Advanced Peptide Nanofibers in Delivery of Therapeutic Agents: Recent Trends, Limitations, and Critical Properties
Abstract
1. Introduction
1.1. Peptide Nanofiber Fabrication Strategies
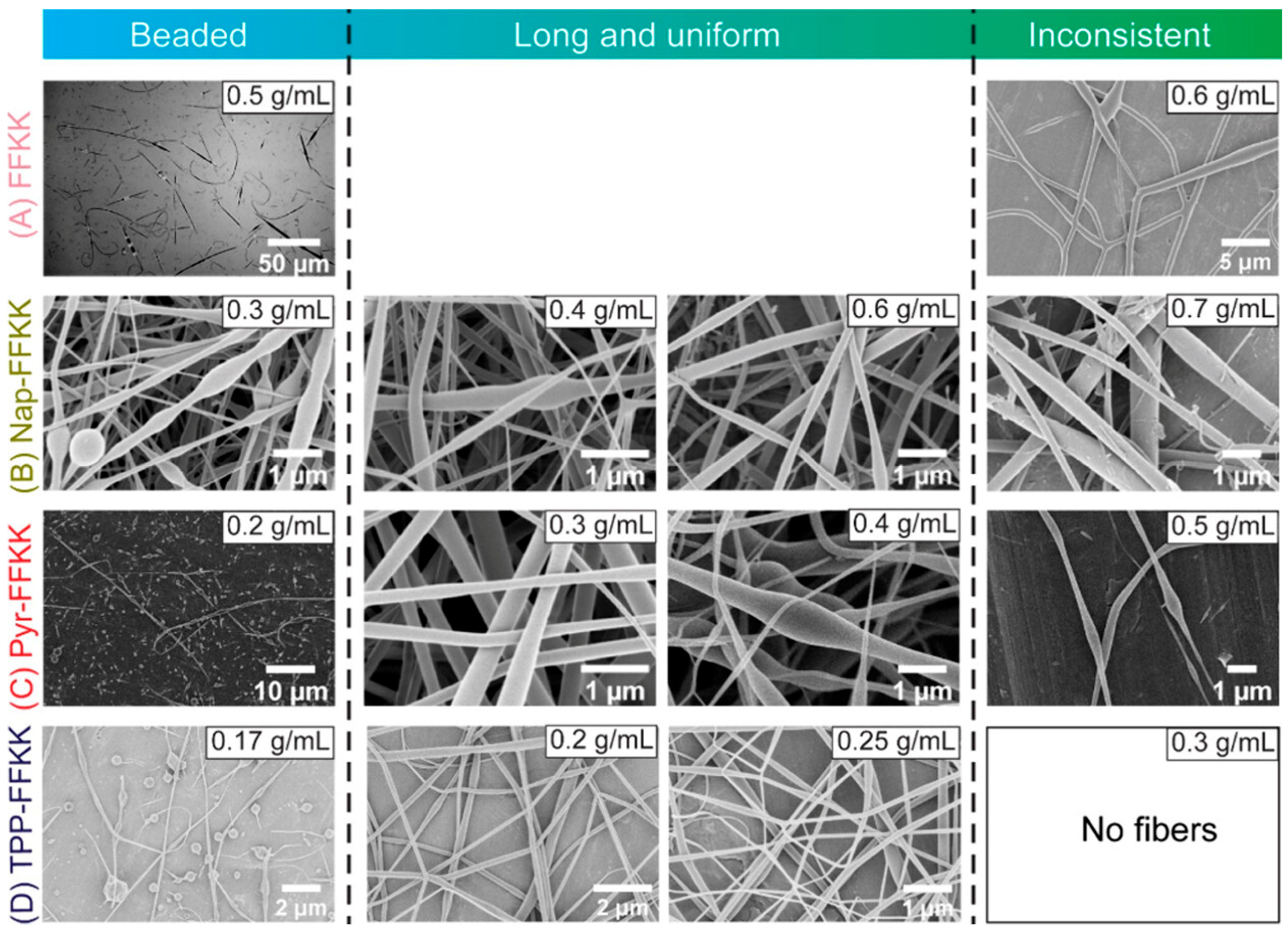
1.1.1. Electrospinning Strategy for PNF Fabrication
1.1.2. Force Spinning Strategy for PNF Fabrication
1.1.3. Drawing Strategy for PNF Fabrication
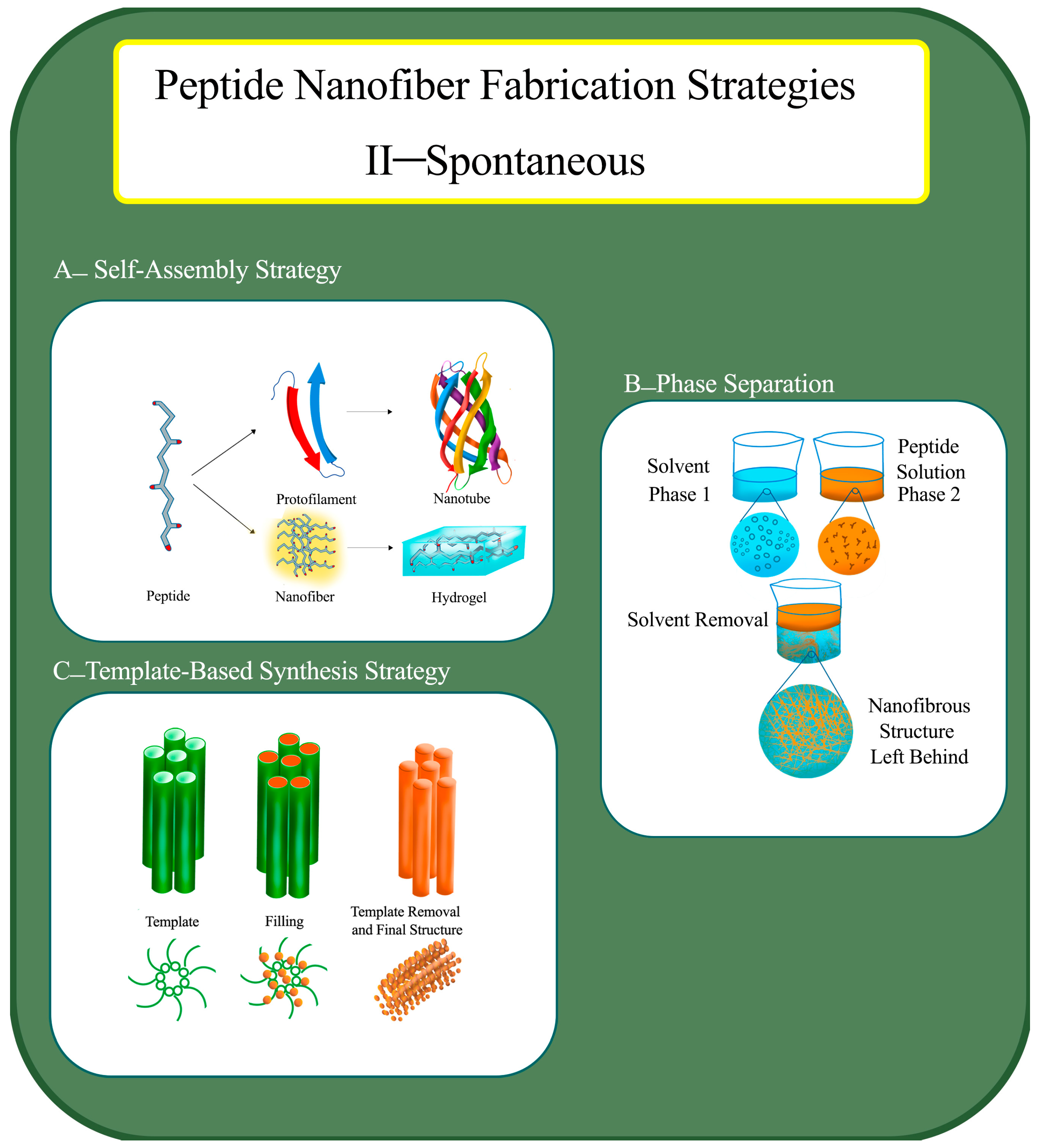
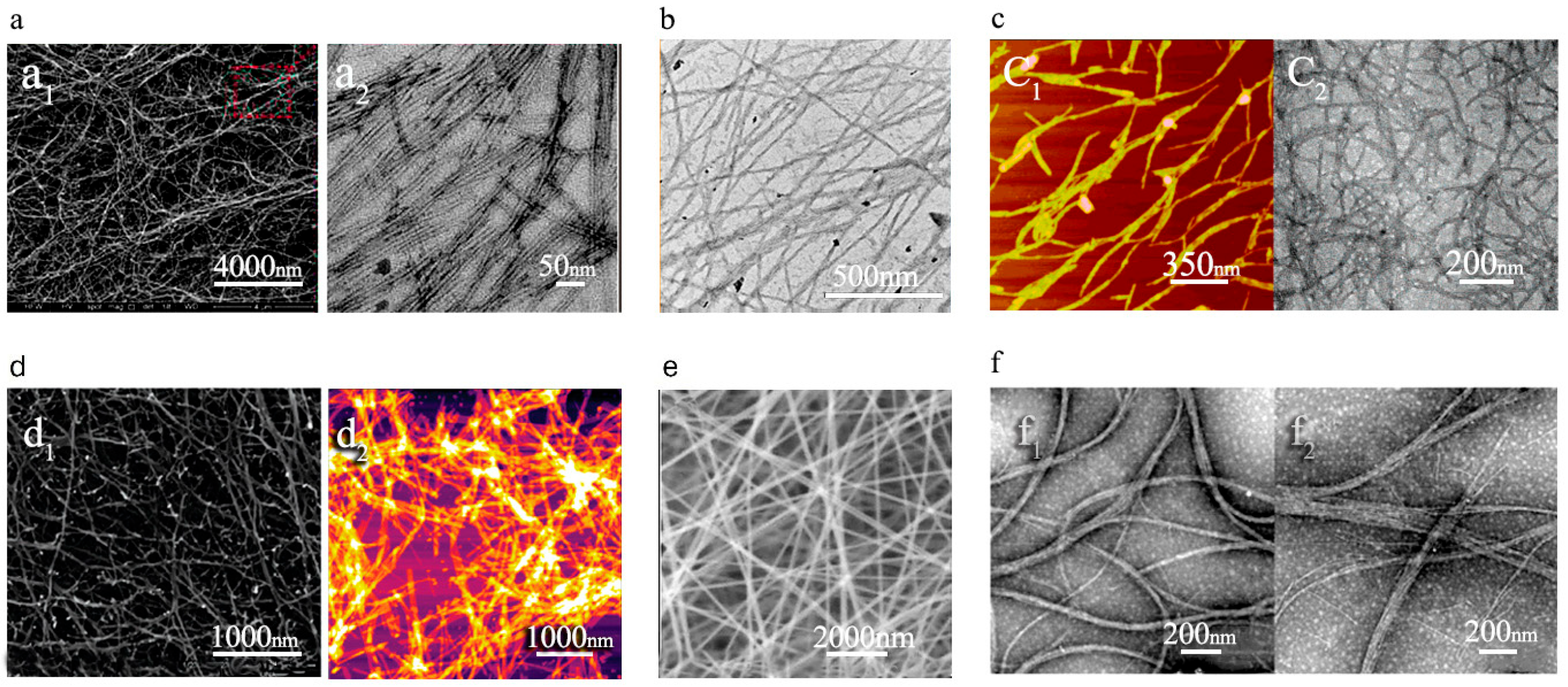
1.1.4. Self-Assembly Strategy for PNF Fabrication
1.1.5. Phase Separation Strategy for PNF Fabrication
1.1.6. Template-Based Synthesis Strategy for PNF Fabrication
1.2. The Role of Secondary Structure of Peptides in Nanofiber Formation
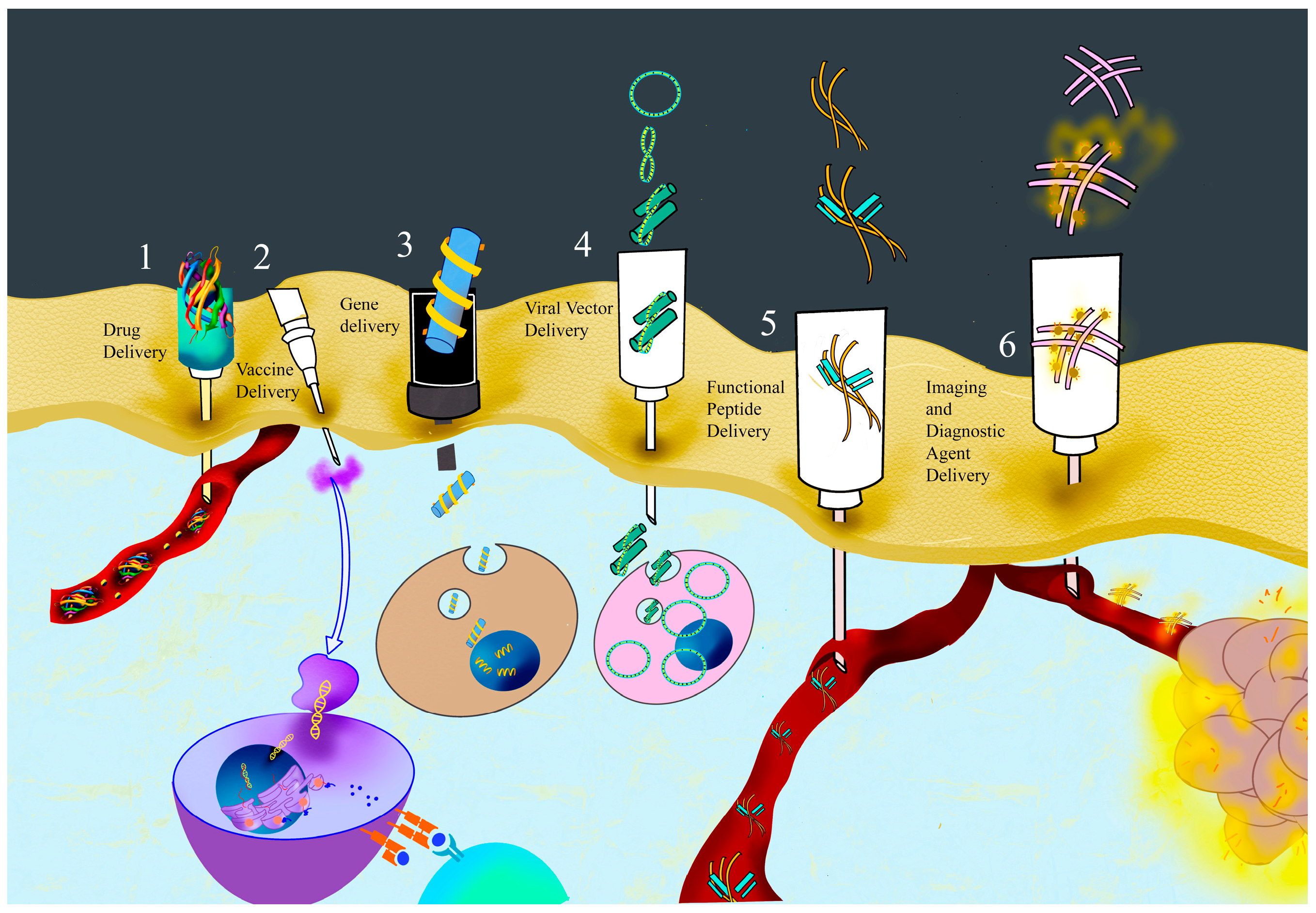
1.3. Application of Peptide Nanofibers
1.3.1. Drug Delivery by PNFs
1.3.2. Vaccine Delivery by PNFs
1.3.3. Gene Delivery by PNFs
1.3.4. Viral Vector Delivery by PNFs
1.3.5. Functional Peptides’ Delivery by PNFs
1.3.6. Diagnostic and Imaging Agents’ Delivery by PNFs
1.4. Delivery Strategies for Peptide Nanofibers:
1.4.1. Systemic Delivery Strategy
1.4.2. Local Delivery Strategy
1.4.3. Controlled-Release Strategy
1.4.4. Stimulus-Responsive Delivery Strategy
1.4.5. Targeted Delivery Strategy
2. Drawbacks, Limitations, and Critical Properties
3. Conclusions and Future Perspectives of Peptide Nanofibers in Delivery
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIE | Aggregation-induced emission |
| AFM | Atomic force microscopy |
| APCs | Anticancer peptides |
| ATPS | Aqueous two-phase system |
| AMPs | Antimicrobial peptides |
| BMSCs | Bone marrow-derived stem cells |
| BSA | Bovine serum albumin |
| CA | Cellulose acetate |
| CAP | Chondrocyte-affinity peptide |
| CNS | Central nervous system |
| CTL | Cytotoxic T lymphocyte |
| DOTA | Derivative of 1,4,7,10-tetraazacyclododecane-1,4,7,10 tetraacetic acid |
| DOX | Doxorubicin |
| ECM | Extracellular matrix |
| ELPs | Elastin-like polypeptides |
| FITC | Fluorescein isothiocyanate |
| FRAP | Fluorescence recovery after photobleaching |
| f-CNOs | Functionalized carbon nano-onions |
| HFIP | 1,1,1,3,3,3-Hexafluoro-2-propanol |
| IFN-γ | Interferon gamma |
| IGF-1 | Insulin-like growth factor 1 |
| LLPS | Liquid–liquid phase separation |
| MMP | Matrix metalloproteinases |
| MRI | Magnetic resonance imaging |
| ODN | Oligo deoxy nucleotide |
| PAN | Polyacrylonitrile |
| PAs | Peptide amphiphiles |
| PBS | Phosphate-buffered saline |
| PLA | Polylactic acid |
| PCL | Poly-ε-caprolactone |
| PLGA | Poly lactic-co-glycolic acid |
| PNFs | Peptide nanofibers |
| PTA | Phosphotungstic acid |
| pDNA | Plasmid DNA |
| PVA | Polyvinyl alcohol |
| RT | Room temperature |
| SAP | Self-assembling peptide |
| SEM | Scanning electron microscope |
| SiO2 | Silicon dioxide |
| SPH | Soy protein hydrolysate |
| TEM | Transmission electron microscopy |
| TFA | Trifluoroacetic acid |
| TFE | 2,2,2-Trifluoroethanol |
| TNBC | Triple-negative breast cancer |
| TPE | Tetraphenylethene |
| VNTRs | Variable-number tandem repeats |
References
- Yıldız, A.; Kara, A.A.; Acartürk, F. Peptide-Protein Based Nanofibers in Pharmaceutical and Biomedical Applications. Int. J. Biol. Macromol. 2020, 148, 1084–1097. [Google Scholar] [CrossRef]
- Kalayil, N.; Budar, A.A.; Dave, R.K. Nanofibers for Drug Delivery: Design and Fabrication Strategies. BIO Integr. 2024, 5, 978. [Google Scholar] [CrossRef]
- Taghizadeh Pirposhteh, R.; Arefian, E.; Arashkia, A.; Mohajel, N. Nona-Arginine Mediated Anti-E6 ShRNA Delivery Suppresses the Growth of Hela Cells in Vitro. Iran. Biomed. J. 2023, 27, 349–356. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Pennington, M.W.; Zell, B.; Bai, C.J. Commercial manufacturing of current good manufacturing practice peptides spanning the gamut from neoantigen to commercial large-scale products. Med. Drug Discov. 2021, 9, 100071. Available online: https://www.sciencedirect.com/science/article/pii/S2590098620300580 (accessed on 1 October 2024). [CrossRef]
