Physical, Chemical, and Mechanical Characterization of Natural Bark Fibers (NBFs) Reinforced Polymer Composites: A Bibliographic Review
Abstract
1. Introduction
2. Bark Fiber Characterization
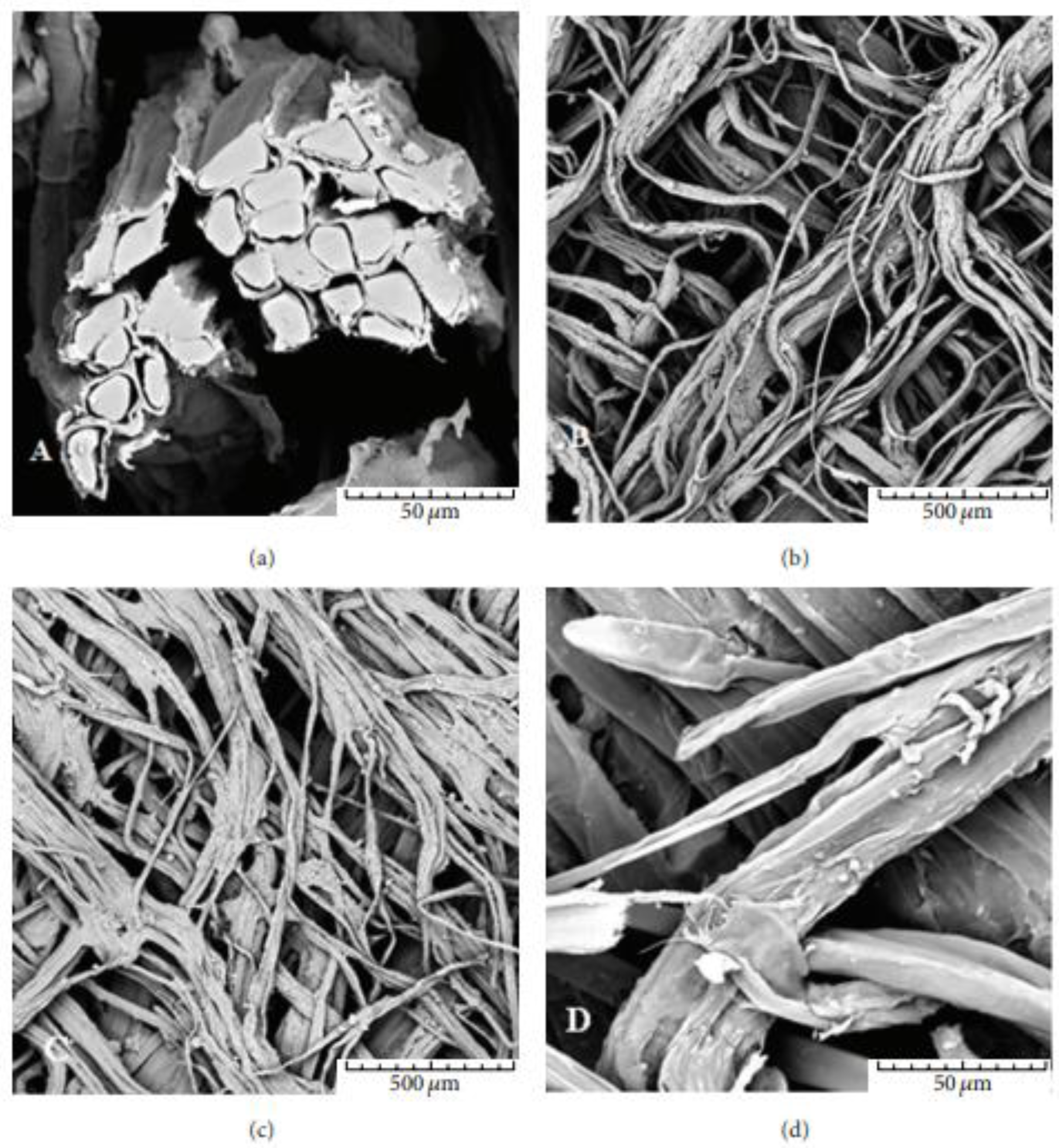
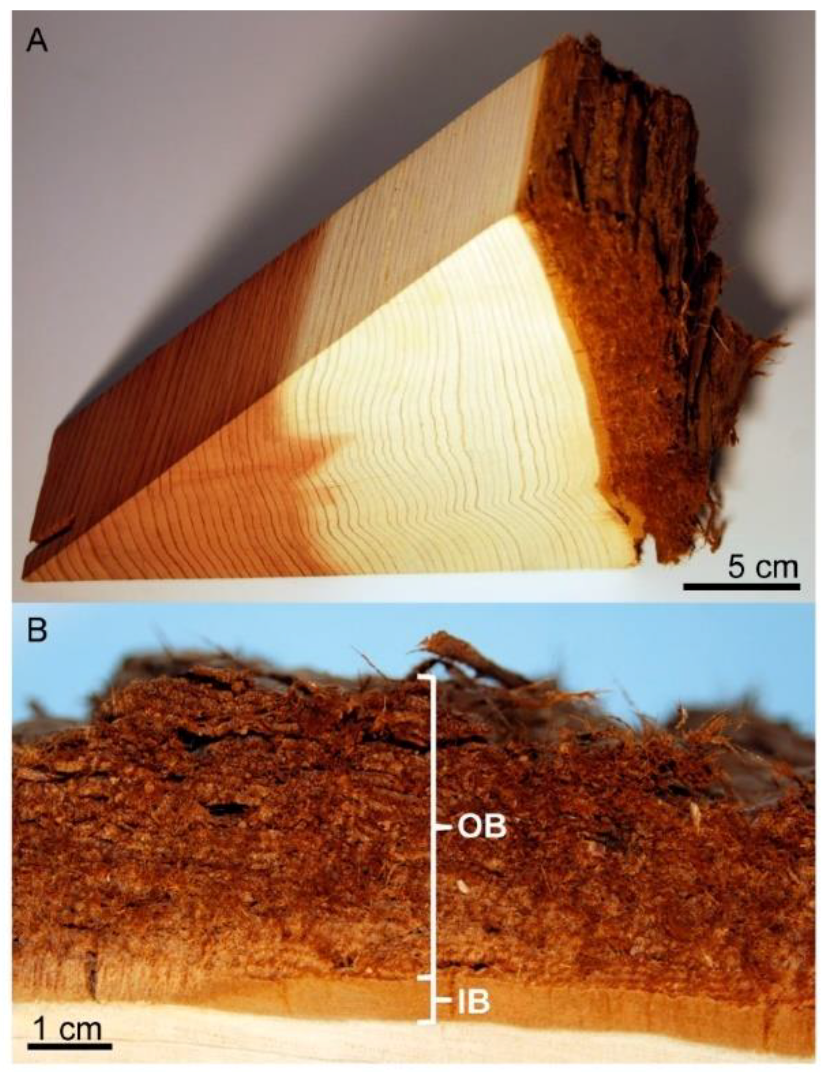
2.1. Bark Fiber Extraction
2.2. Chemical Composition of Bark Fibers
| Fiber | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Ref. |
|---|---|---|---|---|
| Acacia caesia | 37 | 20 | 18 | [72] |
| Acacia nilotica | 56.46 | 14.14 | 8.33 | [74] |
