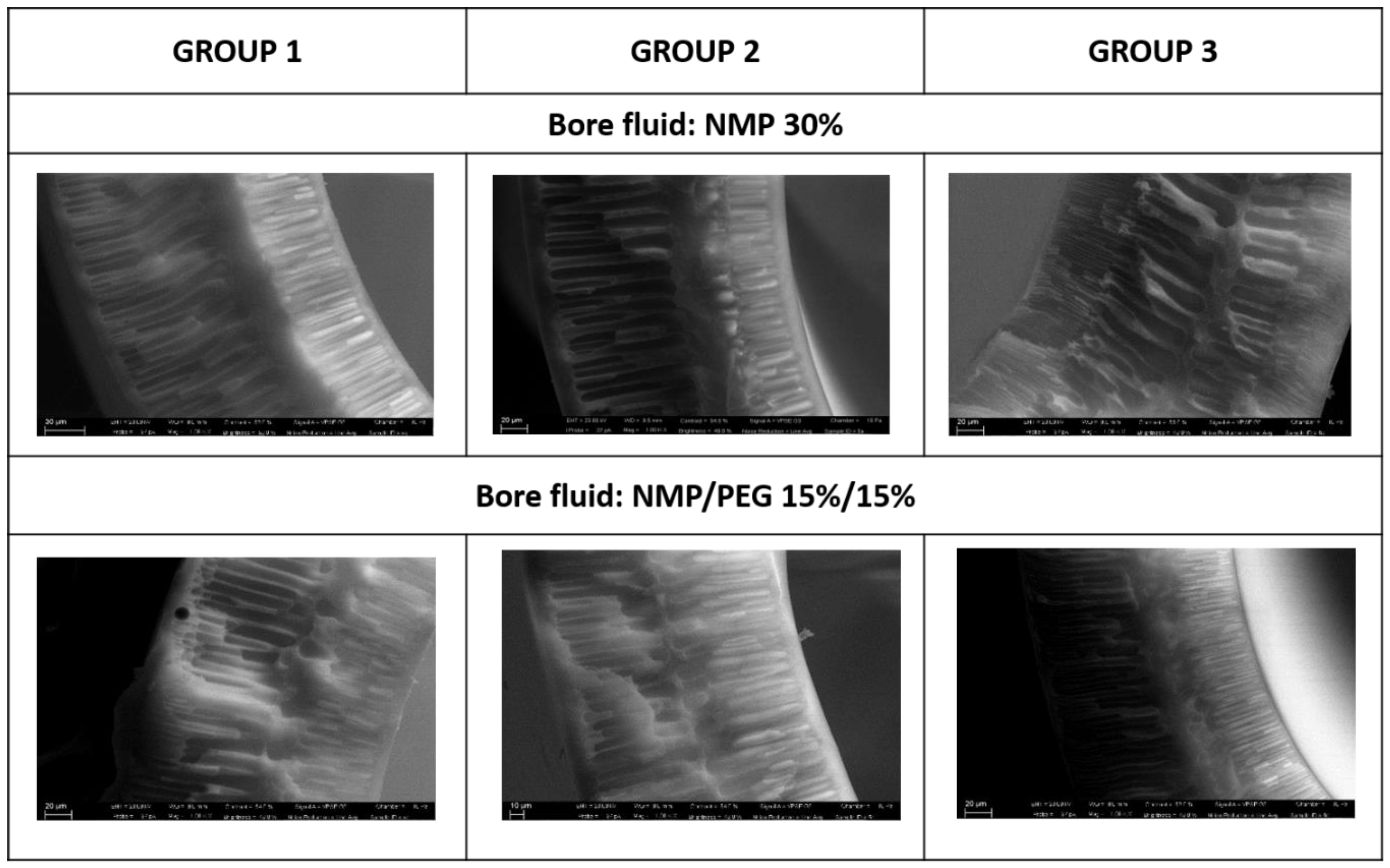
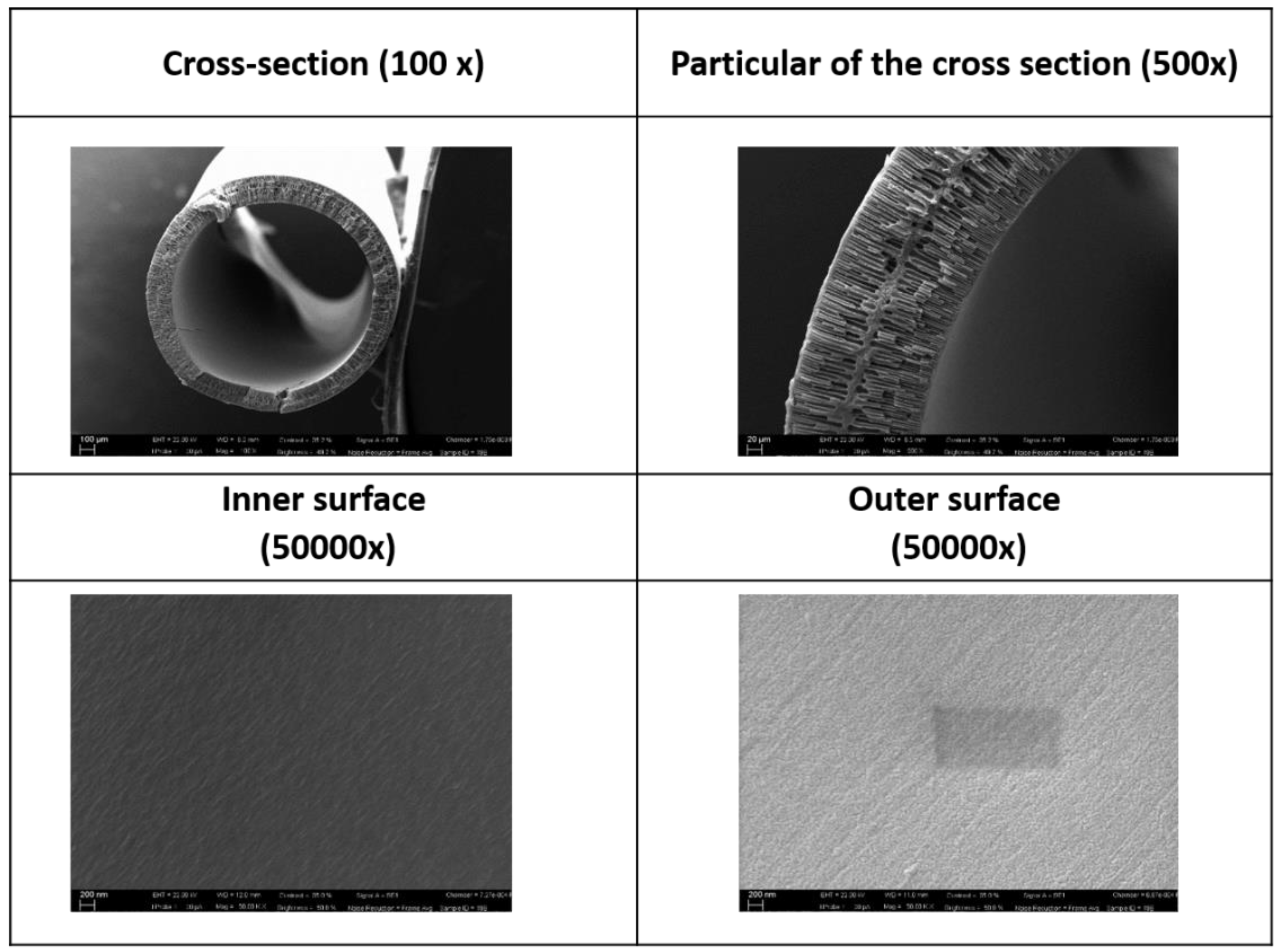
3.2. Blank PES Fiber Spinning Experiments and Optimization of Dope Composition
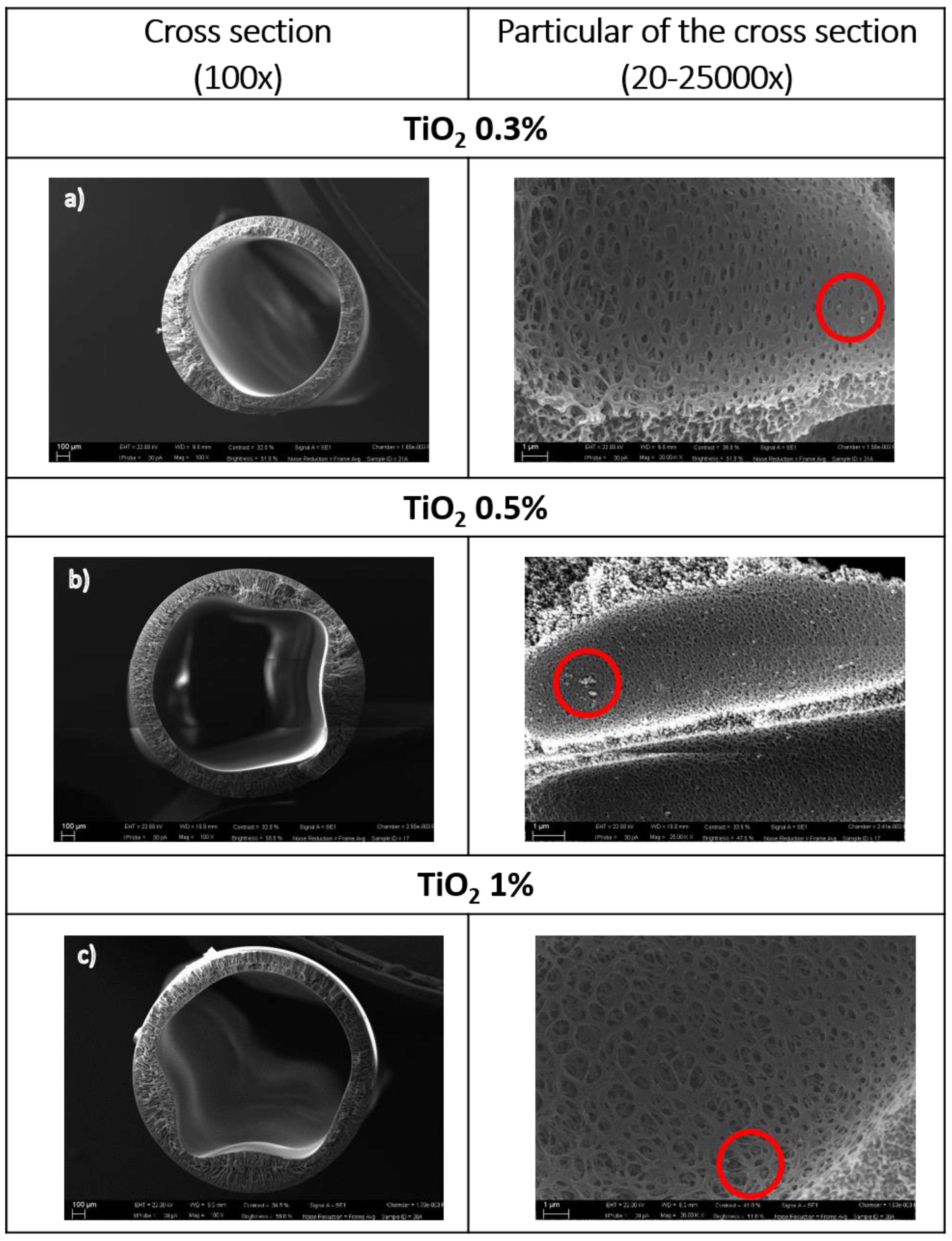
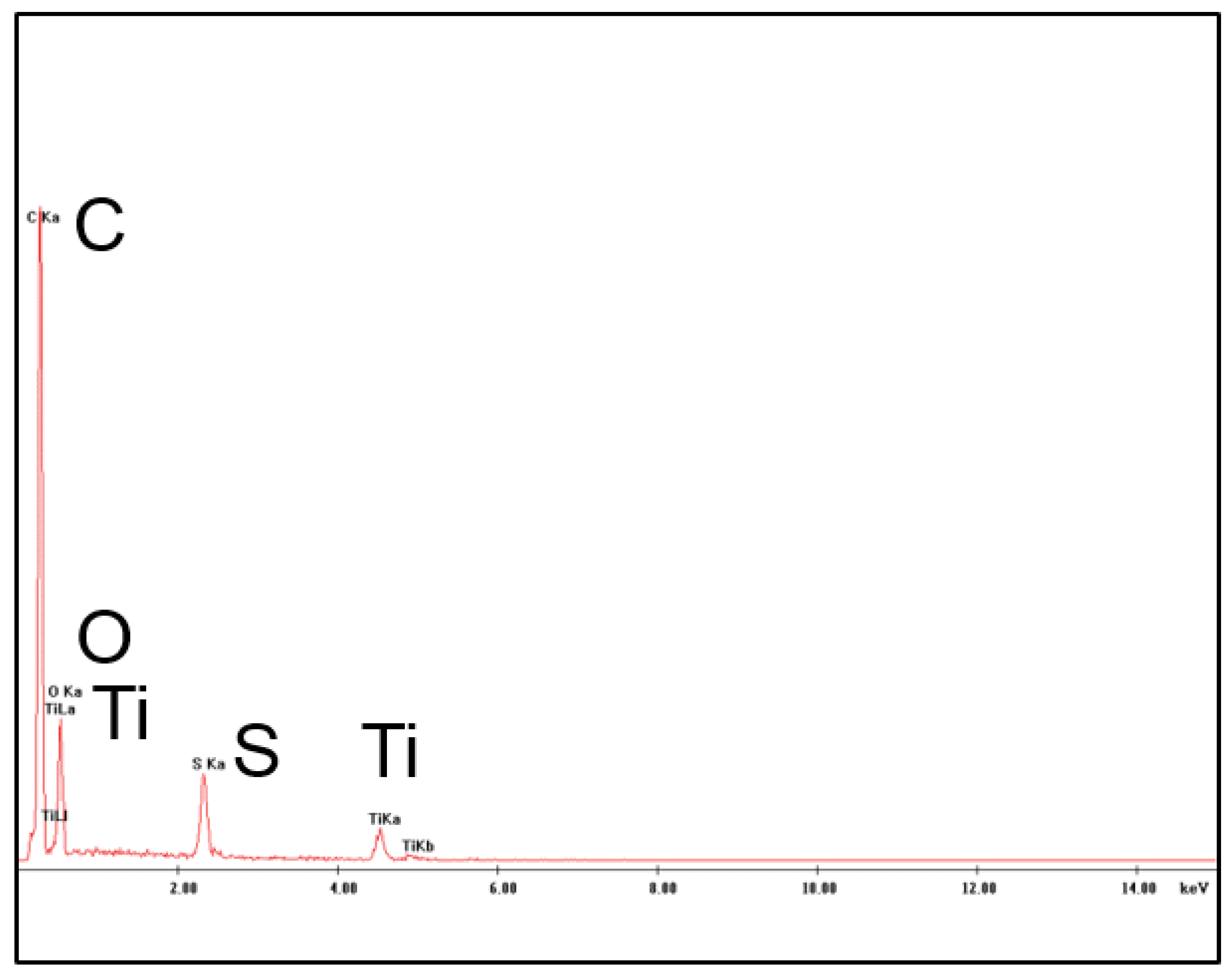
3.3. Preparation and Characterization of PES-TiO2 Hollow Fibers