- Nayak, R.; Padhye, R.; Kyratzis, I.L.; Truong, Y.B.; Arnold, L. Recent Advances in Nanofibre Fabrication Techniques. Text. Res. J. 2012, 82, 129–147. [Google Scholar] [CrossRef]
- Alghoraibi, I.; Alomari, S. Different Methods for Nanofiber Design and Fabrication. In Handbook of Nanofibers; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Jalili, M.; Mozaffari, A.; Gashti, M.; Parsania, M. Electrospinning Nanofibers Gelatin Scaffolds: Nanoanalysis of Properties and Optimizing the Process for Tissue Engineering Functional. J. Nanoanal. 2019, 6, 289–298. [Google Scholar]
- Mozaffari, A.; Gashti, M.P.; Mirjalili, M.; Parsania, M. Argon and Argon–Oxygen Plasma Surface Modification of Gelatin Nanofibers for Tissue Engineering Applications. Membranes 2021, 11, 31. [Google Scholar] [CrossRef]
- McMurtrey, R.J. Patterned and Functionalized Nanofiber Scaffolds in Three-Dimensional Hydrogel Constructs Enhance Neurite Outgrowth and Directional Control. J. Neural Eng. 2014, 11, 066009. [Google Scholar] [CrossRef]
- Gu, X.; Ding, F.; Williams, D.F. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 2014, 35, 6143–6156. Available online: https://www.sciencedirect.com/science/article/pii/S0142961214004621 (accessed on 1 October 2024). [CrossRef]
- Yuan, Y.; Chen, L.; Kong, L.; Qiu, L.; Fu, Z.; Sun, M.; Liu, Y.; Cheng, M.; Ma, S.; Wang, X.; et al. Histidine Modulates Amyloid-like Assembly of Peptide Nanomaterials and Confers Enzyme-like Activity. Nat. Commun. 2023, 14, 5808. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids; StatPearls: Treasure Island, IL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557845/ (accessed on 22 March 2025).
- Mazza, M.; Patel, A.; Pons, R.; Bussy, C.; Kostarelos, K. Peptide Nanofibres as Molecular Transporters: From Self-Assembly to in Vivo Degradation. Faraday Discuss. 2013, 166, 181–194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bucci, R.; Georgilis, E.; Bittner, A.M.; Gelmi, M.L.; Clerici, F. Peptide-Based Electrospun Fibers: Current Status and Emerging Developments. Nanomaterials 2021, 11, 1262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, X.; Li, Y.; Su, Z.; Jandt, K.D.; Wei, G. Protein-mimetic peptide nanofibers: Motif design, self-assembly synthesis, and sequence-specific biomedical applications. Prog. Polym. Sci. 2018, 80, 94–124. Available online: https://www.sciencedirect.com/science/article/pii/S007967001730254X (accessed on 2 October 2024). [CrossRef]
- DeFrates, K.G.; Moore, R.; Borgesi, J.; Lin, G.; Mulderig, T.; Beachley, V.; Hu, X. Protein-Based Fiber Materials in Medicine: A Review. Nanomaterials 2018, 8, 457. [Google Scholar] [CrossRef]
- Moore, A.N.; Hartgerink, J.D. Self-Assembling Multidomain Peptide Nanofibers for Delivery of Bioactive Molecules and Tissue Regeneration. Acc. Chem. Res. 2017, 50, 714–722. [Google Scholar] [CrossRef]
- Li, S.; Duan, G.; Zhang, G.; Yang, H.; Hou, H.; Dai, Y.; Sun, Y.; Jiang, S. Electrospun Nanofiber Nonwovens and Sponges towards Practical Applications of Waterproofing, Thermal Insulation, and Electromagnetic Shielding/Absorption. Mater. Today Nano 2024, 25, 100452. [Google Scholar] [CrossRef]
- He, J.H. On the Height of Taylor Cone in Electrospinning. Results Phys. 2020, 17, 103096. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of Nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Agrahari, V.; Agrahari, V.; Meng, J.; Mitra, A.K. Electrospun nanofibers in drug delivery: Fabrication, advances, and biomedical applications. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; Available online: https://www.sciencedirect.com/science/article/pii/B9780323429788000097 (accessed on 21 November 2024).