| Albizia lebbeck | 72.59 | 9.69 | 10.08 | [92] |
| Albizia saman | 59.43 | 12.78 | 14.64 | [93] |
| Calotropis gigantea | 57 | 19 | 18 | [76] |
| Carica papaya | 58.71 | 11.8 | 14.26 | [94] |
| Ceiba pentandra | 60 | 17 | 23 | [77] |
| Cissus populnea | 61.8 | 14.74 | 11.52 | [95] |
| Ficus racemosa | 72.36 | 11.21 | 10.45 | [79] |
| Grewia monticola | 55.74 | 14.65 | 15.39 | [80] |
| Hibiscus tiliaceus | 58.63 | 18 | 23.35 | [96] |
| Napier grass | 45.66 | 33.67 | 20.60 | [97] |
| Pithecellobium dulce | 75.15 | 10.23 | 12.14 | [98] |
| Prosopis juliflora | 61.65 | 16.14 | 17.11 | [83] |
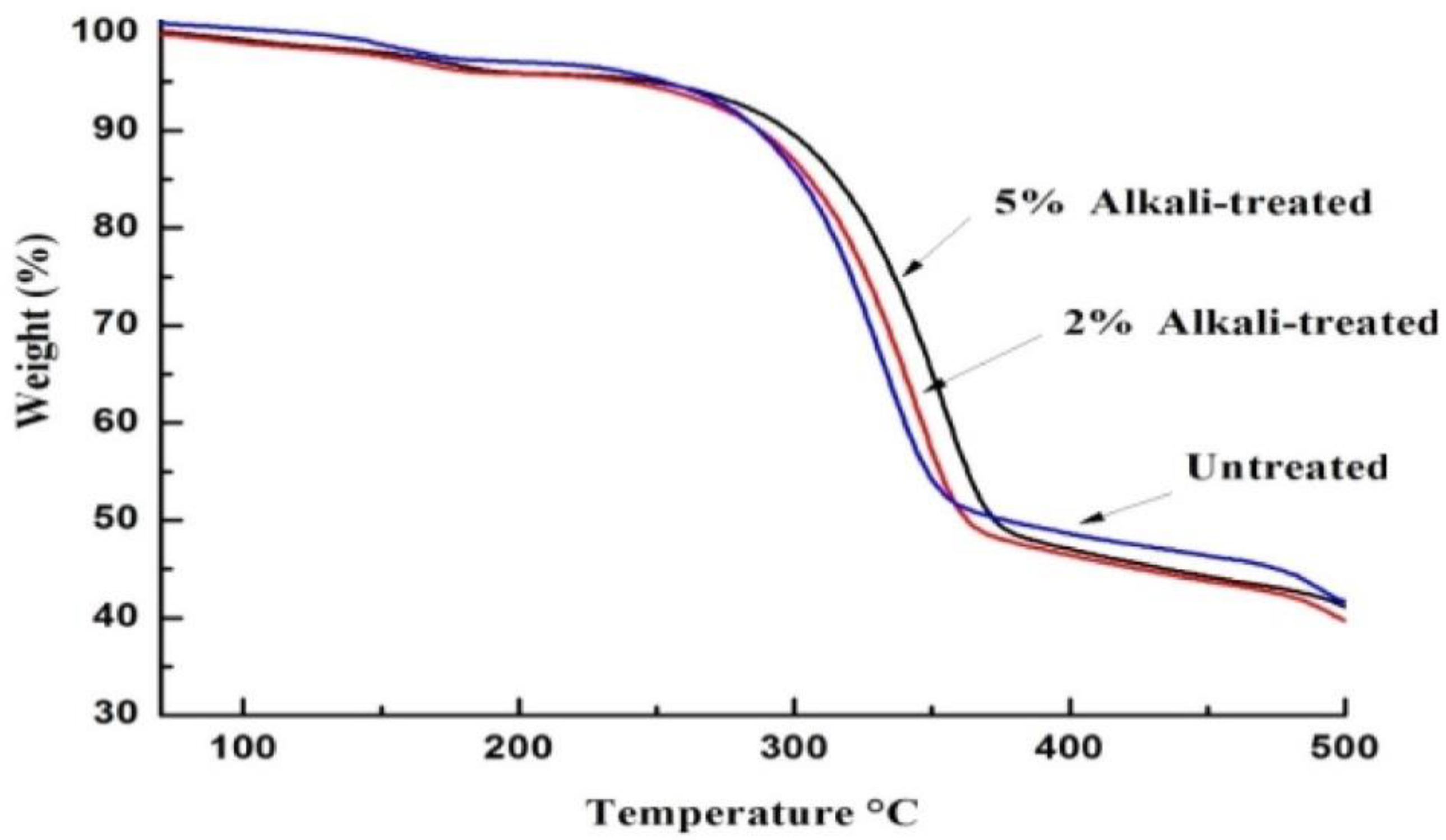
2.3. Thermal Properties of Bark Fibers
2.4. Mechanical Properties of Bark Fibers
3. Bark Fiber Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chahal, A.; Ciolkosz, D. A review of wood-bark adhesion: Methods and mechanics of debarking for woody biomass. Wood Fiber Sci. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Heebink, B.G. Particleboards from Lodgepole Pine Forest Residue; Forest Products Lab: Madison WI USA, 1974.