3.4. Molecular Weight Cut-off
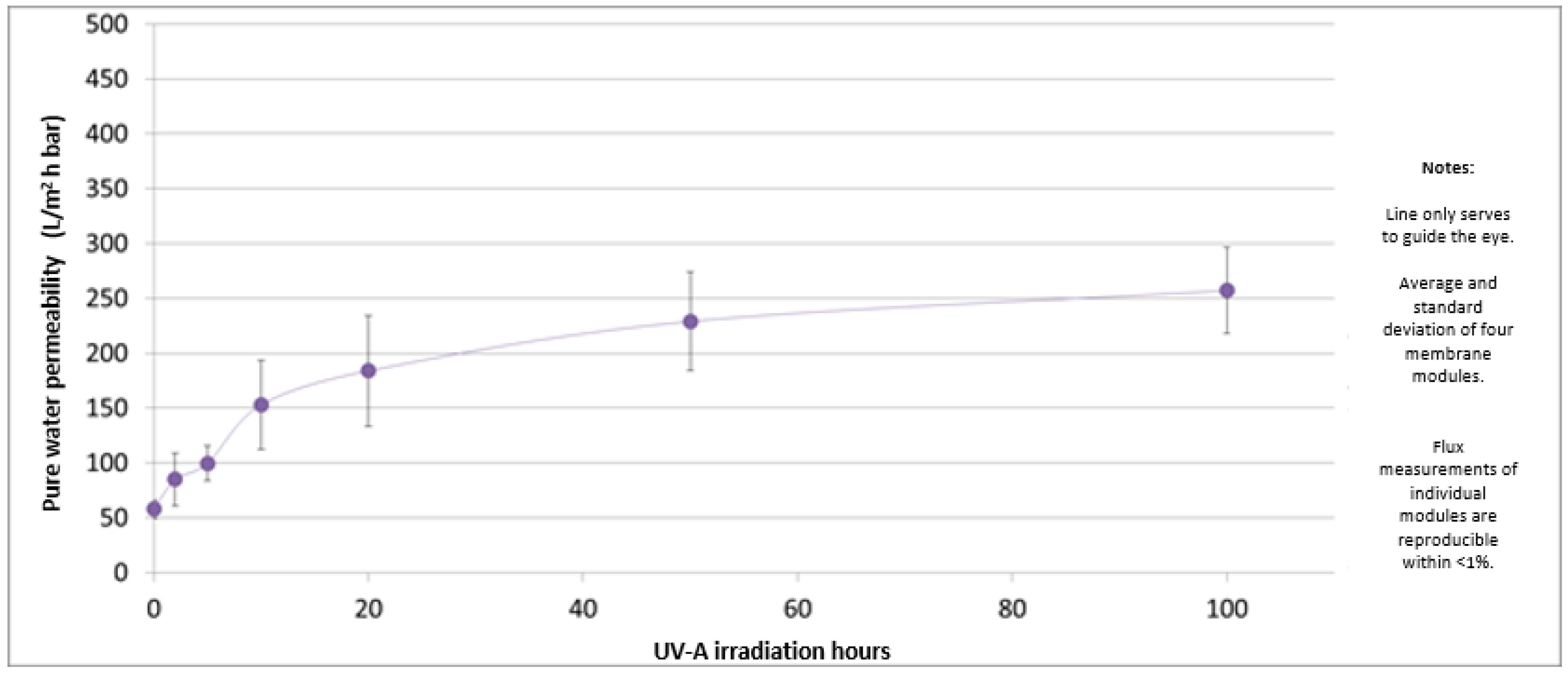
3.5. UV-A Irradiation Stability Test Results
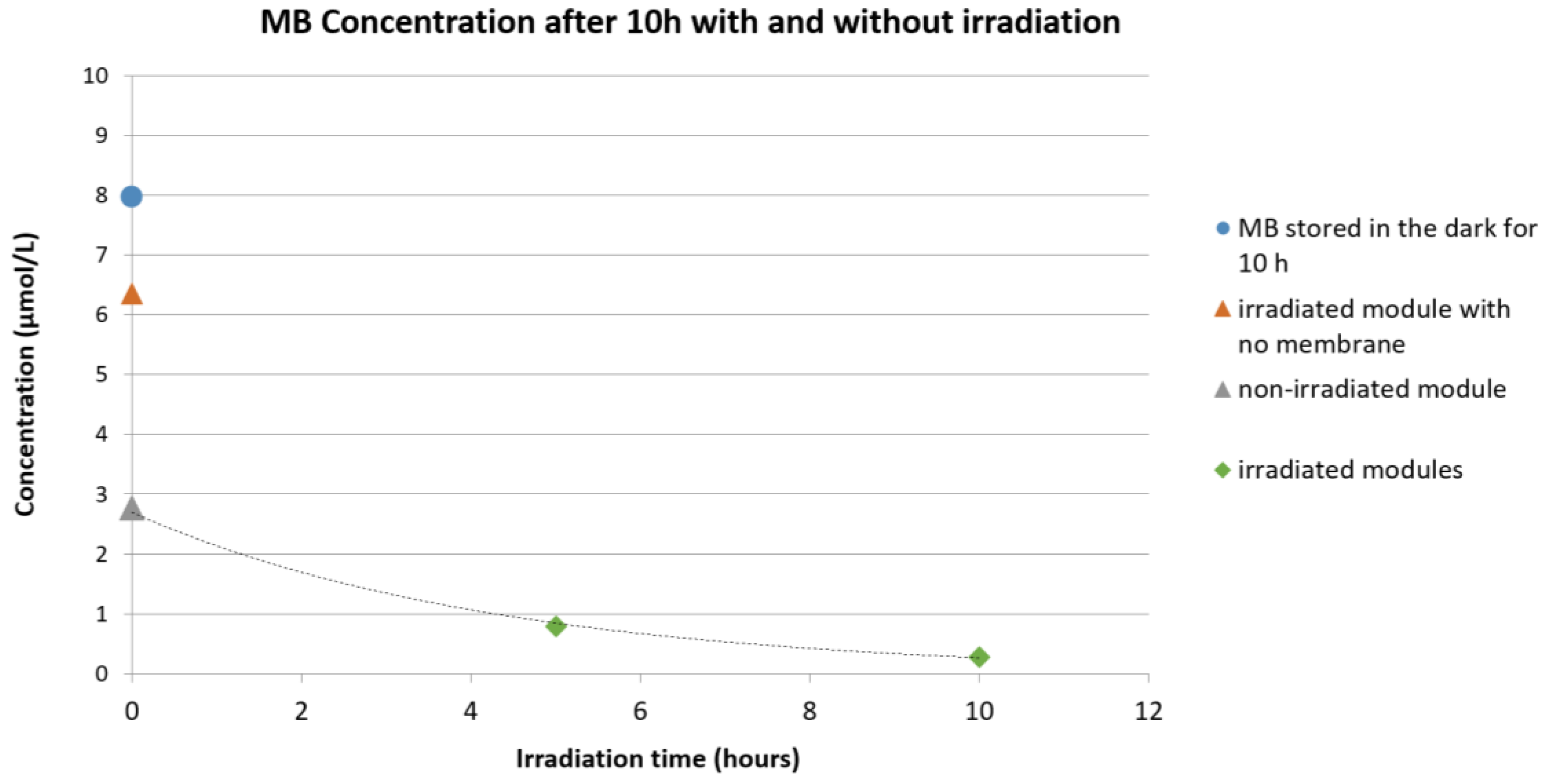
3.6. Qualitative Methylene Blue (MB) Degradation Test
5. Conclusions
- Fiber morphology and, in particular, the growth of finger-like macrovoids vs. sponge like morphology, are clearly affected by the dope viscosity and bore fluid composition.
- Fiber properties are dependent on the morphology, with fibers having a thicker sponge-like layer showing reduced PWP.
- Slight reduction of the polymer concentration, the combination of Pluronic® and water as additives, and the use of PEG 400 in the bore fluid resulted in the fibers with best properties.
- Increase of the humidity percentage in the air gap further improved the fiber permeability.
- High NP concentrations in the dope impaired fiber coagulation, giving rise to irregular morphologies.
