The Design of Temperature-Responsive Nanofiber Meshes for Cell Storage Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Nanofiber Fabrication
2.3. Characterizations
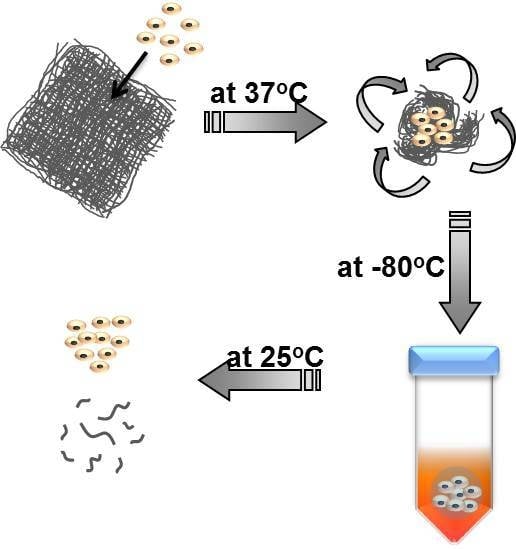
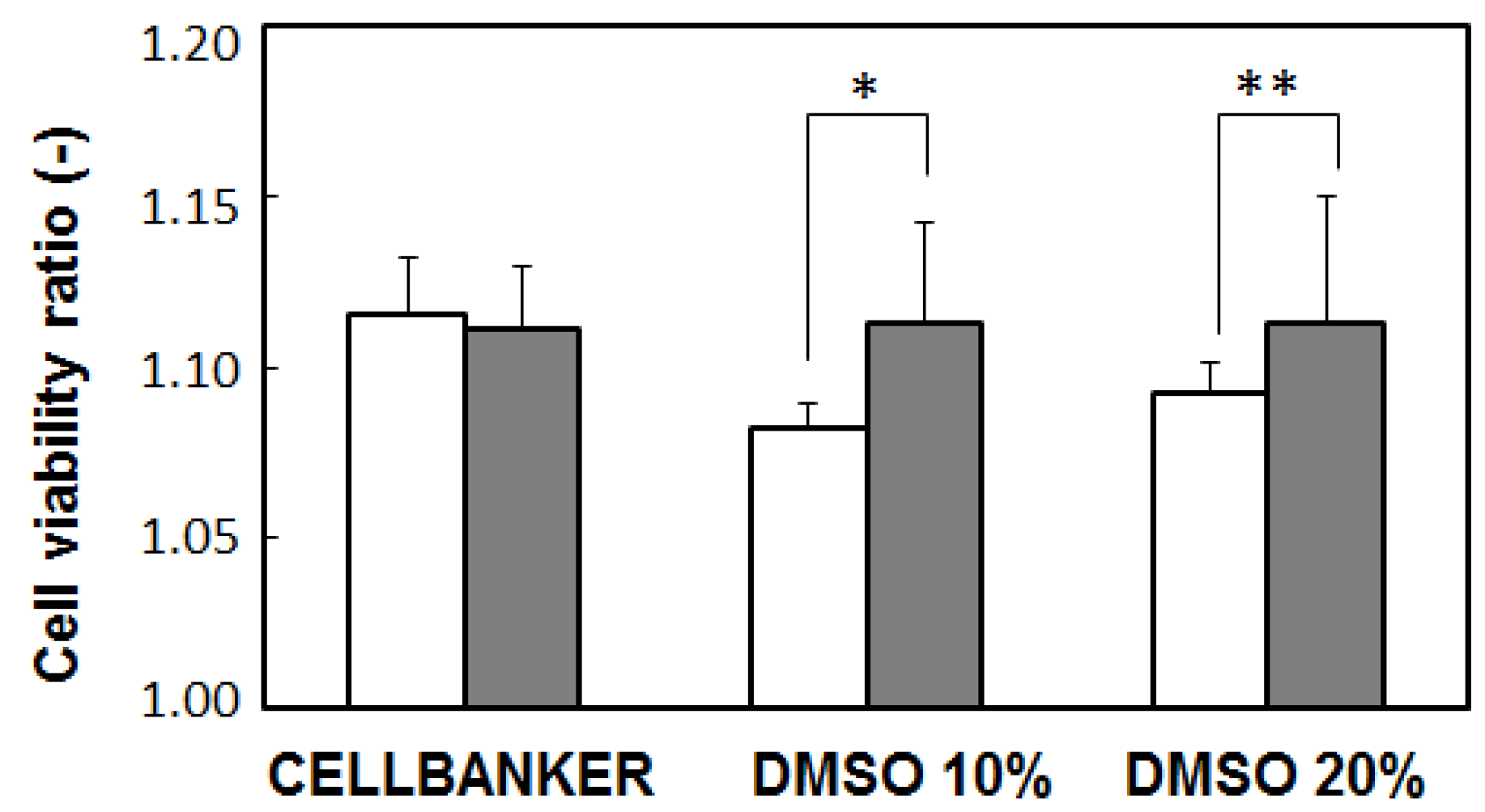
2.4. Cell Culture
3. Results and Discussion
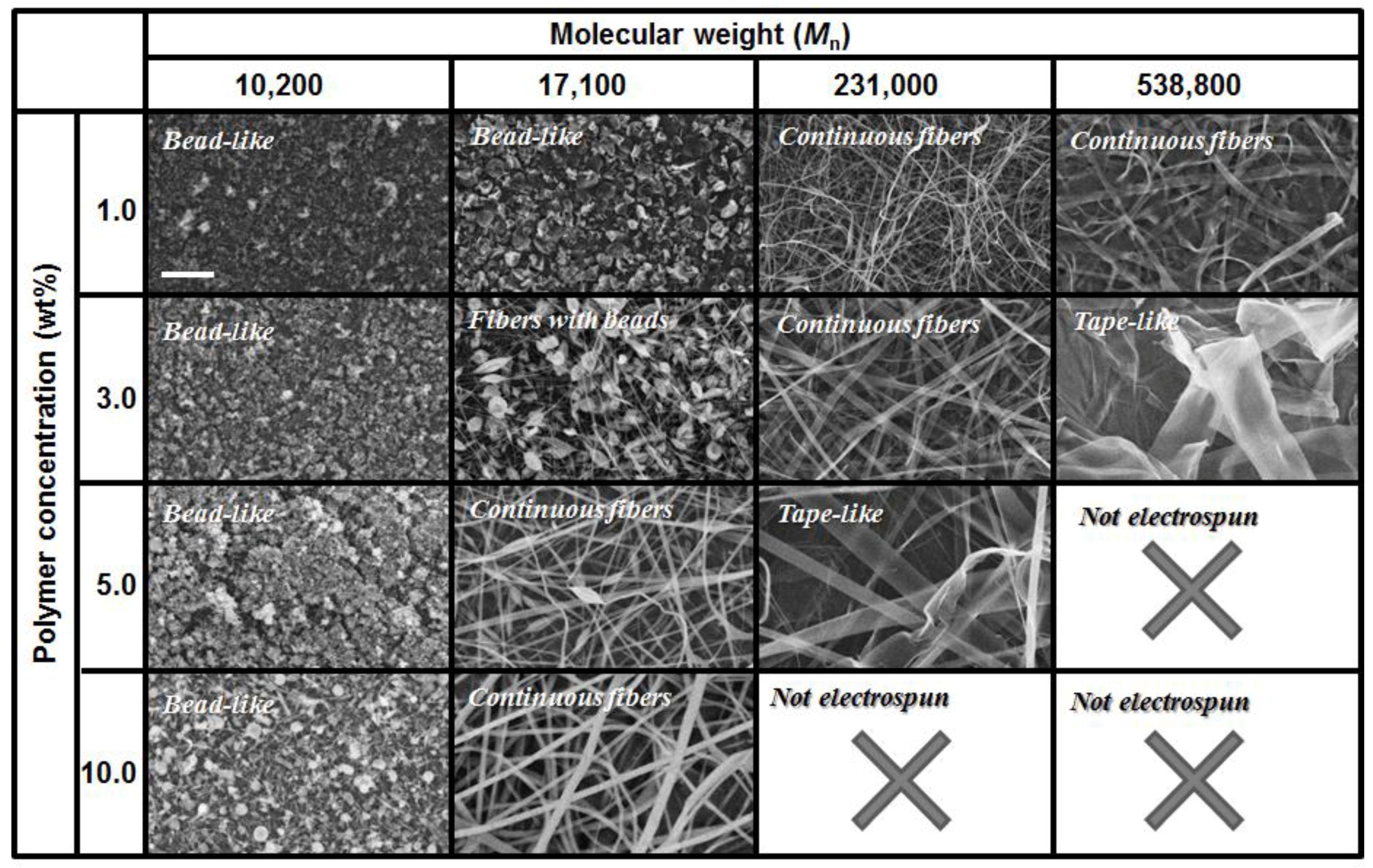
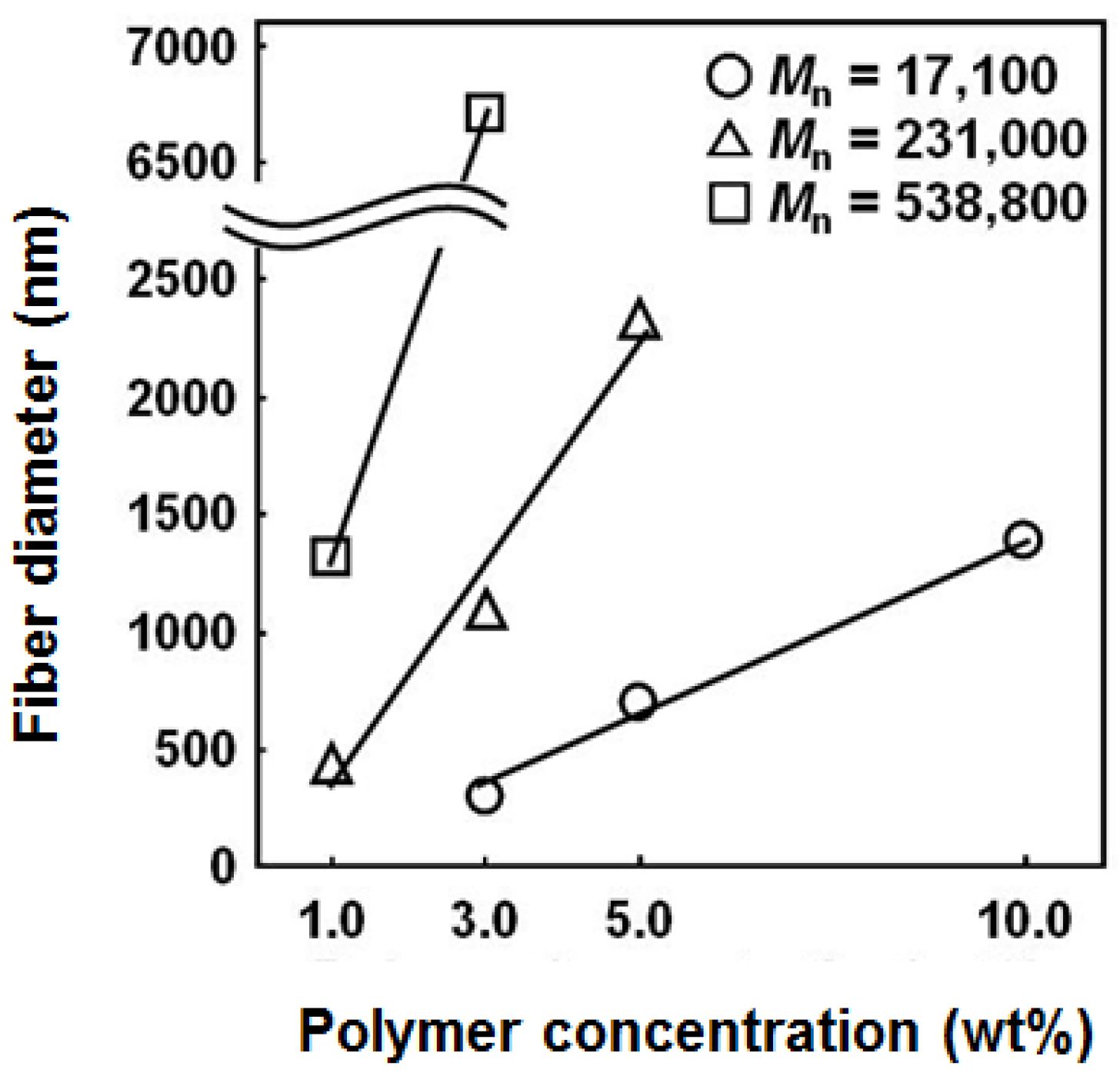
3.1. Effects of Electrospun Conditions on the Fiber Morphology
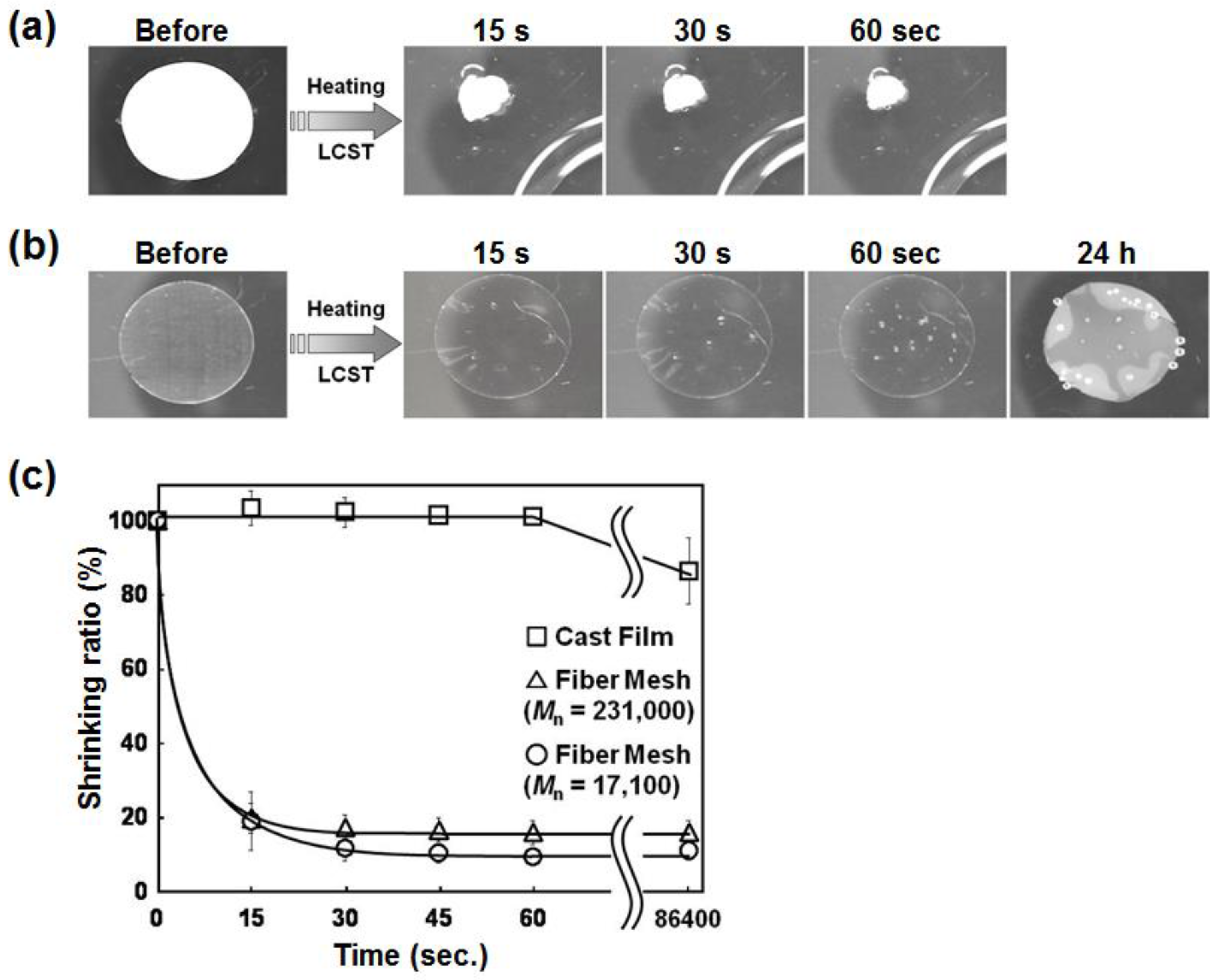
3.2. Temperature-Responsive Behavior
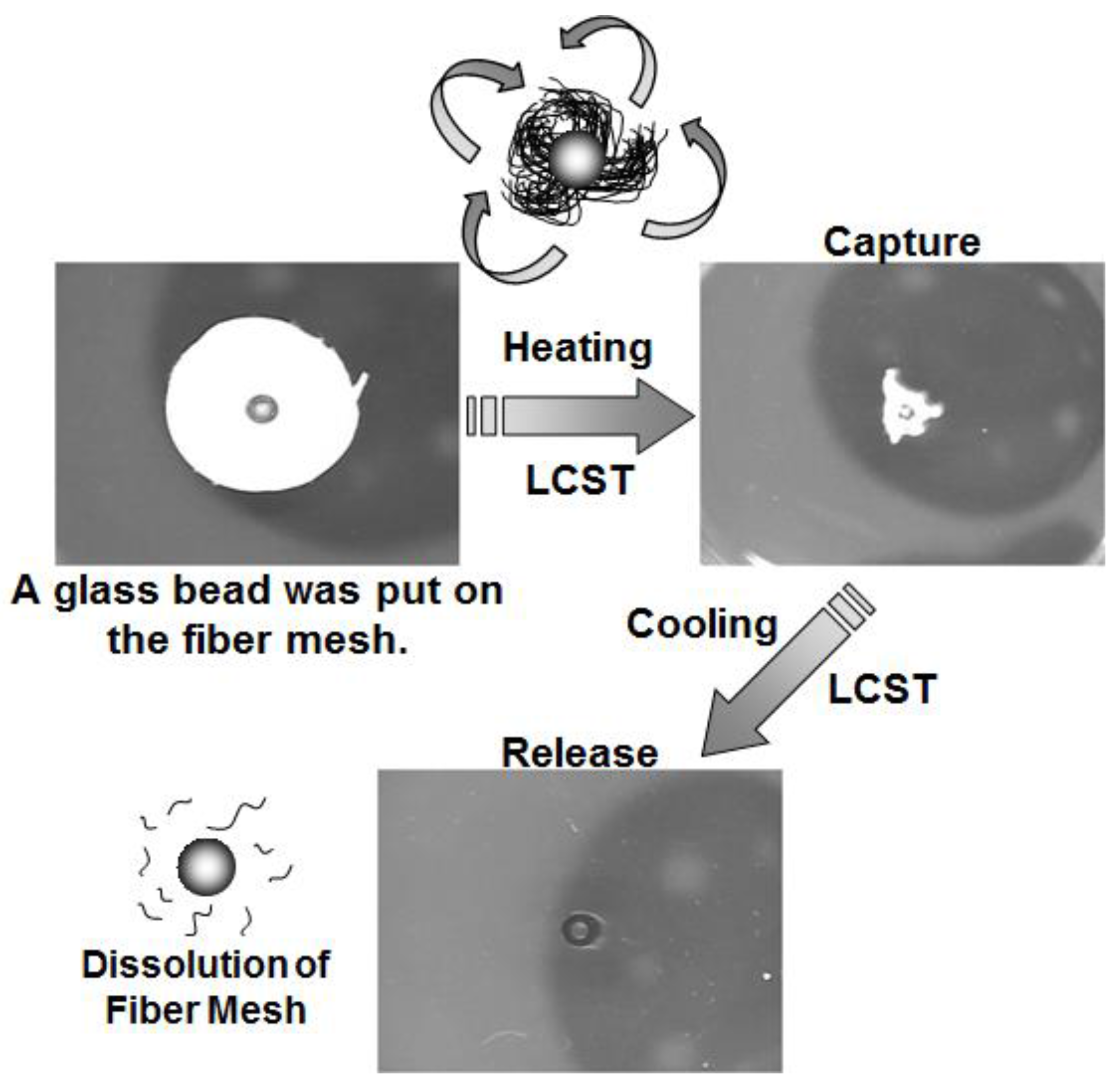
3.3. Capture and Release of Cells
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Kurimoto, R.; Niiyama, E.; Ebara, M. Fibrous Materials. In Biomaterials Nanoarchitectonics; Ebara, M., Ed.; Elsevier: Oxford, UK, 2016; pp. 267–278. [Google Scholar]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef]
- Zhou, F.-L.; Gong, R.-H. Manufacturing technologies of polymeric nanofibres and nanofibre yarns. Polym. Int. 2008, 57, 837–845. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew.Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef]