- Rathore, P.; Montz, B.; Hung, S.H.; Pandey, P.K.; Perry, S.L.; Emrick, T.; Schiffman, J.D. Electrospinning of Self-Assembling Oligopeptides into Nanofiber Mats: The Impact of Peptide Composition and End Groups. Biomacromolecules 2025, 26, 1604–1613. [Google Scholar] [CrossRef]
- Tao, Y.; Luo, P.; Jing, F.; Liu, T.; Tan, X.; Lyu, Z.; VeerleBernaerts, K.; Zhang, T.; Jia, R. Collagen-Inspired 3D Printing Electrospinning Biomimetic Patch for Abdominal Wall Defect Regeneration. Adv. Fiber Mater. 2025, 7, 1177–1194. [Google Scholar] [CrossRef]
- Mosayebi, V.; Fathi, M.; Shahedi, M.; Soltanizadeh, N.; Emam-Djomeh, Z. Fast-Dissolving Antioxidant Nanofibers Based on Spirulina Protein Concentrate and Gelatin Developed Using Needleless Electrospinning. Food Biosci. 2022, 47, 101759. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2019, 50, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Locarno, S.; Eleta-Lopez, A.; Lupo, M.G.; Gelmi, M.L.; Clerici, F.; Bittner, A.M. Electrospinning of Pyrazole-Isothiazole Derivatives: Nanofibers from Small Molecules. RSC Adv. 2019, 9, 20565. [Google Scholar] [CrossRef]
- Nuansing, W.; Frauchiger, D.; Huth, F.; Rebollo, A.; Hillenbrand, R.; Bittner, A.M. Electrospinning of peptide and protein fibres: Approaching the molecular scale. Faraday Discuss. 2013, 166, 209–221. Available online: https://pubs.rsc.org/en/content/articlehtml/2013/fd/c3fd00069a (accessed on 3 October 2024). [CrossRef]
- Maleki, M.; Natalello, A.; Pugliese, R.; Gelain, F. Fabrication of Nanofibrous Electrospun Scaffolds from a Heterogeneous Library of Co- and Self-Assembling Peptides. Acta Biomater. 2017, 51, 268–278. [Google Scholar] [CrossRef]
- Sousa, M.G.C.; Rezende, T.M.B.; Franco, O.L. Nanofibers as Drug-Delivery Systems for Antimicrobial Peptides. Drug Discov. Today 2021, 26, 2064–2074. [Google Scholar] [CrossRef]
- Mu, B.; Xu, H.; Li, W.; Xu, L.; Yang, Y. Spinnability and Rheological Properties of Globular Soy Protein Solution. Food Hydrocoll. 2019, 90, 443–451. [Google Scholar] [CrossRef]
- Singh, G.; Bittner, A.M.; Loscher, S.; Malinowski, N.; Kern, K. Electrospinning of Diphenylalanine Nanotubes. Adv. Mater. 2008, 20, 2332–2336. [Google Scholar] [CrossRef]
- Tayi, A.S.; Pashuck, E.T.; Newcomb, C.J.; McClendon, M.T.; Stupp, S.I. Electrospinning Bioactive Supramolecular Polymers from Water. Biomacromolecules 2014, 15, 1323–1327. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, T.H.B.; Skovsen, E.; Fojan, P. Release of antimicrobial peptides from electrospun nanofibres as a drug delivery system. J. Biomed. Nanotechnol. 2013, 9, 492–498. Available online: https://www.ingentaconnect.com/contentone/asp/jbn/2013/00000009/00000003/art00020 (accessed on 21 November 2024). [CrossRef] [PubMed]
- He, Y.; Jin, Y.; Wang, X.; Yao, S.; Li, Y.; Wu, Q.; Ma, G.; Cui, F.; Liu, H. An antimicrobial peptide-loaded gelatin/chitosan nanofibrous membrane fabricated by sequential layer-by-layer electrospinning and electrospraying Techniques. Nanomaterials 2018, 8, 327. Available online: https://www.mdpi.com/2079-4991/8/5/327 (accessed on 21 November 2024). [CrossRef] [PubMed]
- Yadav, N.; Chauhan, M.K.; Chauhan, V.S. Short to ultrashort peptide-based hydrogels as a platform for biomedical applications. Biomater. Sci. 2020, 8, 84–100. Available online: https://pubs.rsc.org/en/content/articlehtml/2019/bm/c9bm01304k (accessed on 1 October 2024). [CrossRef]
- Wu, S. Chain Structure and Entanglement. J. Polym. Sci. Part B Polym. Phys. 1989, 27, 723–741. [Google Scholar] [CrossRef]
- Vigneswari, S.; Murugaiyah, V.; Kaur, G.; Khalil, H.P.S.A.; Amirul, A. Simultaneous dual syringe electrospinning system using benign solvent to fabricate nanofibrous P (3HB-co-4HB)/collagen peptides construct as potential leave-on wound dressing. Mater. Sci. Eng. C 2016, 66, 147–155. Available online: https://www.sciencedirect.com/science/article/pii/S0928493116302909 (accessed on 3 October 2024). [CrossRef]
- Unalan, I.; Endlein, S.J.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Detsch, R.; Boccaccini, A.R. Evaluation of electrospun poly(ε-caprolactone)/gelatin nanofiber mats containing clove essential oil for antibacterial wound dressing. Pharmaceutics 2019, 11, 570. [Google Scholar] [CrossRef]
- Vogt, L.; Rivera, L.R.; Liverani, L.; Piegat, A.; El Fray, M.; Boccaccini, A.R. Poly(ε-caprolactone)/poly(glycerol sebacate) electrospun scaffolds for cardiac tissue engineering using benign solvents. Mater. Sci. Eng. C 2019, 103, 109712. Available online: https://www.sciencedirect.com/science/article/pii/S0928493119305429 (accessed on 2 October 2024). [CrossRef]
- Lenci, E.; Trabocchi, A. Peptidomimetic toolbox for drug discovery. Chem. Soc. Rev. 2020, 49, 3262–3277. Available online: https://pubs.rsc.org/en/content/articlehtml/2020/cs/d0cs00102c (accessed on 3 October 2024). [CrossRef]
- Bucci, R.; Contini, A.; Clerici, F.; Beccalli, E.M.; Formaggio, F.; Maffucci, I.; Pellegrino, S.; Gelmi, M.L. Fluoro-Aryl Substituted α,β 2,3 -Peptides in the Development of Foldameric Antiparallel β-Sheets: A Conformational Study. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Bruggeman, K.F.; Wang, Y.; Maclean, F.L.; Parish, C.L.; Williams, R.J.; Nisbet, D.R. Temporally Controlled Growth Factor Delivery from a Self-Assembling Peptide Hydrogel and Electrospun Nanofibre Composite Scaffold. Nanoscale 2017, 9, 13661–13669. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.S.; Chu, H.S.; Kim, G.W.; Won, J.I.; Jang, J.H. Electrospun Nanofibrous Scaffolds for Controlled Release of Adeno-Associated Viral Vectors. Acta Biomater. 2011, 7, 3868–3876. [Google Scholar] [CrossRef] [PubMed]
- John, J.V.; Choksi, M.; Chen, S.; Boda, S.K.; Su, Y.; McCarthy, A.; Teusink, M.J.; Reinhardt, R.A.; Xie, J. Tethering Peptides onto Biomimetic and Injectable Nanofiber Microspheres to Direct Cellular Response. Nanomed. Nanotechnol. Biol. Med. 2019, 22, 102081. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Zuníga, A.E.; Villela-Castrejón, J. Engineering and Evaluation of Forcespun Functionalized Carbon Nano-Onions Reinforced Poly (ε-Caprolactone) Composite Nanofibers for PH-Responsive Drug Release. Mater. Sci. Eng. C. Mater. Biol. Appl. 2020, 112, 110928. [Google Scholar] [CrossRef] [PubMed]
- Marjuban, S.M.H.; Rahman, M.; Duza, S.S.; Ahmed, M.B.; Patel, D.K.; Rahman, M.S.; Lozano, K. Recent Advances in Centrifugal Spinning and Their Applications in Tissue Engineering. Polymers 2023, 15, 1253. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.J.; Stoyanov, S.D.; Stride, E.; Pelan, E.; Edirisinghe, M. Electrospinning versus Fibre Production Methods: From Specifics to Technological Convergence. Chem. Soc. Rev. 2012, 41, 4708–4735. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y. Centrifugal Spinning: An Alternative Approach to Fabricate Nanofibers at High Speed and Low Cost. Polym. Rev. 2014, 54, 677–701. [Google Scholar] [CrossRef]
- Weitz, R.T.; Harnau, L.; Rauschenbach, S.; Burghard, M.; Kern, K. Polymer Nanofibers via Nozzle-Free Centrifugal Spinning. Nano Lett. 2008, 8, 1187–1191. [Google Scholar] [CrossRef]
- Ondarçuhu, T.; Joachim, C. Drawing a Single Nanofibre over Hundreds of Microns. Europhys. Lett. 1998, 42, 215. [Google Scholar] [CrossRef]
- Sakpal, D.; Gharat, S.; Momin, M. Recent Advancements in Polymeric Nanofibers for Ophthalmic Drug Delivery and Ophthalmic Tissue Engineering. Biomater. Adv. 2022, 141, 213124. [Google Scholar] [CrossRef]
- Nuraje, N.; Bai, H.; Su, K. Bolaamphiphilic Molecules: Assembly and Applications. Prog. Polym. Sci. 2013, 38, 302–343. [Google Scholar] [CrossRef]
- Sorrenti, A.; Illa, O.; Ortuño, R.M. Amphiphiles in Aqueous Solution: Well beyond a Soap Bubble. Chem. Soc. Rev. 2013, 42, 8200–8219. [Google Scholar] [CrossRef] [PubMed]
- Fujino, K.; Yamamoto, N.; Yoshimura, Y.; Yokota, A.; Hirano, Y.; Neo, M. Repair Potential of Self-assembling Peptide Hydrogel in a Mouse Model of Anterior Cruciate Ligament Reconstruction. J. Exp. Orthop. 2024, 11, e12061. [Google Scholar] [CrossRef]
- Pentlavalli, S.; Coulter, S.; Laverty, G. Peptide Nanomaterials for Drug Delivery Applications. Curr. Protein Pept. Sci. 2020, 21, 401–412. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Polymer Nanofibrous Structures: Fabrication, Biofunctionalization, and Cell Interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef]
- Li, W.; Shanti, R.M.; Tuan, R.S. Electrospinning Technology for Nanofibrous Scaffolds in Tissue Engineering. Nanotechnol. Life Sci. 2007, 125–144. [Google Scholar] [CrossRef]