- An NP concentration of 0.3 wt % gave the best results in terms of fiber morphology and properties.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Drioli, E.; Stankiewicz, A.I.; Macedonio, F. Membrane engineering in process intensification—An overview. J. Membr. Sci. 2011, 380, 1–8. [Google Scholar] [CrossRef]
- Drioli, E.; Macedonio, F. Membrane engineering for water engineering. Ind. Eng. Chem. Res. 2012, 51, 10051–10056. [Google Scholar] [CrossRef]
- Flemming, H.C.; Schaule, G.; Griebe, T.; Schmitt, J.; Tamachkiarowa, A. Biofouling—The Achilles heel of membrane processes. Desalination 1997, 113, 215–225. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z.V.P. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Shen, Y.; Lua, A.C. Preparation and characterization of mixed matrix membranes based on PVDF and three inorganic fillers (fumed nonporous silica, zeolite 4A and mesoporous MCM-41) for gas separation. Chem. Eng. J. 2012, 192, 201–210. [Google Scholar] [CrossRef]
- Anadao, P.; Sato, L.F.; Wiebeck, H.; Valenzuela-Diaz, F.R. Montmorillonite as a component of polysulfone nanocomposite membranes. Appl. Clay. Sci. 2010, 48, 127–132. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xia, J.; Zhang, F.; Li, F.; Xia, Y.; Li, Y. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Z.; Shan, M.; Min, C.; Zhou, B.; Li, Y.; Li, B.; Liu, L.; Qian, X. Effect of graphite oxide and multi-walled carbon nanotubes on the microstructure and performance of PVDF membranes. Sep. Purif. Technol. 2013, 103, 78–83. [Google Scholar] [CrossRef]
- Kou, L.; Gao, C. Making silica nanoparticle-covered graphene oxide nanohybrids as general building blocks for large-area superhydrophilic coatings. Nanoscale 2011, 3, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-graphene composite as a high performance photocatalyst. ACS Nano 2009, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Safarpour, M.; Vatanpour, V.; Khataee, A. Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 2016, 393, 65–78. [Google Scholar] [CrossRef]
- Bet-Moushoul, E.; Mansourpanah, Y.; Farhadi, K.; Tabatabaei, M. TiO2 nanocomposite based polymeric membranes: A review on performance improvement for various applications in chemical engineering processes. Chem. Eng. J. 2016, 283, 29–46. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Rahimpour, A. Effect of type of solvent and non-solvents on morphology and performance of polysulfone and polyethersulfone ultrafiltration membranes for milk concentration. Polym. Adv. Technol. 2005, 16, 717–724. [Google Scholar] [CrossRef]
- Idris, A.; Zain, N.M.; Noordin, M.Y. Synthesis, characterization and performance of asymmetric polyethersulfone (PES) ultrafiltration membranes with polyethylene glycol of different molecular weights as additives. Desalination 2007, 207, 324–339. [Google Scholar] [CrossRef]
- Li, J.F.; Xu, Z.L.; Yang, H. Microporous polyethersulfone membranes prepared under the combined precipitation conditions with non-solvent additives. Polym. Adv. Technol. 2008, 19, 251–257. [Google Scholar] [CrossRef]
- Susanto, H.; Stahra, N.; Ulbricht, M. High performance polyethersulfone microfiltration membranes having high flux and stable hydrophilic property. J. Membr. Sci. 2009, 342, 153–164. [Google Scholar] [CrossRef]
- Susanto, H.; Ulbricht, M. Characteristics, performance and stability of polyethersulfone ultrafiltration membranes prepared by phase separation method using different macromolecular additives. J. Membr. Sci. 2009, 327, 125–135. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Salih, H.A.; Simone, S.; Zablouk, M.; Drioli, E.; Figoli, A. Poly (ether sulfone) (PES) hollow-fiber membranes prepared from various spinning parameters. Desalination 2014, 345, 21–35. [Google Scholar] [CrossRef]
- Li, J.F.; Xu, Z.L.; Yang, H.; Yu, L.Y.; Liu, M. Effect of TiO2 nanoparticles on the surface morphology and performance of microporous PES membrane. Appl. Surf. Sci. 2009, 255, 4725–4732. [Google Scholar] [CrossRef]
- Razmjou, A.; Mansouri, J.; Chen, V. The effect of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Wu, G.P.; Gan, S.Y.; Cui, L.Z.; Xu, Y.Y. Preparation and characterization of PES/TiO2 composite membranes. Appl. Surf. Sci. 2008, 254, 7080–7086. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Khataee, A.R.; Salehi, E.; Zinadini, S.; Monfared, H.A. TiO2 embedded mixed matrix PES nanocomposite membranes: Influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 2012, 292, 19–29. [Google Scholar] [CrossRef]
- Razmjou, A.; Resosudarmo, A.; Holmes, R.L.; Li, H.; Mansouri, J.; Chen, V. The effect of modified TiO2 nanoparticles on the polyethersulfone ultrafiltration hollow fiber membranes. Desalination 2012, 287, 271–280. [Google Scholar] [CrossRef]