- Wagner, K.; Roth, C.; Willför, S.; Musso, M.; Petutschnigg, A.; Oostingh, G.J.; Schnabel, T. Identification of antimicrobial compounds in different hydrophilic larch bark extracts. BioResources 2019, 14, 5807. [Google Scholar]
- Kulikowska, D.; Bernat, K. Waste willow-bark from salicylate extraction successfully reused as an amendment for sewage sludge composting. Sustainability 2021, 13, 6771. [Google Scholar] [CrossRef]
- Nicolai, V. The bark of trees: Thermal properties, microclimate and fauna. Oecologia 1986, 69, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Lawes, M.J. Quantifying carbon in tree bark: The importance of bark morphology and tree size. Methods Ecol. Evol. 2021, 12, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Wingfield, M.J.; de Beer, C. Gummosis and wilt of Acacia mearnsii in South Africa caused by Ceratocystis fimbriata. Plant Pathol. 1993, 42, 814–817. [Google Scholar] [CrossRef]
- Miranda, I.; Pereira, H. Variation of wood and bark density and production in coppiced Eucalyptus globulus trees in a second rotation. iForest 2015, 9, 270. [Google Scholar] [CrossRef]
- Wassenberg, M.; Chiu, H.S.; Guo, W.; Spiecker, H. Analysis of wood density profiles of tree stems: Incorporating vertical variations to optimize wood sampling strategies for density and biomass estimations. Trees 2015, 29, 551–561. [Google Scholar] [CrossRef]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crops Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- Nahrstedt, A.; Schmidt, M.; Jäggi, R.; Metz, J.; Khayyal, M.T. Willow bark extract: The contribution of polyphenols to the overall effect. Wien. Med. Wochenschr. 2007, 157, 348–351. [Google Scholar] [CrossRef]
- Ghitescu, R.E.; Volf, I.; Carausu, C.; Bühlmann, A.M.; Gilca, I.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Filbakk, T.; Jirjis, R.; Nurmi, J.; Høibø, O. The effect of bark content on quality parameters of Scots pine (Pinus sylvestris L.) pellets. Biomass Bioenergy 2011, 35, 3342. [Google Scholar] [CrossRef]
- Fodor, F. Fásult személyi-Az árulkodó fakéreg. Természetbúvár 2004, 59, 2. [Google Scholar]
- Ghosh, D. Bark is the hallmark. Resonance 2006, 11, 41. [Google Scholar] [CrossRef]
- Graça, J. Suberin: The biopolyester at the frontier of plants. Front Chem. 2015, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.C. Materials for Construction and Civil Engineering; Gonçalves, M.C., Margarido, F., Eds.; Springer: Cham, Switzerland, 2015; pp. 585–627. [Google Scholar]
- Sudakova, I.G.; Ivanov, I.P.; Ivanchenko, N.M.; Kuznetsov, B.N. Protective compositions for wood on the basis of the suberin of birch bark. Chem. Plant Raw Mater. 2005, 1, 59–63. [Google Scholar]
- De Oliveira, H.; Yoon, B.; Michaud, V.; Nam, J.D.; Suhr, J. All natural cork composites with suberin-based polyester and lignocellulosic residue. Ind. Crops Prod. 2017, 109, 843–849. [Google Scholar] [CrossRef]
- Fernandes, E.M.; Mano, J.F.; Reis, R.L. Hybrid cork–polymer composites containing sisal fibre: Morphology, effect of the fibre treatment on the mechanical properties and tensile failure prediction. Comp. Struct. 2013, 105, 153–162. [Google Scholar] [CrossRef]
- Dhakal, H.N.; Jiang, C.; Sit, M.; Zhang, Z.; Khalfallah, M.; Grossmann, E. Moisture Absorption Effects on the Mechanical Properties of Sandwich Biocomposites with Cork Core and Flax/PLA Face Sheets. Molecules 2021, 26, 7295. [Google Scholar] [CrossRef]
- Gandini, A.; Neto, C.P.; Silvestre, A.J. Suberin: A promising renewable resource for novel macromolecular materials. Prog. Polym. Sci. 2006, 31, 878–892. [Google Scholar] [CrossRef]
- González-López, M.E.; Robledo-Ortíz, J.R.; Manríquez-González, R.; Silva-Guzmán, J.A.; Pérez-Fonseca, A.A. Polylactic acid functionalization with maleic anhydride and its use as coupling agent in natural fiber biocomposites: A review. Compos. Interfaces 2018, 25, 515–538. [Google Scholar] [CrossRef]