- Zhang, S.; Holmes, T.; Lockshin, C.; Rich, A. Spontaneous Assembly of a Self-Complementary Oligopeptide to Form a Stable Macroscopic Membrane. Proc. Natl. Acad. Sci. USA 1993, 90, 3334–3338. [Google Scholar] [CrossRef]
- Habibi, N.; Kamaly, N.; Memic, A.; Shafiee, H. Self-Assembled Peptide-Based Nanostructures: Smart Nanomaterials toward Targeted Drug Delivery. Nano Today 2016, 11, 41–60. [Google Scholar] [CrossRef]
- Arslan, E.; Garip, I.C.; Gulseren, G.; Tekinay, A.B.; Guler, M.O. Bioactive Supramolecular Peptide Nanofibers for Regenerative Medicine. Adv. Healthc. Mater. 2014, 3, 1357–1376. [Google Scholar] [CrossRef]
- Arias, F.J.; Reboto, V.; Martín, S.; López, I.; Rodríguez-Cabello, J.C. Tailored Recombinant Elastin-like Polymers for Advanced Biomedical and Nano(Bio)Technological Applications. Biotechnol. Lett. 2006, 28, 687–695. [Google Scholar] [CrossRef]
- Yang, C.Y.; Song, B.; Ao, Y.; Nowak, A.P.; Abelowitz, R.B.; Korsak, R.A.; Havton, L.A.; Deming, T.J.; Sofroniew, M.V. Biocompatibility of Amphiphilic Diblock Copolypeptide Hydrogels in the Central Nervous System. Biomaterials 2009, 30, 2881–2898. [Google Scholar] [CrossRef]
- Davis, M.E.; Hsieh, P.C.H.; Takahashi, T.; Song, Q.; Zhang, S.; Kamm, R.D.; Grodzinsky, A.J.; Anversa, P.; Lee, R.T. Local Myocardial Insulin-like Growth Factor 1 (IGF-1) Delivery with Biotinylated Peptide Nanofibers Improves Cell Therapy for Myocardial Infarction. Proc. Natl. Acad. Sci. USA 2006, 103, 8155–8160. [Google Scholar] [CrossRef]
- Jankoski, P.E.; Masoud, A.-R.; Dennis, J.; Trinh, S.; DiMartino, L.R.; Shrestha, J.; Marrero, L.; Hobden, J.; Carter, J.; Schoen, J.; et al. Bioactive Supramolecular Polymers for Skin Regeneration Following Burn Injury. Biomacromolecules 2025, 26, 5471–5482. [Google Scholar] [CrossRef]
- Barlek, M.H.; Gillis, D.C.; Egner, S.A.; Maragos, S.L.; Karver, M.R.; Stupp, S.I.; Tsihlis, N.D.; Kibbe, M.R. Systemic Peptide Amphiphile Nanofiber Delivery Following Subcutaneous Injection. Biomaterials 2023, 303, 122401. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Xu, H.; Zhang, Y.; Chu, L.; Liu, Q.; Song, N.; Yang, C. Novel Tumor-Targeting, Self-Assembling Peptide Nanofiber as a Carrier for Effective Curcumin Delivery. Int. J. Nanomed. 2014, 9, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Fujihara, K.; Teo, W.E.; Lim, T.C.; Ma, Z. An Introduction to Electrospinning and Nanofibers. In An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005; pp. 1–382. [Google Scholar] [CrossRef]
- Titus, A.R.; Madeira, P.P.; Ferreira, L.A.; Chernyak, V.Y.; Uversky, V.N.; Zaslavsky, B.Y. Mechanism of Phase Separation in Aqueous Two-Phase Systems. Int. J. Mol. Sci. 2022, 23, 14366. [Google Scholar] [CrossRef]
- Yuan, C.; Li, Q.; Xing, R.; Li, J.; Yan, X. Peptide Self-Assembly through Liquid-Liquid Phase Separation. Chem 2023, 9, 2425–2445. [Google Scholar] [CrossRef]
- Kumar, P. Effect of Colletor on Electrospinning to Fabricate Aligned Nano Fiber. Bachelor’s Thesis, National Institute of Technology, Rourkela, India, May 2012. [Google Scholar]
- Criado-Gonzalez, M.; Fores, J.R.; Carvalho, A.; Blanck, C.; Schmutz, M.; Kocgozlu, L.; Schaaf, P.; Jierry, L.; Boulmedais, F. Phase Separation in Supramolecular Hydrogels Based on Peptide Self-Assembly from Enzyme-Coated Nanoparticles. Langmuir 2019, 35, 10838–10845. [Google Scholar] [CrossRef]
- Jarak, I.; Silva, I.; Domingues, C.; Santos, A.I.; Veiga, F.; Figueiras, A. Nanofiber Carriers of Therapeutic Load: Current Trends. Int. J. Mol. Sci. 2022, 23, 8581. [Google Scholar] [CrossRef]
- Pérez-Page, M.; Yu, E.; Li, J.; Rahman, M.; Dryden, D.M.; Vidu, R.; Stroeve, P. Template-Based Syntheses for Shape Controlled Nanostructures. Adv. Colloid Interface Sci. 2016, 234, 51–79. [Google Scholar] [CrossRef]
- Kim, Y.M.; Ahn, K.R.; Sung, Y.B.; Rai, S.J. Manufacturing Device and the Method of Preparing for the Nanofibers via Electro-Blown Spinning Process. U.S. Patent US7618579B2, 20 November 2009. Available online: https://lens.org/152-379-730-698-289 (accessed on 7 June 2025).
- Dalton, P.D.; Grafahrend, D.; Klinkhammer, K.; Klee, D.; Möller, M. Electrospinning of Polymer Melts: Phenomenological Observations. Polymer 2007, 48, 6823–6833. [Google Scholar] [CrossRef]
- Ebrahimi, I.; Gashti, M.P. Chemically Reduced versus Photo-Reduced Clay-Ag-Polypyrrole Ternary Nanocomposites: Comparing Thermal, Optical, Electrical and Electromagnetic Shielding Properties. Mater. Res. Bull. 2016, 83, 96–107. [Google Scholar] [CrossRef]
- Ebrahimi, I.; Gashti, M.P. Polypyrrole-MWCNT-Ag Composites for Electromagnetic Shielding: Comparison between Chemical Deposition and UV-Reduction Approaches. J. Phys. Chem. Solids 2018, 118, 80–87. [Google Scholar] [CrossRef]
- Ikegame, M.; Tajima, K.; Aida, T. Template Synthesis of Polypyrrole Nanofibers Insulated within One-Dimensional Silicate Channels: Hexagonal versus Lamellar for Recombination of Polarons into Bipolarons. Angew. Chem. Int. Ed. 2003, 42, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.L.; Desai, T.A. Aligned Arrays of Biodegradable Poly(Epsilon-Caprolactone) Nanowires and Nanofibers by Template Synthesis. Nano Lett. 2007, 7, 1463–1468. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Wang, Y.; Qi, W.; Su, R.; He, Z. Peptide-Templated Synthesis of TiO2 Nanofibers with Tunable Photocatalytic Activity. Chemistry 2018, 24, 18123–18129. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, S.W.; Kang, K.; Park, C.B. Synthesis of Diphenylalanine/Cobalt Oxide Hybrid Nanowires and Their Application to Energy Storage. ACS Nano 2010, 4, 159–164. [Google Scholar] [CrossRef]
- Chen, C.L.; Zhang, P.; Rosi, N.L. A New Peptide-Based Method for the Design and Synthesis of Nanoparticle Superstructures: Construction of Highly Ordered Gold Nanoparticle Double Helices. J. Am. Chem. Soc. 2008, 130, 13555–13557. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, F.; Xu, H.; Yaseen, M.; Shan, H.; Hauser, C.A.E.; Zhang, S.; Lu, J.R. Molecular Self-Assembly and Applications of Designer Peptide Amphiphiles. Chem. Soc. Rev. 2010, 39, 3480–3498. [Google Scholar] [CrossRef]
- Farsheed, A.C.; Thomas, A.J.; Pogostin, B.H.; Hartgerink, J.D. 3D Printing of Self-Assembling Nanofibrous Multidomain Peptide Hydrogels. Adv. Mater. 2023, 35, e2210378. [Google Scholar] [CrossRef]
- Sinha-Ray, S.; Zhang, Y.; Yarin, A.L.; Davis, S.C.; Pourdeyhimi, B. Solution Blowing of Soy Protein Fibers. Biomacromolecules 2011, 12, 2357–2363. [Google Scholar] [CrossRef]
- Jun, H.W.; Paramonov, S.E.; Hartgerink, J.D. Biomimetic Self-Assembled Nanofibers. Soft Matter 2006, 2, 177–181. [Google Scholar] [CrossRef]
- Ahmed, J.; Gultekinoglu, M.; Edirisinghe, M. Recent developments in the use of centrifugal spinning and pressurized gyration for biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1916. [Google Scholar] [CrossRef]
- Mehta, P.P.; Pawar, V.S. Electrospun nanofiber scaffolds: Technology and applications. In Applications of Nanocomposite Materials in Drug Delivery; Woodhead Publishing: London, UK, 2018; pp. 509–573. [Google Scholar] [CrossRef]
- Jeong, H.E.; Lee, S.H.; Kim, P.; Suh, K.Y. Stretched Polymer Nanohairs by Nanodrawing. Nano Lett. 2006, 6, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Dobry, A.; Boyer-Kawenoki, F. Phase Separation in Polymer Solution. J. Polym. Sci. 1947, 2, 90–100. [Google Scholar] [CrossRef]
- Hulteen, J.C.; Martin, C.R. A General Template-Based Method for the Preparation Ofnanomaterials. J. Mater. Chem. 1997, 7, 1075–1087. [Google Scholar] [CrossRef]
- Martin, C.R.; Van Dyke, L.S.; Cai, Z. Template-Synthesis—a Method for Enhancing the Ionic and Electronic Conductivity in Electronically Conductive Polymers. Electrochim. Acta 1992, 37, 1611–1613. [Google Scholar] [CrossRef]
- Holmes, T.C. Novel Peptide-Based Biomaterial Scaffolds for Tissue Engineering. Trends Biotechnol. 2002, 20, 16–21. [Google Scholar] [CrossRef]
- Zhang, S. Emerging Biological Materials through Molecular Self-Assembly. Biotechnol. Adv. 2002, 20, 321–339. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, J.; Lu, S.; Igweze, J.; Xu, W.; Kuang, D.; Zealey, C.; Liu, D.; Gregor, A.; Bozorgzad, A.; et al. Self-Assembling Peptide for Co-Delivery of HIV-1 CD8+ T Cells Epitope and Toll-like Receptor 7/8 Agonists R848 to Induce Maturation of Monocyte Derived Dendritic Cell and Augment Polyfunctional Cytotoxic T Lymphocyte (CTL) Response. J. Control. Release 2016, 236, 22–30. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Hou, Z.; Cui, X.; Zhao, Y.; Xu, H. Rational Design of Short Peptide-Based Hydrogels with MMP-2 Responsiveness for Controlled Anticancer Peptide Delivery. Biomacromolecules 2017, 18, 3563–3571. [Google Scholar] [CrossRef]