- Thompson, C.J.; Chase, G.G.; Yarin, A.L.; Reneker, D.H. Effects of parameters on nanofiber diameter determined from electrospinning model. Polymer 2007, 48, 6913–6922. [Google Scholar] [CrossRef]
- Rajala, J.; Shin, H.; Lolla, D.; Chase, G. Core-Shell Electrospun Hollow Aluminum Oxide Ceramic Fibers. Fibers 2015, 3, 450–462. [Google Scholar] [CrossRef]
- Uyar, T.; Havelund, R.; Hacaloglu, J.; Besenbachert, F.; Kingshott, P. Functional electrospun polystyrene nanofibers incorporating α-, β-, and γ-cyclodextrins: compoarison of molecular filter performance. ACS Nano 2010, 4, 5121–5130. [Google Scholar] [CrossRef] [PubMed]
- Bou, S.; Ellis, A.V.; Ebara, M. Synthetic stimuli-responsive ‘smart’ fibers. Curr. Opin. Biotechnol. 2016, 39, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Garrett, R.; Niiyama, E.; Kotsuchibashi, Y.; Uto, K.; Ebara, M. Biodegradable nanofiber for delivery of immunomodulating agent in the treatment of basal cell carcinoma. Fibers 2015, 3, 478–490. [Google Scholar] [CrossRef]
- Lin, Y.; Yao, Y.; Yang, X.; Wei, N.; Li, X.; Gong, P.; Li, R.; Wu, D. Preparation of poly(ether sulfone) nanofibers by gas-jet/electrospinning. J. Appl. Polym. Sci. 2008, 107, 909–917. [Google Scholar] [CrossRef]