- Liang, C.-Y.; Uchytil, P.; Petrychkovych, R.; Lai, Y.-C.; Friess, K.; Sipek, M.; Reddy, M.M.; Suen, S.-Y. A comparison on gas separation between PES (polyethersulfone)/MMT (Na-montmorillonite) and PES/TiO2 mixed matrix membranes. Sep. Purif. Technol. 2012, 92, 57–63. [Google Scholar] [CrossRef]
- Mansourpanah, Y.; Madaeni, S.S.; Rahimpour, A. Formation of appropriate sites on nanofiltration membrane surface for binding TiO2 photo-catalyst: Performance, characterization and fouling resistant capability. J. Membr. Sci. 2009, 330, 297–306. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Taheri, A.H.; Mansourpanah, Y. Coupling TiO2 nanoparticles with UV irradiation for modification of polyethersulfone ultrafiltration membranes. J. Membr. Sci. 2008, 313, 158–169. [Google Scholar] [CrossRef]
- Razmjou, A.; Mansouri, J.; Chen, V.; Lim, M.; Amal, R. Titania nanocomposite polyethersulfone ultrafiltration membranes fabricated using a low temperature hydrothermal coating process. J. Membr. Sci. 2011, 380, 98–113. [Google Scholar] [CrossRef]
- Luo, M.-L.; Zhao, J.-Q.; Tang, W.; Pu, C.-S. Hydrophilic modification of poly(ether sulfone) ultrafiltration membrane surface by self-assembly of TiO2 nanoparticles. Appl. Surf. Sci. 2005, 249, 76–84. [Google Scholar] [CrossRef]
- Pourjafar, S.; Rahimpour, A.; Jahanshahi, M. Synthesis and characterization of PVA/PES thin film composite nanofiltration membrane modified with TiO2 nanoparticles for better performance and surface properties. J. Ind. Eng. Chem. 2012, 18, 1398–1405. [Google Scholar] [CrossRef]
- Chung, T.S.; Qin, J.J.; Gu, J. Effect of shear rate within the spinneret on morphology, separation performance and mechanical properties of ultrafiltration polyethersulfone hollow fiber membranes. Chem. Eng. Sci. 2000, 55, 1077–1091. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F.; Matsuura, T.; Kassim, M.A.; Abdullah, M.S. Effect of modified PVDF hollow fiber submerged ultrafiltration membrane for refinery wastewater treatment. Desalination 2011, 283, 214–220. [Google Scholar] [CrossRef]
- Han, L.F.; Xu, Z.L.; Yu, L.Y.; Wei, Y.M.; Cao, Y. Performance of PVDF/Multi-nanoparticles composite hollow fibre ultrafiltration membranes. Iran. Polym. J. (Engl. Ed.) 2010, 19, 553–565. [Google Scholar]
- Chiang, C.Y.; Jaipal Reddy, M.; Chu, P.P. Nano-tube TiO2 composite PVDF/LiPF6 solid membranes. Solid State Ionics 2004, 175, 631–635. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Shen, H.-M.; Xu, Z.-L. PVDF–TiO2 composite hollow fiber ultrafiltration membranes prepared by TiO2 sol–gel method and blending method. J. Appl. Polym. Sci. 2009, 113, 1763–1772. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H. Photocatalytic degradation of nonylphenol by immobilized TiO2 in dual layer hollow fibre membranes. Chem. Eng. J. 2015, 269, 255–261. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Stability study of PVDF/TiO2 dual layer hollow fibre membranes under long-term UV irradiation exposure. J. Water Process Eng. 2016, in press. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Fan, X.; Chen, S.; Yu, H.; Quan, X. A controlled wet-spinning and dip-coating process for preparation of high-permeable TiO2 hollow fiber membranes. Water Sci. Technol. 2016, 73, 725–733. [Google Scholar] [PubMed]
- Figoli, A.; Simone, S.; Criscuoli, A.; Al-Jlil, S.A.; Al Shabouna, F.S.; Al-Romaih, H.S.; Di Nicolò, E.; Al-Harbi, O.A.; Drioli, E. Hollow fibers for seawater desalination from blends of PVDF with different molecular weights: Morphology, properties and VMD performance. Polymer 2014, 55, 1296–1306. [Google Scholar] [CrossRef]
- Simone, S.; Figoli, A.; Criscuoli, A.; Carnevale, M.C.; Alfadul, S.; Al-Romaih, H.; Al Shabouna, F.; Al-Harbi, O.A.; Drioli, E. Effect of selected spinning parameters on PVDF hollow fibers morphology for potential application in desalination by VMD. Desalination 2014, 344, 28–35. [Google Scholar] [CrossRef]
- Simone, S.; Figoli, A.; Criscuoli, A.; Carnevale, M.C.; Rosselli, A.; Drioli, E. Preparation of hollow fibre membranes from PVDF/PVP blends and their application in VMD. J. Membr. Sci. 2010, 364, 219–232. [Google Scholar] [CrossRef]
- Wang, D. Polyethersulfone Hollow Fiber Gas Separation Membranes Prepared from Solvent Systems Containing Nonsolvent-Additives. Ph.D. Thesis, Department of Chemical Engineering, National University of Singapore, Singapore, 1995. [Google Scholar]
- Li, Q.; Xu, Z.L.; Yu, L.-Y. Effects of mixed solvents and PVDF types on performances of PVDF microporous membranes. J. Appl. Polym. Sci. 2010, 115, 2277–2287. [Google Scholar] [CrossRef]
- Lee, K.-W.; Se, B.-K.; Nam, S.-T.; Han, M.-J. Trade-off between thermodynamic enhancement and kinetic hindrance during phase inversion in the preparation of polysulfone membranes. Desalination 2003, 159, 289–296. [Google Scholar] [CrossRef]
- Guillen, G.R.; Ramon, G.Z.; Pirouz Kavehpour, H.; Kaner, R.B.; Hoek, E.M.V. Direct microscopic observation of membrane formation by nonsolvent induced phase separation. J. Membr. Sci. 2013, 431, 212–220. [Google Scholar] [CrossRef]