- Mazza, M.; Hadjidemetriou, M.; De Lázaro, I.; Bussy, C.; Kostarelos, K. Peptide Nanofiber Complexes with SiRNA for Deep Brain Gene Silencing by Stereotactic Neurosurgery. ACS Nano 2015, 9, 1137–1149. [Google Scholar] [CrossRef]
- Briuglia, M.L.; Urquhart, A.J.; Lamprou, D.A. Sustained and Controlled Release of Lipophilic Drugs from a Self-Assembling Amphiphilic Peptide Hydrogel. Int. J. Pharm. 2014, 474, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, T.; Jin, S.; Xue, X.; Yang, X.; Gong, N.; Zhang, J.; Wang, P.C.; Tian, J.H.; Xing, J.; et al. Virus-Inspired Self-Assembled Nanofibers with Aggregation-Induced Emission for Highly Efficient and Visible Gene Delivery. ACS Appl. Mater. Interfaces 2017, 9, 4425–4432. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Toumia, Y.; Cerroni, B.; Pandolfi, D.; Paradossi, G.; Palocci, C. Biofabrication of Genipin-Crosslinked Peptide Hydrogels and Their Use in the Controlled Delivery of Naproxen. N. Biotechnol. 2017, 37, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ehlers, M.; Schlesiger, S.; Zellermann, E.; Knauer, S.K.; Schmuck, C. Incorporation of a Non-Natural Arginine Analogue into a Cyclic Peptide Leads to Formation of Positively Charged Nanofibers Capable of Gene Transfection. Angew. Chem. Int. Ed. Engl. 2016, 55, 598–601. [Google Scholar] [CrossRef]
- Kim, J.K.; Anderson, J.; Jun, H.W.; Repka, M.A.; Jo, S. Self-Assembling Peptide Amphiphile-Based Nanofiber Gel for Bioresponsive Cisplatin Delivery. Mol. Pharm. 2009, 6, 978–985. [Google Scholar] [CrossRef]
- Pi, Y.; Zhang, X.; Shi, J.; Zhu, J.; Chen, W.; Zhang, C.; Gao, W.; Zhou, C.; Ao, Y. Targeted Delivery of Non-Viral Vectors to Cartilage in Vivo Using a Chondrocyte-Homing Peptide Identified by Phage Display. Biomaterials 2011, 32, 6324–6332. [Google Scholar] [CrossRef]
- Cinar, G.; Ceylan, H.; Urel, M.; Erkal, T.S.; Deniz Tekin, E.; Tekinay, A.B.; Dâna, A.; Guler, M.O. Amyloid Inspired Self-Assembled Peptide Nanofibers. Biomacromolecules 2012, 13, 3377–3387. [Google Scholar] [CrossRef]
- Huang, Z.H.; Shi, L.; Ma, J.W.; Sun, Z.Y.; Cai, H.; Chen, Y.X.; Zhao, Y.F.; Li, Y.M. A Totally Synthetic, Self-Assembling, Adjuvant-Free MUC1 Glycopeptide Vaccine for Cancer Therapy. J. Am. Chem. Soc. 2012, 134, 8730–8733. [Google Scholar] [CrossRef]
- Soukasene, S.; Toft, D.J.; Moyer, T.J.; Lu, H.; Lee, H.K.; Standley, S.M.; Cryns, V.L.; Stupp, S.I. Antitumor Activity of Peptide Amphiphile Nanofiber-Encapsulated Camptothecin. ACS Nano 2011, 5, 9113–9121. [Google Scholar] [CrossRef]
- Leach, D.G.; Dharmaraj, N.; Piotrowski, S.L.; Lopez-Silva, T.L.; Lei, Y.L.; Sikora, A.G.; Young, S.; Hartgerink, J.D. STINGel: Controlled Release of a Cyclic Dinucleotide for Enhanced Cancer Immunotherapy. Biomaterials 2018, 163, 67–75. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, H.; Liu, Y.; Mao, L.; Chen, W.; Zhu, Z.; Liu, W.; Zheng, W.; Zhao, Y.; Kong, D.; et al. A Peptide-Based Nanofibrous Hydrogel as a Promising DNA Nanovector for Optimizing the Efficacy of HIV Vaccine. Nano Lett. 2014, 14, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wen, H.; Bernstein, Z.J.; Hainline, K.M.; Blakney, T.S.; Congdon, K.L.; Snyder, D.J.; Sampson, J.H.; Sanchez-Perez, L.; Collier, J.H. Multiepitope Supramolecular Peptide Nanofibers Eliciting Coordinated Humoral and Cellular Antitumor Immune Responses. Sci. Adv. 2022, 8, eabm7833. [Google Scholar] [CrossRef]
- Files, M.A.; Naqvi, K.F.; Saito, T.B.; Clover, T.M.; Rudra, J.S.; Endsley, J.J. Self-Adjuvanting Nanovaccines Boost Lung-Resident CD4+ T Cell Immune Responses in BCG-Primed Mice. NPJ Vaccines 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Peptide-Amphiphile Nanofibers: A Versatile Scaffold for the Preparation of Self-Assembling Materials. Proc. Natl. Acad. Sci. USA 2002, 99, 5133–5138. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.L.; Bruggeman, K.F.; Wang, Y.; Wang, T.Y.; Williams, R.J.; Parish, C.L.; Nisbet, D.R. Using Minimalist Self-Assembling Peptides as Hierarchical Scaffolds to Stabilise Growth Factors and Promote Stem Cell Integration in the Injured Brain. J. Tissue Eng. Regen. Med. 2018, 12, e1571–e1579. [Google Scholar] [CrossRef]
- Mendanha, K.; Colherinhas, G. Molecular Dynamics Simulations of Self-Assembled E2(SW)6E2 Peptide Nanofibers: Implications for Drug Delivery and Biomimetic Material Design. ACS Phys. Chem. Au 2025, 5, 302–315. [Google Scholar] [CrossRef]
- Bellavita, R.; Barra, T.; Braccia, S.; Prisco, M.; Valiante, S.; Lombardi, A.; Leone, L.; Pisano, J.; Esposito, R.; Nastri, F.; et al. Engineering Multifunctional Peptide-Decorated Nanofibers for Targeted Delivery of Temozolomide across the Blood-Brain Barrier. Mol. Pharm. 2025, 22, 1920–1938. [Google Scholar] [CrossRef]
- Li, S.; Guo, C.; Zhang, X.; Liu, X.; Mu, J.; Liu, C.; Peng, Y.; Chang, M. Self-Assembling Modified Neuropeptide S Enhances Nose-to-Brain Penetration and Exerts a Prolonged Anxiolytic-like Effect. Biomater. Sci. 2021, 9, 4765–4777. [Google Scholar] [CrossRef]
- Cinar, G.; Ozdemir, A.; Hamsici, S.; Gunay, G.; Dana, A.; Tekinay, A.B.; Guler, M.O. Local Delivery of Doxorubicin through Supramolecular Peptide Amphiphile Nanofiber Gels. Biomater. Sci. 2016, 5, 67–76. [Google Scholar] [CrossRef]
- Serdar, N.G.; Pospišil, T.; Šišić, M.; Crnolatac, I.; Maleš, P.; Frkanec, R.; Frkanec, L. Self-Assembled Ac-FFA-NH2 Based Hydrogels with Strong Immunostimulating Activity for Vaccine Delivery. Nanoscale Adv. 2025, 7, 4660–4672. [Google Scholar] [CrossRef]
- Rauch-Wirth, L.; Renner, A.; Kaygisiz, K.; Weil, T.; Zimmermann, L.; Rodriguez-Alfonso, A.A.; Schütz, D.; Wiese, S.; Ständker, L.; Weil, T.; et al. Optimized Peptide Nanofibrils as Efficient Transduction Enhancers for in Vitro and Ex Vivo Gene Transfer. Front. Immunol. 2023, 14, 1270243. [Google Scholar] [CrossRef] [PubMed]
- Kaygisiz, K.; Dutta, A.; Rauch-Wirth, L.; Synatschke, C.V.; Münch, J.; Bereau, T.; Weil, T. Inverse Design of Viral Infectivity-Enhancing Peptide Fibrils from Continuous Protein-Vector Embeddings. Biomater. Sci. 2023, 11, 5251–5261. [Google Scholar] [CrossRef] [PubMed]
- Tarvirdipour, S.; Huang, X.; Mihali, V.; Schoenenberger, C.A.; Palivan, C.G. Peptide-Based Nanoassemblies in Gene Therapy and Diagnosis: Paving the Way for Clinical Application. Molecules 2020, 25, 3482. [Google Scholar] [CrossRef] [PubMed]
- Handelman, A.; Natan, A.; Rosenman, G. Structural and Optical Properties of Short Peptides: Nanotubes-to-Nanofibers Phase Transformation. J. Pept. Sci. 2014, 20, 487–493. [Google Scholar] [CrossRef]
- Paramonov, S.E.; Jun, H.W.; Hartgerink, J.D. Self-Assembly of Peptide-Amphiphile Nanofibers: The Roles of Hydrogen Bonding and Amphiphilic Packing. J. Am. Chem. Soc. 2006, 128, 7291–7298. [Google Scholar] [CrossRef]
- Velichko, Y.S.; Stupp, S.I.; De La Cruz, M.O. Molecular Simulation Study of Peptide Amphiphile Self-Assembly. J. Phys. Chem. B 2008, 112, 2326–2334. [Google Scholar] [CrossRef]
- Cui, H.; Webber, M.J.; Stupp, S.I. Self-Assembly of Peptide Amphiphiles: From Molecules to Nanostructures to Biomaterials. Pept. Sci. 2010, 94, 1–18. [Google Scholar] [CrossRef]
- Song, Z.; Chen, X.; You, X.; Huang, K.; Dhinakar, A.; Gu, Z.; Wu, J. Self-Assembly of Peptide Amphiphiles for Drug Delivery: The Role of Peptide Primary and Secondary Structures. Biomater. Sci. 2017, 5, 2369–2380. [Google Scholar] [CrossRef]
- Jiang, H.; Guler, M.O.; Stupp, S.I. The Internal Structure of Self-Assembled Peptide Amphiphiles Nanofibers. Soft Matter 2007, 3, 454–462. [Google Scholar] [CrossRef]
- Gelain, F.; Luo, Z.; Zhang, S. Self-Assembling Peptide EAK16 and RADA16 Nanofiber Scaffold Hydrogel. Chem. Rev. 2020, 120, 13434–13460. [Google Scholar] [CrossRef] [PubMed]
- Coulter, S.M.; Pentlavalli, S.; An, Y.; Vora, L.K.; Cross, E.R.; Moore, J.V.; Sun, H.; Schweins, R.; McCarthy, H.O.; Laverty, G. In Situ Forming, Enzyme-Responsive Peptoid-Peptide Hydrogels: An Advanced Long-Acting Injectable Drug Delivery System. J. Am. Chem. Soc. 2024, 146, 21401–21416. [Google Scholar] [CrossRef] [PubMed]
- Bond, C.W.; Angeloni, N.L.; Harrington, D.A.; Stupp, S.I.; McKenna, K.E.; Podlasek, C.A. Peptide Amphiphile Nanofiber Delivery of Sonic Hedgehog Protein to Reduce Smooth Muscle Apoptosis in the Penis After Cavernous Nerve Resection. J. Sex. Med. 2011, 8, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Matson, J.B.; Newcomb, C.J.; Bitton, R.; Stupp, S.I. Nanostructure-Templated Control of Drug Release from Peptide Amphiphile Nanofiber Gels. Soft Matter 2012, 8, 3586–3595. [Google Scholar] [CrossRef]