- Ellison, C.J.; Phatak, A.; Giles, D.W.; Macosko, C.W.; Bates, F.S. Melt blown nanofibers: Fiber diameter distributions and onset of fiber breakup. Polymer 2007, 48, 3306–3316. [Google Scholar] [CrossRef]
- Okada, T.; Niiyama, E.; Uto, K.; Aoyagi, T.; Ebara, M. Inactivated Sendai Virus (HVJ-E) immobilized electrospun nanofiber for cancer therapy. Materials 2016, 9, 12. [Google Scholar] [CrossRef]
- Che, H.-L.; Lee, H.J.; Uto, K.; Ebara, M.; Kim, W.J.; Aoyagi, T.; Park, I.-K. Simultaneous drug and gene delivery from the biodegradable poly(ε-caprolactone) nanofibers for the treatment of liver cancer. J. Nanosci. Nanotechnol. 2015, 15, 7971–7975. [Google Scholar] [CrossRef] [PubMed]
- Namekawa, K.; Tokoro Schreiber, M.; Aoyagi, T.; Ebara, M. Fabrication of zeolite-polymer comosite nanofibers for removal of uremic toxins from kidney failure patients. Biomater. Sci. 2014, 2, 674–679. [Google Scholar] [CrossRef]
- Takai, R.; Kurimoto, R.; Nakagawa, Y.; Kotsuchibashi, Y.; Namekawa, K.; Ebara, M. Towards a rational design of zeolite-polymer composite nanofibers for efficient adsorption of creatinine. J. Nanomater. 2016, 2016, 5638905. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wang, X.; Feng, Y.; Li, J.; Lim, C.T.; Ramakrishna, S. Coaxial electrospinning of (fluorescein isothiocyanate-conjugated bovine serum albumin)-encapsulated poly(epsilon-caprolactone) nanofibers for sustained release. Biomacromolecules 2006, 7, 1049. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.D.; Xu, L.Q.; Yao, F.; Zhang, K.; Wang, X.F.; Zhu, M.F.; Nie, S.Z. Smart nanofibers from combined living radical polymerization, click chemistry, and electrospinning. ACS Appl. Mater. Interfaces 2009, 1, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Heskins, M.; Guillet, J.E. Solution properties of poly(N-isopropylacrylamide). J. Macromol. Sci. Part A 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Stayton, P.S.; Shimoboji, T.; Long, C.; Chilkoti, A.; Chen, G.; Harris, J.M.; Hoffman, A.S. Control of protein-ligand recognition using a stimuli-responsive polymer. Nature 1995, 378, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.J.; Hoffman, J.M.; Ebara, M.; Hoffman, A.S.; Estournes, C.; Wattiaux, A.; Stayton, P.S. Dual magnetic-/temperature-responsive nanoparticles for microfluidic separatrions and assays. Langmuir 2007, 23, 7385–7391. [Google Scholar] [CrossRef] [PubMed]