- Li, X.-M.; He, T. Does more solvent in bore liquid create more open inner surface in hollow fiber membranes? Polym. Adv. Technol. 2008, 19, 801–806. [Google Scholar] [CrossRef]
- Wang, H.T.; Yu, T.; Zhao, C.Y.; Du, Q.Y. Improvement of hydrophilicity and blood compatibility on polyethersulfone membrane by adding polyvinylpyrrolidone. Fiber Polym. 2009, 10, 1–5. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wang, T.; Su, Y.L.; Peng, F.B.; Wu, H.; Jiang, Z.Y. Protein-adsorption-resistance and permeation property of polyethersulfone and soybean phosphatidylcholine blend ultrafiltration membranes. J. Membr. Sci. 2006, 270, 108–114. [Google Scholar] [CrossRef]
- Barzin, J.; Feng, C.; Khulbe, K.C.; Matsuura, T.; Madaeni, S.S.; Mirzadeh, H. Characterization of polyethersulfone hemodialysis membrane by ultrafiltration and atomic force microscopy. J. Membr. Sci. 2004, 237, 77–85. [Google Scholar] [CrossRef]
- Mosqueda-Jimenez, D.B.; Narbaitz, R.M.; Matsuura, T. Effects of preparation conditions on the surface modification and performance of polyethersulfone ultrafiltration membranes. J. Appl. Polym. Sci. 2006, 99, 2978–2988. [Google Scholar] [CrossRef]
- Su, B.H.; Fu, P.; Li, Q.; Tao, Y.; Li, Z.; Zhao, C.S. Evaluation of polyethersulfone high flux hemodialysis membrane in vitro and in vivo. J. Mater. Sci. Mater. Med. 2008, 19, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Su, Y.L.; Li, C.; Shi, Q.; Ning, X.; Jiang, Z.Y. Fabrication of antifouling polyethersulfone ultrafiltration membranes using Pluronic F127 as both surface modifier and pore-forming agent. J. Membr. Sci. 2008, 318, 405–412. [Google Scholar] [CrossRef]
- Tasselli, F.; Jansen, J.C.; Drioli, E. PEEKWC Ultrafiltration Hollow-Fiber Membranes: Preparation, Morphology, and Transport Properties. J. Appl. Polym. Sci. 2004, 91, 841–853. [Google Scholar] [CrossRef]
- Tasselli, F.; Jansen, J.C.; Sidari, F.; Drioli, E. Morphology and transport property control of modified poly(ether ether ketone) (PEEKWC) hollow fiber membranes prepared from PEEKWC/PVP blends: Influence of the relative humidity in the air gap. J. Membr. Sci. 2005, 255, 13–22. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Mollahosseini, A.; Rajaeian, B. Structural and performance properties of UV-assisted TiO2 deposited nano-composite PVDF/SPES membranes. Desalination 2012, 285, 31–38. [Google Scholar] [CrossRef]
- Hu, W.; Yin, J.; Deng, B.; Hu, Z. Application of nano TiO2 modified hollow fiber membranes in algal membrane bioreactors for high-density algae cultivation and wastewater polishing. Bioresour. Technol. 2015, 193, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Ismail, A.F.; Rahbari-Sisakht, M. Synthesis and characterization of thin film nanocomposite forward osmosis membrane with hydrophilic nanocomposite support to reduce internal concentration polarization. J. Membr. Sci. 2014, 449, 74–85. [Google Scholar] [CrossRef]



| TiO2 Type | Membrane Preparation and Particle Immobilization Technique | Main Results/Application | REF |
|---|---|---|---|
| NANOPARTICLES IN MEMBRANE MATRIX | |||
| TiO2 (21 nm Degussa) | Combination of vapor and non solvent induced phase inversion (VIPS/NIPS) | Improved hydrophilicity and permeability; higher breaking strength and lower elongation ratio. | [21] |
| TiO2 (20 nm Degussa) Mechanically or mechanically/chemically modified | NIPS | Improved fouling resistance; optimal 2% concentration of TiO2; good dispersion thanks to chemical and mechanical modifications of particles. | [22] |
| TiO2 (30 nm, Hangzhou Dayang Chemical) Chemically modified | NIPS | Enhanced hydrophilicity, thermal stability, mechanical strength, and anti-fouling properties until 0.5% of TiO2. | [23] |
| TiO2 (20 nm Degussa) Mechanically or mechanically/chemically modified | Dry-jet/wet spinning technique (fibers) | Improved hydrophilicity, good dispersion thanks to chemical and mechanical modifications of particles. | [24] |
| TiO2 (20 nm Degussa) (8 or 15–25 nm Millennium Inorganics) | NIPS | Improved hydrophilicity and better resistance to fouling. | [25] |
| TiO2 (Rutile content = 61.2%, particle size = 70 nm) | Solvent evaporation (dense membranes) | Improved CO2/CH4 selectivity (optimal TiO2 concentration of 4%). | [26] |
| NANOPARTICLES ON MEMBRANE SURFACE | |||
| TiO2 (25 nm Degussa) | NIPS/treatment with DEA/dipping in aqueous TiO2 suspension. | Improved hydrophilicity and flux; increment in flux recovery ratio; uniform settlement of TiO2 nanoparticles thanks to diethyladipate (DEA). | [27] |
| TiO2 (25 nm Degussa) | NIPS/dipping in aqueous TiO2 suspension. | Antifouling property and long term flux stability further improved by UV irradiation. Coating gave better results than nanoparticles (NP) incorporation. | [28] |