- Xie, C.; Li, X.; Luo, X.; Yang, Y.; Cui, W.; Zou, J.; Zhou, S. Release Modulation and Cytotoxicity of Hydroxycamptothecin-Loaded Electrospun Fibers with 2-Hydroxypropyl-Beta-Cyclodextrin Inoculations. Int. J. Pharm. 2010, 391, 55–64. [Google Scholar] [CrossRef]
- Bellavita, R.; Piccolo, M.; Leone, L.; Ferraro, M.G.; Dardano, P.; De Stefano, L.; Nastri, F.; Irace, C.; Falanga, A.; Galdiero, S. Tuning Peptide-Based Nanofibers for Achieving Selective Doxorubicin Delivery in Triple-Negative Breast Cancer. Int. J. Nanomed. 2024, 19, 6057–6084. [Google Scholar] [CrossRef]
- Bull, S.R.; Guler, M.O.; Bras, R.E.; Meade, T.J.; Stupp, S.I. Self-Assembled Peptide Amphiphile Nanofibers Conjugated to MRI Contrast Agents. Nano Lett. 2005, 5, 1–4. [Google Scholar] [CrossRef]
- Bulut, S.; Erkal, T.S.; Toksoz, S.; Tekinay, A.B.; Tekinay, T.; Guler, M.O. Slow Release and Delivery of Antisense Oligonucleotide Drug by Self-Assembled Peptide Amphiphile Nanofibers. Biomacromolecules 2011, 12, 3007–3014. [Google Scholar] [CrossRef]
- Furuno, K.; Elvitigala, K.C.M.L.; Suzuki, K.; Sakai, S. Local Delivery of Adeno-Associated Viral Vectors with Electrospun Gelatin Nanofiber Mats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2024, 112, e35345. [Google Scholar] [CrossRef]
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide Vaccine: Progress and Challenges. Vaccines 2014, 2, 515–536. [Google Scholar] [CrossRef] [PubMed]
- Rudra, J.S.; Tian, Y.F.; Jung, J.P.; Collier, J.H. A Self-Assembling Peptide Acting as an Immune Adjuvant. Proc. Natl. Acad. Sci. USA 2010, 107, 622–627. [Google Scholar] [CrossRef]
- Zhang, S. Discovery and Design of Self-Assembling Peptides. Interface Focus 2017, 7, 20170028. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Trinh, T.H.T.; Yoo, M.; Shin, J.; Lee, H.; Kim, J.; Hwang, E.; Lim, Y.B.; Ryou, C. Self-Assembling Peptides and Their Application in the Treatment of Diseases. Int. J. Mol. Sci. 2019, 20, 5850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, Y.-C.; Song, W.-Y.; Liu, X.-X.; Peng, H.-H.; Sun, Y. Rationally Designed Self-Assembled Peptide Nanofibers Provoke Robust Humoral Immunity against Nervous Necrosis Virus. J. Virol. 2025, 99, e0031925. [Google Scholar] [CrossRef]
- Yang, P.; Song, H.; Qin, Y.; Huang, P.; Zhang, C.; Kong, D.; Wang, W. Engineering Dendritic-Cell-Based Vaccines and PD-1 Blockade in Self-Assembled Peptide Nanofibrous Hydrogel to Amplify Antitumor T-Cell Immunity. Nano Lett. 2018, 18, 4377–4385. [Google Scholar] [CrossRef]
- Rudra, J.S.; Mishra, S.; Chong, A.S.; Mitchell, R.A.; Nardin, E.H.; Nussenzweig, V.; Collier, J.H. Self-Assembled Peptide Nanofibers Raising Durable Antibody Responses against a Malaria Epitope. Biomaterials 2012, 33, 6476–6484. [Google Scholar] [CrossRef]
- Wu, Y.; Kelly, S.H.; Sanchez-Perez, L.; Sampson, J.H.; Collier, J.H. Comparative Study of α-Helical and β-Sheet Self-Assembled Peptide Nanofiber Vaccine Platforms: Influence of Integrated T-Cell Epitopes. Biomater. Sci. 2020, 8, 3522–3535. [Google Scholar] [CrossRef]
- Roe, E.F.; Freire Haddad, H.; Lazar, K.M.; Liu, P.; Collier, J.H. Tuning Helical Peptide Nanofibers as a Sublingual Vaccine Platform for a Variety of Peptide Epitopes. Adv. Healthc. Mater. 2025, 14, e2402055. [Google Scholar] [CrossRef]
- Curvino, E.J.; Woodruff, M.E.; Roe, E.F.; Freire Haddad, H.; Cordero Alvarado, P.; Collier, J.H. Supramolecular Peptide Self-Assemblies Facilitate Oral Immunization. ACS Biomater. Sci. Eng. 2024, 10, 3041–3056. [Google Scholar] [CrossRef]
- Hudalla, G.A.; Sun, T.; Gasiorowski, J.Z.; Han, H.; Tian, Y.F.; Chong, A.S.; Collier, J.H. Gradated Assembly of Multiple Proteins into Supramolecular Nanomaterials. Nat. Mater. 2014, 13, 829–836. [Google Scholar] [CrossRef]
- Pompano, R.R.; Chen, J.; Verbus, E.A.; Han, H.; Fridman, A.; Mcneely, T.; Collier, J.H.; Chong, A.S. Titrating T-Cell Epitopes within Self-Assembled Vaccines Optimizes CD4+ Helper T Cell and Antibody Outputs. Adv. Healthc. Mater. 2014, 3, 1898–1908. [Google Scholar] [CrossRef]
- Myers, K.J.; Dean, N.M. Sensible Use of Antisense: How to Use Oligonucleotides as Research Tools. Trends Pharmacol. Sci. 2000, 21, 19–23. [Google Scholar] [CrossRef]
- Hao, Y.; Hou, D.Y.; Zhou, L.; Fan, Y.L.; Wu, X.H.; Liu, Y.X.; Xu, Y.S.; Song, B.L.; Yi, L.; Qiao, Z.Y.; et al. Tumor-Specific Protein Induced in Situ Self-Assembly of Peptide Drugs for Synergistic Mitochondria Disruption. Adv. Mater. 2025, 37, e2413069. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.J.; Steinman, L.; Kim, D.T.; Fathman, C.G.; Rothbard, J.B. Polyarginine Enters Cells More Efficiently than Other Polycationic Homopolymers. J. Pept. Res. 2000, 56. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Nakazawa, H.; Miura, D.; Umetsu, M. Enzymatic Ligation of Antibody and Cell-Penetrating Peptide for Ecient and Cell-Specic SiRNA Delivery. Res. Sq. 2021, 11, 1–22. [Google Scholar] [CrossRef]
- Oba, M.; Demizu, Y.; Yamashita, H.; Kurihara, M.; Tanaka, M. Plasmid DNA Delivery Using Fluorescein-Labeled Arginine-Rich Peptides. Bioorg. Med. Chem. 2015, 23, 4911–4918. [Google Scholar] [CrossRef]
- Taghizadeh Pirposhteh, R.; Mohajel, N.; Arashkia, A.; Azadmanesh, K.; Masoumi, M. Central Position of Histidine in the Sequence of Designed Alternating Polarity Peptides Enhances PH-Responsive Assembly with DNA. BMC Biotechnol. 2025, 25, 54. [Google Scholar] [CrossRef]
- Mulholland, E.J.; McErlean, E.M.; Dunne, N.; McCarthy, H.O. Design of a Novel Electrospun PVA Platform for Gene Therapy Applications Using the CHAT Peptide. Int. J. Pharm. 2021, 598, 120366. [Google Scholar] [CrossRef]
- Rujitanaroj, P.O.; Jao, B.; Yang, J.; Wang, F.; Anderson, J.M.; Wang, J.; Chew, S.Y. Controlling Fibrous Capsule Formation through Long-Term down-Regulation of Collagen Type I (COL1A1) Expression by Nanofiber-Mediated SiRNA Gene Silencing. Acta Biomater. 2013, 9, 4513–4524. [Google Scholar] [CrossRef]
- Bombin, A.D.J.; Dunne, N.; McCarthy, H.O. Delivery of a Peptide/MicroRNA Blend via Electrospun Antimicrobial Nanofibres for Wound Repair. Acta Biomater. 2023, 155, 304–322. [Google Scholar] [CrossRef]
- Pinese, C.; Lin, J.; Milbreta, U.; Li, M.; Wang, Y.; Leong, K.W.; Chew, S.Y. Sustained Delivery of SiRNA/Mesoporous Silica Nanoparticle Complexes from Nanofiber Scaffolds for Long-Term Gene Silencing. Acta Biomater. 2018, 76, 164–177. [Google Scholar] [CrossRef]
- Bellavita, R.; Braccia, S.; Piccolo, M.; Bialecki, P.; Ferraro, M.G.; Graziano, S.F.; Esposito, E.; Donadio, F.; Bryszewska, M.; Irace, C.; et al. Shielding SiRNA by Peptide-Based Nanofibers: An Efficient Approach for Turning off EGFR Gene in Breast Cancer. Int. J. Biol. Macromol. 2025, 292, 139219. [Google Scholar] [CrossRef]
- Pourzadegan, F.; Shariati, L.; Taghizadeh, R.; Khanahmad, H.; Mohammadi, Z.; Tabatabaiefar, M.A. Using Intron Splicing Trick for Preferential Gene Expression in Transduced Cells: An Approach for Suicide Gene Therapy. Cancer Gene Ther. 2016, 23, 7–12. [Google Scholar] [CrossRef]
- Kaygisiz, K.; Rauch-Wirth, L.; Iscen, A.; Hartenfels, J.; Kremer, K.; Münch, J.; Synatschke, C.V.; Weil, T. Peptide Amphiphiles as Biodegradable Adjuvants for Efficient Retroviral Gene Delivery. Adv. Healthc. Mater. 2024, 13, 2301364. [Google Scholar] [CrossRef] [PubMed]
- Puhl, D.L.; Mohanraj, D.; Nelson, D.W.; Gilbert, R.J. Designing Electrospun Fiber Platforms for Efficient Delivery of Genetic Material and Genome Editing Tools. Adv. Drug Deliv. Rev. 2022, 183, 114161. [Google Scholar] [CrossRef] [PubMed]
- Münch, J.; Rücker, E.; Ständker, L.; Adermann, K.; Goffinet, C.; Schindler, M.; Wildum, S.; Chinnadurai, R.; Rajan, D.; Specht, A.; et al. Semen-Derived Amyloid Fibrils Drastically Enhance HIV Infection. Cell 2007, 131, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Kaygisiz, K.; Rauch-Wirth, L.; Dutta, A.; Yu, X.; Nagata, Y.; Bereau, T.; Münch, J.; Synatschke, C.V.; Weil, T. Data-Mining Unveils Structure–Property–Activity Correlation of Viral Infectivity Enhancing Self-Assembling Peptides. Nat. Commun. 2023, 14, 5121. [Google Scholar] [CrossRef]
- Fan, T.; Yu, X.; Shen, B.; Sun, L. Peptide Self-Assembled Nanostructures for Drug Delivery Applications. J. Nanomater. 2017, 2017, 4562474. [Google Scholar] [CrossRef]
- Dart, A.; Bhave, M.; Kingshott, P. Antimicrobial Peptide-Based Electrospun Fibers for Wound Healing Applications. Macromol. Biosci. 2019, 19, e1800488. [Google Scholar] [CrossRef]
- Kirbas, Z.; Altay, F. Uniaxial Electrospinning Encapsulation of Bioactive Peptides into Green Nanofibers Containing Pullulan-Alginate-CaCl2. Int. J. Pept. Res. Ther. 2025, 31, 19. [Google Scholar] [CrossRef]