- Halperin, A.; Kroger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) phase diagrams: Fifty years of research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef] [PubMed]
- Okuzaki, H.; Kobayashi, K.; Yan, H. Non-woven fabric of poly(N-isopropylacrylamide) nanofibers fabricated by electrospinning. Synth. Met. 2009, 159, 2273–2276. [Google Scholar] [CrossRef]
- Okuzaki, H.; Kobayashi, K.; Yan, H. Thermo-resonsive nanofiber mats. Macromolecules 2009, 42, 5916–5918. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Ebara, M.; Aoyagi, T. A smart hyperthermia nanofiber with switchable drug release for inducing cancer apoptosis. Adv. Funct. Mater. 2013, 23, 5753–5761. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Ebara, M.; Aoyagi, T. Temperature-responsive electrospun nanofibers for “on–off” switchable release of dextran. Sci. Technol. Adv. Mater. 2012, 12, 064203. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Ebara, M.; Aoyagi, T. A smart nanofiber web that captures and releases cells. Angew. Chem. Int. Ed. 2012, 51, 10537–10541. [Google Scholar] [CrossRef] [PubMed]
- Lolla, D.; Lolla, M.; Abutaleb, A.; Shin, H.U.; Reneker, D.H.; Chase, G.G. Fabrication, Polarization of Electrospun Polyvinylidene Fluoride Electret Fibers and Effect on Capturing Nanoscale Solid Aerosols. Materials 2016, 9, 671. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Chase, D.B.; Akins, R.E., Jr.; Rabolt, J.F. Characterization of electrospun poly(N-isopropyl acrylamide) fibers. Polymer 2008, 49, 4025–4032. [Google Scholar] [CrossRef]
- Nartetamrongsutt, K.; Chase, G.G. The influence of salt and solvent concentrations on electrospun polyvinylpyrrolidone fiber diameters and bead formation. Polymer 2013, 54, 2166–2173. [Google Scholar] [CrossRef]
- Zheng, J.; Zhuang, M.; Yu, Z.; Zheng, G.; Zhao, Y.; Wang, H.; Sun, D. The Effect of Surfactants on the Diameter and Morphology of Electrospun Ultrafine Nanofiber. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- Peppas, N.A.; Stauffer, S.R. Reinforced uncrosslinked poly (vinyl alcohol) gels produced by cyclic freezing-thawing processes: A short review. J. Contr. Release 1991, 16, 305–310. [Google Scholar] [CrossRef]
- Tiktopulo, E.I.; Uversky, V.N.; Lushchik, V.B.; Klenin, S.I.; Bychkova, V.E.; Ptitsyn, O.B. “Domain” coil-globule transition in homopolymers. Macromolecules 1995, 28, 7519–7524. [Google Scholar] [CrossRef]