| TiO2 nanoparticles were synthesized from titanium (IV) iso-propoxide (TTIP) | NIPS/coating by low temperature hydrothermal (LTH) process. | Low protein adsorption, photocatalytic activity, long term hydrophilicity, improvement in fouling performance, and increase in flux recovery after filtration of HA. Uniform and stable NP layer. | [29] |
| TiO2 nanoparticles were synthesized from titanium (IV) iso-propoxide (TTIP) (Particles 40 nm). | NIPS/dipping in aqueous TiO2 suspension. | Improved membrane hydrophilicity, anti-fouling ability, good separation performance (tests on PEG-5000). | [30] |
| TiO2 (20 nm Aldrich) | NIPS (PES)/coating with polyvinyl alcohol (PVA) cross-linked by glutaraldehyde/immersion in TiO2 suspension/heat treatment. | Superior performance in terms of flux and NaCl salt rejection. Optimal TiO2 concentration of 0.1%. | [31] |
| PES (wt %) | Additives (Name and Range of wt %) | NMP (wt %) |
|---|---|---|
| Measurements at 85 °C | ||
| 20 | PEG400/H2O/PVP K-17 30/0-5/0-10 | 35 |
| 20 | PEG400/H2O/PVP K-30 30/0-2.5/0-2.5 | 45 |
| 20 | PEG400/H2O/Plu F-127 30/0-2.5/0-2.5 | 45 |
| Measurements as a Function of Temperature (between 40 and 85 °C) | ||
| 20 | PEG400/H2O/PVP K-17 30/1.25-2.5/5 | 43.75–42.5 |
| 20 | PEG400/H2O/PVP K-30 30/1.25/1.25-2.5 | 47.5–46.25 |
| 20 | PEG400/H2O/Plu F-127 30/0-1.25/5 | 45 |
| PES (wt %) | Additives (Name and Range of wt %) | NMP (wt %) |
|---|---|---|
| Group 1 | ||
| 20 | PEG 400/H2O/PVP K-17 30/1.25/5 | 43.75 |
| Group 2 | ||
| 20 | PEG 400/H2O/PVP K-17 40/1.25/5 | 33.75 |
| Group 3 | ||
| 20 | PEG 400/Plu F-127 30/5 | 45 |
| Group 4 | ||
| 18 | PEG 400/H2O/Plu F-127 30/2/5 | 45 |
| Group 5 | ||
| 18 | PEG 400/H2O/Plu F-127/TiO2 30/2/5/0.3-1 | 44.7–44 |
| Common to All Experiments | |
| Bore fluid temperature | 25 °C |
| Outer coagulant | Tap water at room temperature |
| Air gap (cm) | 25 |
| Spinneret dimensions (cm) | O.D./I.D. 1.6/0.6 |
| Group 1 | |
| Dope temperature | Preparation 65 °C; spinning 40 °C |
| Viscosity at 40 °C | ~11,500 Cp |
| Dope flow rate (g/min) | 10.6 |
| Bore fluid composition and flow rate | a) NMP 30%, 13 mL/min |
| b) NMP 50%, 13 mL/min | |
| c) NMP 15%, PEG 15%, 13 mL/min | |
| d) NMP 30%, PEG 15%, 13 mL/min | |
| e) NMP 30%, PEG 30%, 13 mL/min | |
| Group 2 | |
| Dope temperature | Preparation 65 °C; spinning 40 °C |
| Viscosity at 40 °C | ~26,000 Cp |
| Dope flow rate (g/min) | 10.12 |
| Bore fluid composition and flow rate | a) NMP 30%, 13 mL/min |
| b) NMP 50%, 13 mL/min | |
| c) NMP 15%, PEG 15%, 13 mL/min | |
| d) NMP 30%, PEG 15%, 13 mL/min | |
| e) NMP 30%, PEG 30%, 13 mL/min | |
| f) NMP 30%, PEG 30%, 18 mL/min | |
| g) NMP 50%, 18 mL/min | |
| h) NMP 30%, PEG 15%, 18 mL/min | |
| Group 3 | |
| Dope temperature | Preparation 65 °C; spinning 40 °C |
| Viscosity at 40 °C | ~15,500 Cp |
| Dope flow rate (g/min) | 10.84 |
| Bore fluid composition and flow rate | a) NMP 30%, 13 mL/min |
| b) NMP 50%, 13 mL/min | |
| c) NMP 15%, PEG 15%, 13 mL/min | |
| d) NMP 30%, PEG 15%, 13 mL/min | |
| Dope Composition | BORE FLUID | O.D. | I.D. | Thickness | Porosity | PWP |
|---|---|---|---|---|---|---|
| (mm) | (mm) | (mm) | (%) | (L/hm2 bar) | ||
| PES/PEG400/H2O/Plu F-127/NMP 18/30/2/5/45 (Group n° 4) | PEG 45%, 13 mL/min | 1.67 ± 0.02 | 1.31 ± 0.01 | 0.18 ± 0.02 | 78.69 ± 0.88 | 185 |
| TiO2 | BORE FLUID | O.D. | I.D. | Thickness | Porosity | PWP |
|---|---|---|---|---|---|---|
| (wt %) | (mm) | (mm) | (mm) | (%) | (L/hm2 bar) | |
| 0.3 | PEG 40%, 13 mL/min | 1.63 ± 0.2 | 1.25 ± 0.03 | 0.19 ± 0.02 | 77.05 ± 1.59 | 75 |
| 0.5 | PEG 45%, 13 mL/min | 1.88 ± 0.03 | 1.49 ± 0.09 | 0.20 ± 0.06 | 71.02 ± 1.99 | 45 |
| 1 | PEG 45%, 13 mL/min | 1.97 ± 0.01 | 1.57 ± 0.06 | 0.20 ± 0.04 | 81.93 ± 2.39 | 10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simone, S.; Galiano, F.; Faccini, M.; Boerrigter, M.E.; Chaumette, C.; Drioli, E.; Figoli, A. Preparation and Characterization of Polymeric-Hybrid PES/TiO2 Hollow Fiber Membranes for Potential Applications in Water Treatment. Fibers 2017, 5, 14. https://doi.org/10.3390/fib5020014
Simone S, Galiano F, Faccini M, Boerrigter ME, Chaumette C, Drioli E, Figoli A. Preparation and Characterization of Polymeric-Hybrid PES/TiO2 Hollow Fiber Membranes for Potential Applications in Water Treatment. Fibers. 2017; 5(2):14. https://doi.org/10.3390/fib5020014
Chicago/Turabian StyleSimone, Silvia, Francesco Galiano, Mirko Faccini, Marcel E. Boerrigter, Christiane Chaumette, Enrico Drioli, and Alberto Figoli. 2017. "Preparation and Characterization of Polymeric-Hybrid PES/TiO2 Hollow Fiber Membranes for Potential Applications in Water Treatment" Fibers 5, no. 2: 14. https://doi.org/10.3390/fib5020014
APA StyleSimone, S., Galiano, F., Faccini, M., Boerrigter, M. E., Chaumette, C., Drioli, E., & Figoli, A. (2017). Preparation and Characterization of Polymeric-Hybrid PES/TiO2 Hollow Fiber Membranes for Potential Applications in Water Treatment. Fibers, 5(2), 14. https://doi.org/10.3390/fib5020014