- Asif, F.; Zaman, S.U.; Arnab, M.K.H.; Hasan, M.; Islam, M.M. Antimicrobial Peptides as Therapeutics: Confronting Delivery Challenges to Optimize Efficacy. The Microbe 2024, 2, 100051. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Borro, B.C.; Nordström, R.; Malmsten, M. Microgels and Hydrogels as Delivery Systems for Antimicrobial Peptides. Colloids Surfaces B Biointerfaces 2020, 187, 110835. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Cheong, S.; Kim, T.Y.; Choi, H.; Hahn, S.K. Supramolecular Hydrogels for Precisely Controlled Antimicrobial Peptide Delivery for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 16471–16481. [Google Scholar] [CrossRef]
- Chinnadurai, R.K.; Khan, N.; Meghwanshi, G.K.; Ponne, S.; Althobiti, M.; Kumar, R. Current Research Status of Anti-Cancer Peptides: Mechanism of Action, Production, and Clinical Applications. Biomed. Pharmacother. 2023, 164, 114996. [Google Scholar] [CrossRef]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer Peptide: Physicochemical Property, Functional Aspect and Trend in Clinical Application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef]
- Silva, G.A.; Czeisler, C.; Niece, K.L.; Beniash, E.; Harrington, D.A.; Kessler, J.A.; Stupp, S.I. Selective Differentiation of Neural Progenitor Cells by High-Epitope Density Nanofibers. Science 2004, 303, 1352–1355. [Google Scholar] [CrossRef]
- Song, Y.; Wu, C.; Zhang, X.; Bian, W.; Liu, N.; Yin, S.; Yang, M.F.; Luo, M.; Tang, J.; Yang, X. A Short Peptide Potentially Promotes the Healing of Skin Wound. Biosci. Rep. 2019, 39, BSR20181734. [Google Scholar] [CrossRef]
- Md Fadilah, N.I.; Shahabudin, N.A.; Mohd Razif, R.A.; Sanyal, A.; Ghosh, A.; Baharin, K.I.; Ahmad, H.; Maarof, M.; Motta, A.; Fauzi, M.B. Discovery of Bioactive Peptides as Therapeutic Agents for Skin Wound Repair. J. Tissue Eng. 2024, 15. [Google Scholar] [CrossRef]
- Shenoy, D.; Chivukula, S.; Erdogan, N.; Chiesa, E.; Pellegrino, S.; Reches, M.; Genta, I. Self-Assembled Peptide-Based Nanofibers for Cardiovascular Tissue Regeneration. J. Mater. Chem. B 2025, 13, 844–857. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, X.; Liang, G.; Sun, X. Self-Assembly of Peptide Nanofibers for Imaging Applications. Nanoscale 2021, 13, 15142–15150. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.F.; Cui, H. Protease-Sensitive Nanomaterials for Cancer Therapeutics and Imaging. Ind. Eng. Chem. Res. 2017, 56, 5761–5777. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Y.; Chen, S.; Wang, Y.; Zheng, Y. VEGF Mimetic Peptide-Conjugated Nanoparticles for Magnetic Resonance Imaging and Therapy of Myocardial Infarction. J. Control. Release 2023, 360, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Karavasili, C.; Panteris, E.; Vizirianakis, I.S.; Koutsopoulos, S.; Fatouros, D.G. Chemotherapeutic Delivery from a Self-Assembling Peptide Nanofiber Hydrogel for the Management of Glioblastoma. Pharm. Res. 2018, 35, 166. [Google Scholar] [CrossRef]
- Sharma, R.; Kwon, S. New Applications of Nanoparticles in Cardiovascular Imaging. J. Exp. Nanosci. 2007, 2, 115–126. [Google Scholar] [CrossRef]
- Lou, P.; Liu, S.; Wang, Y.; Pan, C.; Xu, X.; Zhao, M.; Liao, G.; Yang, G.; Yuan, Y.; Li, L.; et al. Injectable Self-Assembling Peptide Nanofiber Hydrogel as a Bioactive 3D Platform to Promote Chronic Wound Tissue Regeneration. Acta Biomater. 2021, 135, 100–112. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Y.; Lu, H.; Zhang, X.; Zhu, Y.; Liu, S.; Zhang, Z.; Ye, J.; Yang, W. Injectable Hydrogel Encapsulated with VEGF-Mimetic Peptide-Loaded Nanoliposomes Promotes Peripheral Nerve Repair in Vivo. Acta Biomater. 2023, 160, 225–238. [Google Scholar] [CrossRef]
- Coulter, S.M.; Pentlavalli, S.; Vora, L.K.; An, Y.; Cross, E.R.; Peng, K.; McAulay, K.; Schweins, R.; Donnelly, R.F.; McCarthy, H.O.; et al. Enzyme-Triggered l-α/d-Peptide Hydrogels as a Long-Acting Injectable Platform for Systemic Delivery of HIV/AIDS Drugs. Adv. Healthc. Mater. 2023, 12, 2203198. [Google Scholar] [CrossRef]
- Wu, J.; Jones, N.; Hohenwarter, L.; Zhao, F.; Chan, V.; Tan, Z.; Carlaw, T.; Morin, T.; Li, J.; Kaur, T.; et al. Systemic Delivery of Proteins Using Novel Peptides via the Sublingual Route. J. Control. Release 2024, 368, 290–302. [Google Scholar] [CrossRef]
- Yaylaci, S.; Dinç, E.; Aydın, B.; Tekinay, A.B.; Guler, M.O. Peptide Nanofiber System for Sustained Delivery of Anti-VEGF Proteins to the Eye Vitreous. Pharmaceutics 2023, 15, 1264. [Google Scholar] [CrossRef] [PubMed]
- Karavasili, C.; Fatouros, D.G. Self-Assembling Peptides as Vectors for Local Drug Delivery and Tissue Engineering Applications. Adv. Drug Deliv. Rev. 2021, 174, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Khosravimelal, S.; Chizari, M.; Farhadihosseinabadi, B.; Moosazadeh Moghaddam, M.; Gholipourmalekabadi, M. Fabrication and Characterization of an Antibacterial Chitosan/Silk Fibroin Electrospun Nanofiber Loaded with a Cationic Peptide for Wound-Dressing Application. J. Mater. Sci. Mater. Med. 2021, 32, 114. [Google Scholar] [CrossRef] [PubMed]
- Kyser, A.J.; Fotouh, B.; Harris, V.; Patel, R.; Maners, C.; Frieboes, H.B. Electrospun Nanofibers: Focus on Local Therapeutic Delivery Targeting Infectious Disease. J. Drug Deliv. Sci. Technol. 2025, 104, 106520. [Google Scholar] [CrossRef]
- Altunbas, A.; Pochan, D.J. Peptide-Based and Polypeptide-Based Hydrogels for Drug Delivery and Tissue Engineering. Top. Curr. Chem. 2012, 310, 135–167. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Gao, M.; Lin, J.; Wu, W.; Wang, J.; Chew, S.Y. Three-Dimensional Aligned Nanofibers-Hydrogel Scaffold for Controlled Non-Viral Drug/Gene Delivery to Direct Axon Regeneration in Spinal Cord Injury Treatment. Sci. Reports 2017, 7, 42212. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wang, X.; Feng, Y.; Li, J.; Lim, C.T.; Ramakrishna, S. Coaxial Electrospinning of (Fluorescein Isothiocyanate-Conjugated Bovine Serum Albumin)-Encapsulated Poly(Epsilon-Caprolactone) Nanofibers for Sustained Release. Biomacromolecules 2006, 7, 1049–1057. [Google Scholar] [CrossRef]
- Hsieh, P.C.H.; Davis, M.E.; Gannon, J.; MacGillivray, C.; Lee, R.T. Controlled Delivery of PDGF-BB for Myocardial Protection Using Injectable Self-Assembling Peptide Nanofibers. J. Clin. Investig. 2006, 116, 237–248. [Google Scholar] [CrossRef]
- Alam, A.; Karmakar, R.; Rengan, A.K.; Khandelwal, M. Nanofiber-Based Systems for Stimuli-Responsive and Dual Drug Delivery: Present Scenario and the Way Forward. ACS Biomater. Sci. Eng. 2023, 9, 3160–3184. [Google Scholar] [CrossRef]
- Wu, H.; Liu, S.; Xiao, L.; Dong, X.; Lu, Q.; Kaplan, D.L. Injectable and PH-Responsive Silk Nanofiber Hydrogels for Sustained Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 17118–17126. [Google Scholar] [CrossRef]
- Jiang, J.; Xie, J.; Ma, B.; Bartlett, D.E.; Xu, A.; Wang, C.H. Mussel-Inspired Protein-Mediated Surface Functionalization of Electrospun Nanofibers for PH-Responsive Drug Delivery. Acta Biomater. 2014, 10, 1324–1332. [Google Scholar] [CrossRef]
- Dehsorkhi, A.; Castelletto, V.; Hamley, I.W. Self-Assembling Amphiphilic Peptides. J. Pept. Sci. 2014, 20, 453–467. [Google Scholar] [CrossRef]
- Lowik, D.W.P.M.; Leunissen, E.H.P.; Van Den Heuvel, M.; Hansen, M.B.; Hest, J.C.M.V. Stimulus Responsive Peptide Based Materials. Chem. Soc. Rev. 2010, 39, 3394–3412. [Google Scholar] [CrossRef]
- Eskandari, S.; Guerin, T.; Toth, I.; Stephenson, R.J. Recent Advances in Self-Assembled Peptides: Implications for Targeted Drug Delivery and Vaccine Engineering. Adv. Drug Deliv. Rev. 2017, 110–111, 169–187. [Google Scholar] [CrossRef]
- Man, Z.; Yin, L.; Shao, Z.; Zhang, X.; Hu, X.; Zhu, J.; Dai, L.; Huang, H.; Yuan, L.; Zhou, C.; et al. The Effects of Co-Delivery of BMSC-Affinity Peptide and RhTGF-Β1 from Coaxial Electrospun Scaffolds on Chondrogenic Differentiation. Biomaterials 2014, 35, 5250–5260. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov Expert Search: Peptide Nanofibers. Available online: https://clinicaltrials.gov/expert-search?term=peptidenanofiber (accessed on 3 April 2025).
- Parvinzadeh Gashti, M.; Hegemann, D.; Stir, M.; Hulliger, J. Thin Film Plasma Functionalization of Polyethylene Terephthalate to Induce Bone-Like Hydroxyapatite Nanocrystals. Plasma Process. Polym. 2014, 11, 37–43. [Google Scholar] [CrossRef]