- Itle, L.J.; Pishko, M.V. Cryopreservation of cell-containing poly(ethylene) glycol hydrogel microarrays. Biotechnol. Prog. 2005, 21, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Vrana, N.E.; O’Grady, A.; Kay, E.; Cahill, P.A.; McGuinness, G.B. Cell encapsulation within PVA-based hydrogels via freeze-thawing: A one-step scaffold formation and cell storage technique. J. Tissue Eng. Regen. Med. 2009, 3, 567–572. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, T.; Kim, Y.-J.; Aoyagi, T.; Ebara, M. The Design of Temperature-Responsive Nanofiber Meshes for Cell Storage Applications. Fibers 2017, 5, 13. https://doi.org/10.3390/fib5010013
Maeda T, Kim Y-J, Aoyagi T, Ebara M. The Design of Temperature-Responsive Nanofiber Meshes for Cell Storage Applications. Fibers. 2017; 5(1):13. https://doi.org/10.3390/fib5010013
Chicago/Turabian StyleMaeda, Tomohiro, Young-Jin Kim, Takao Aoyagi, and Mitsuhiro Ebara. 2017. "The Design of Temperature-Responsive Nanofiber Meshes for Cell Storage Applications" Fibers 5, no. 1: 13. https://doi.org/10.3390/fib5010013
APA StyleMaeda, T., Kim, Y.-J., Aoyagi, T., & Ebara, M. (2017). The Design of Temperature-Responsive Nanofiber Meshes for Cell Storage Applications. Fibers, 5(1), 13. https://doi.org/10.3390/fib5010013