- Parvinzadeh Gashti, M.; Farch, S.; Parvinzadeh Gashti, M.; Pousti, M.; Pakdel, E.; Francisco Martins, A.; Siam, K. Plasma-Assisted Hydroxyapatite/Chitosan Bionanocomposite Films with Improved Thermal Stability, Biomineralization and Optical Absorption Properties. ChemNanoMat 2025, 11, e202400577. [Google Scholar] [CrossRef]
- Mozaffari, A.; Parvinzadeh Gashti, M.; Alimohammadi, F.; Pousti, M. The Impact of Helium and Nitrogen Plasmas on Electrospun Gelatin Nanofiber Scaffolds for Skin Tissue Engineering Applications. J. Funct. Biomater. 2024, 15, 326. [Google Scholar] [CrossRef]


| Parameters | Solvent | Voltage (kV) | Flow Rate (mL/h) | Collector Distance (cm) | Fiber Diameter (nm) | Collector Spinning (rpm) | Scalability (%) | Mechanical Strength | Reference |
|---|---|---|---|---|---|---|---|---|---|
| DIKVAV | PBS | 20 | 2 | 12 | 10–300 | 1000 | 100 | - | [44] |
| ELP/PCL | Aqueous | 13–20 | 1 | 13 | 350–500 | - | - | [45] | |
| LDLK, LKLK, CDLK, LDLD | HFIP, TFE, and TFA | 0–50 | 0.01–0.1 | 5–15 | ~200 | - | 100 | - | [29] |
| VEGF-mimicking | PBS | 4–6 | 2 | 10 | - | - | 100 | 10 MPa | [46] |
| FFKK | Water | 20 | 0.5 | 15 | ~7–35 | 20 | 100 | - | [23] |
| GPO (GP-Hydroxyproline) | Dichloromethane (DCM) | 18 kV | 1 mL/h | 15 cm | 209.3 | - | 100 | 3.5–4 MPa | [24] |
| Parameters | Temperature | Solvent | Charge | wt.% | Encapsulation Efficiency | Yields | Scalability | Degradability | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Fmoc–F–F | 23°C | Water | Positive | - | - | >90% | Laboratory scale | After 180 days | [12] |
| Palmitoyl-GGGAAAR | - | Water | Positive | - | 80–95% | >90% | Laboratory scale | After 21 days | [14] |
| Palmitoyl-GGGAAAKRK | - | Water | Positive | - | 80–95% | >90% | Laboratory scale | After 21 days | [14] |
| (AEAEAKAK)2 | - | PBS/water | Positive | - | - | 70–90 | Laboratory scale | After 4 h | [60] |
| KLTWQELYQLKYKGI-NH 2 | - | Water | Positive | - | - | >90 | Laboratory scale | - | [62] |
| RAD16-II | - | Water | Positive | 1% (wt/vol) | - | >90 | Laboratory scale | [65] | |
| Poly(VPGVG) (simplest form of ELPs) | 40 °C< | Water | Neutral | - | - | >90 | Laboratory scale | [63] | |
| K180L20 | 40 °C | NaHCO3 | Positive | 33 wt.% | - | >90 | Laboratory scale | [64] | |
| C16V3A3E3G | Water | Negative | - | 20–30% | >90 | Laboratory scale | - | [66] | |
| KKAAVVKC12, | RT | Water | Positive | 5% | >90 | Laboratory scale | - | [67] | |
| KKGGAAKC12 | RT | Water | Positive | 5% | >90 | Laboratory scale | After 72 h | [67] | |
| EEAAVVKC12 | RT | Water | Negative | 5% | >90 | Laboratory scale | [67] | ||
| EEGGAAKC12 | RT | Water | Negative | 5% | >90 | Laboratory scale | [67] | ||
| Nap-GFFYG-RGE | 40 °C | PBS | Neutral | 32% | >90 | Laboratory scale | [68] |
| Fabrication Strategy | Nanofiber Size | Process Initiation | Year of Emerging | Encapsulation Efficiency | Yields of Fabrication Process | Scalability | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|---|
| Electrospinning strategy | 2–500 nm | Non-Spontaneous | 1934 | 60–90% | 60–90% | High scalability |
|
|
| Force spinning strategy | 200 nm | Non-Spontaneous | 2001 [89,90] | 50–60% | 40–50% | High scalability |
|
|
| Drawing strategy | 100 nm–1 μm | Non-Spontaneous | 2006 [91] | No data | No data | Laboratory scale |
|
|
| Self-assembly strategy | <200 nm | Spontaneous | 1993 [60] | 50–85% | 60–90% | Laboratory scale |
|
|
| Phase separation strategy | 50–500 nm | Spontaneous | 1947 [92] | <60% | >70% | Laboratory scale |
|
|
| Template-based synthesis | 1 nm–1 μm | Spontaneous | 1990s [75,93,94] | 60–80% | >70% | Laboratory scale |
|
|
| Peptide Sequence | Application | Fabrication Method | Amino Acids | Reference |
|---|---|---|---|---|
| RGD | Targeted delivery | Self-assembly | R, G, D | [95,96] |
| Nap-GFFYG-RGD | Drug delivery | Self-assembly | G, F, Y, R, D | [68] |
| EAK16-II (AEAEAKAKAEAEAKAK) | Vaccine delivery | Self-assembly | E, A, K | [97] |
| K180L20 | Therapeutic delivery | Self-assembly | K, L | [64] |
| Ac-I3SLKG-NH2 | Anticancer peptide delivery | Self-assembly | I, S, L, K, G | [98] |
| Palmitoyl-GGGAAAR Palmitoyl-GGGAAAKRK | Therapeutic delivery | Self-assembly | G, A, R, K | [14] |
| Palmitoyl-GGGAAAKRK | siRNA delivery | Self-assembly | G, A, K, R | [99] |
| RADA-16 | Tissue engineering/vaccine delivery | Self-assembly | R, A, D | [100] |
| TR4 | Gene delivery | Self-assembly | R | [101] |
| RAD16-II peptide (AcN-RARA-DADARARADADA-CNH2). | Growth factor delivery | Self-assembly | R, A, D | [65] |
| FmocFFF | Drug delivery | Self-assembly | F | [102] |
| (KA)4-GCP | Gene delivery | Self-assembly | K, A | [103] |
| GTAGLIGQRGDS | Targeted drug delivery | Self-assembly | G, T, A, L, I, Q, R, G, D | [104] |
| DWRVIIPPRPSA | Targeted delivery | Self-assembly | D, W, R, V, I, P, R, S, A | [105] |
| Ac-KFFAAK-Am | - | Self-assembly | K, F, A | [106] |
| Ac-EFFAAE-Am | - | Self-assembly | E, F, A | [106] |
| Q11 (QQKFQFQFEQQ) | Vaccine delivery | Self-assembly | Q, K, F, E | [107] |
| palmitoyl-A4G3E3 | Drug delivery | Self-assembly | A, G, E | [108] |
| K2 (SL)6K2 | Vaccine delivery | - | K, S, L | [109] |
| G-NMe | Vaccine delivery | - | G | [110] |
| QARILEADAEILRAYARILEAHAEILRAD | Vaccine delivery | - | Q, A, R, I, L, E, D, Y, H | [111] |
| KFE8 (FKFEFKFE-GGAAYFQDAYNAAGGHNAVF) | Vaccine delivery | - | K, F, E, G, A, Y, Q, D, H, N, V, F | [112] |
| IKVAV | siRNA delivery | Self-assembly | I, K, V, A | [113] |
| VEGF-mimicking peptides | Growth factor delivery | Self-assembly | - | [46] |
| DIKVAV | Growth factor controlled release | Electrospinning | D, I, K, V, A | [44,114] |
| ELP | Viral vector delivery | Electrospinning | V, P, G | [45] |
| LDLK | Peptide-based nanofiber scaffold | Electrospinning | L, D, K | [29] |
| LKLK | Peptide-based nanofiber scaffold | Electrospinning | L, K | [29] |
| CDLK | Peptide-based nanofiber scaffold | Electrospinning | C, D, L, K | [29] |
| LDLD | Peptide-based nanofiber scaffold | Electrospinning | L, D | [29] |
| Fmoc-FFD | Biomedicine | Template synthesis | F, F, D | [82] |
| Fmoc–FFpY | Potential therapeutic applications | Phase separation and enzyme catalysis | F, Y | [73] |
| KKAAVVKC12 | Fluorescent dye delivery | Self-assembly | K, A, V | [67] |
| KKGGAAKC12 | Fluorescent dye delivery | Self-assembly | K, G, A | [67] |
| EEAAVVKC12 | Fluorescent dye delivery | Self-assembly | E, A, V | [67] |
| EEGGAAKC12 | Fluorescent dye delivery | Self-assembly | E, G, A | [67] |
| GPO | Functional peptide delivery | Electrospinning | G, P, Hydroxyproline | [24] |
| EESWSWSWSWSWSWEE | Drug delivery | Self-assembly | E, S, W | [115] |
| gH625 | Drug delivery | Self-assembly | G, H | [116] |
| mNPS (SFRNGVGTGMKKTSFQRAKS) | Drug delivery | Self-assembly | S, F, R, N, G, V, T, M, K, Q, A | [117] |
| Lauryl-VVAGEEE (E3PA) | Drug delivery | Self-assembly | V, A, G, E | [118] |
| Lauryl-VVAGKKK(K3PA) | Drug delivery | Self-assembly | V, A, G, K | [118] |
| Ac-FFA-NH | Vaccine delivery | Self-assembly | F, A | [119] |
| QCKIKQIINMWQ | Viral vector delivery | Self-assembly | Q, C, K, I, N, M, W | [120] |
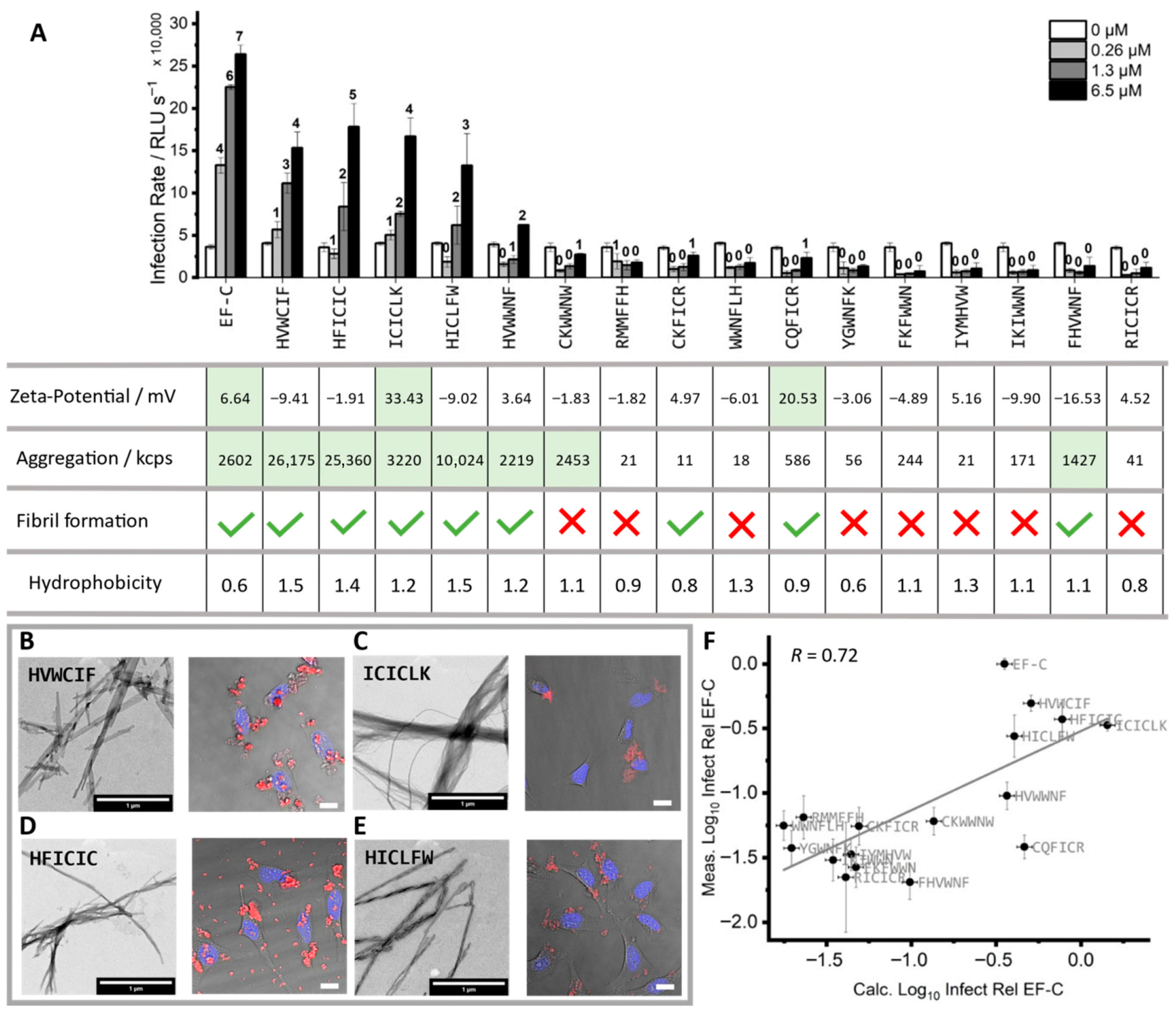
| HVWCIF | Viral vector delivery | Self-assembly | H, I, V, W, C, F | [121] |
| HICLFW | Viral vector delivery | Self-assembly | H, I, C, L, F, W | [121] |
| HFICIC | Viral vector delivery | Self-assembly | H, I, C, F | [121] |
| C16V3A3E3G4REGRT | Functional peptide delivery | Self-assembly | V, A, E, G, R, T | [66] |
| NapFFKY | Drug delivery | Self-assembly | F, K, Y | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghizadeh Pirposhteh, R.; Kheirkhah, O.; Naderi, S.; Borzouee, F.; Bazaz, M.; Parvinzadeh Gashti, M. Advanced Peptide Nanofibers in Delivery of Therapeutic Agents: Recent Trends, Limitations, and Critical Properties. Fibers 2025, 13, 130. https://doi.org/10.3390/fib13100130
Taghizadeh Pirposhteh R, Kheirkhah O, Naderi S, Borzouee F, Bazaz M, Parvinzadeh Gashti M. Advanced Peptide Nanofibers in Delivery of Therapeutic Agents: Recent Trends, Limitations, and Critical Properties. Fibers. 2025; 13(10):130. https://doi.org/10.3390/fib13100130
Chicago/Turabian StyleTaghizadeh Pirposhteh, Razieh, Omolbani Kheirkhah, Shamsi Naderi, Fatemeh Borzouee, Masoume Bazaz, and Mazeyar Parvinzadeh Gashti. 2025. "Advanced Peptide Nanofibers in Delivery of Therapeutic Agents: Recent Trends, Limitations, and Critical Properties" Fibers 13, no. 10: 130. https://doi.org/10.3390/fib13100130
APA StyleTaghizadeh Pirposhteh, R., Kheirkhah, O., Naderi, S., Borzouee, F., Bazaz, M., & Parvinzadeh Gashti, M. (2025). Advanced Peptide Nanofibers in Delivery of Therapeutic Agents: Recent Trends, Limitations, and Critical Properties. Fibers, 13(10), 130. https://doi.org/10.3390/fib13100130