- Chow, S.Z.; Pickles, K.J. Thermal softening and degradation of wood and bark. Wood Fiber Sci. 1971, 3, 166–178. [Google Scholar]
- Popp, M.P.; Johnson, J.D.; Massey, T.L. Stimulation of resin flow in slash and loblolly pine by bark beetle vectored fungi. Can. J. Res. 1991, 21, 1124–1126. [Google Scholar] [CrossRef]
- Bauer, G.; Speck, T.; Blömer, J.; Bertling, J.; Speck, O. Insulation capability of the bark of trees with different fire adaptation. J. Mater. Sci. 2010, 45, 5950–5959. [Google Scholar] [CrossRef]
- Yan, N.; Zhang, B.; Zhao, Y.; Farnood, R.R.; Shi, J. Application of biobased phenol formaldehyde novolac resin derived from beetle infested lodgepole pine barks for thermal molding of wood composites. Ind. Eng. Chem. Res. 2017, 56, 6369–6377. [Google Scholar] [CrossRef]
- Bold, G.; Langer, M.; Börnert, L.; Speck, T. The protective role of bark and bark fibers of the giant sequoia (Sequoiadendron giganteum) during high-energy impacts. Int. J. Mol. Sci. 2020, 21, 3355. [Google Scholar] [CrossRef]
- Catry, F.X.; Rego, F.; Moreira, F.; Fernandes, P.M.; Pausas, J.G. Post-fire tree mortality in mixed forests of central Portugal. Ecol. Manag. 2010, 260, 1184–1192. [Google Scholar] [CrossRef]
- Dickinson, M.B. Heat transfer and vascular cambium necrosis in the boles of trees during surface fires. In Forest Fire Research & Wildland Fire Safety; Viegas, Ed.; Millpress: Rotterdam, The Netherlands, 2002; pp. 1–10. ISBN 90-77017-72-0. [Google Scholar]
- Hengst, G.E.; Dawson, J.O. Bark thermal properties of selected central hardwood species. United States Department of Agriculture Report. In Proceedings of the 9th Central Hardwood Forest Conference: Proceedings of a Meeting, West Lafayette, IN, USA, 8–10 March 1993; pp. 55–75. [Google Scholar]
- Lawes, M.J.; Richards, A.; Dathe, J.; Midgley, J.J. Bark thickness determines fire resistance of selected tree species from fire-prone tropical savanna in north Australia. Plant Ecol. 2011, 212, 2057–2069. [Google Scholar] [CrossRef]
- Wang, G.G.; Wangen, S.R. Does frequent burning affect longleaf pine bark thickness? Can. J. Res. 2011, 41, 1562–1565. [Google Scholar] [CrossRef]
- Valentín, L.; Kluczek-Turpeinen, B.; Willför, S.; Hemming, J.; Hatakka, A.; Steffen, K.; Tuomela, M. Scots pine (Pinus sylvestris) bark composition and degradation by fungi: Potential substrate for bioremediation. Bioresour. Technol. 2003, 101, 2203–2209. [Google Scholar] [CrossRef]
- Tamokou, J.D.; Tala, M.F.; Wabo, H.K.; Kuiate, J.R.; Tane, P. Antimicrobial activities of methanol extract and compounds from stem bark of Vismia rubescens. J. Ethnopharmacol. 2009, 124, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Bhaskara Rao, R.; Murugesan, T.; Pal, M.; Saha, B.P.; Mandal, S.C. Antitussive potential of methanol extract of stem bark of Ficus racemosa Linn. Phytother. Res. 2003, 17, 1117–1118. [Google Scholar] [CrossRef] [PubMed]
- Shehu, A.; Magaji, M.G.; Yau, J.; Mahmud, B.; Ahmed, A. Antidepressant effect of methanol stem bark extract of Adansonia digitata L. Malvaceae in Mice. Trop. J. Nat. Prod. Res. 2018, 2, 87–91. [Google Scholar]
- Malik, H.; Javaid, S.; Fawad Rasool, M.; Samad, N.; Rizwan Ahamad, S.; Alqahtani, F.; Imran, I. Amelioration of scopolamine-induced amnesic, anxiolytic and antidepressant effects of Ficus benghalensis in behavioral experimental models. Medicina 2020, 56, 144. [Google Scholar] [CrossRef]
- Venkateshwarlu, G.; Santhosh, A.; Naik, E.R.; Suma, G.; Swapna, K.; Pranitha, D. Traditional and folklore use of Acacia nilotica (l.) in ayurvedic system. Asian J. Pharm. Technol. 2014, 4, 98–99. [Google Scholar]
- Abdullah, A.H.; Kassim, A.; Zainal, Z.; Hussien, M.Z.; Kuang, D.; Ahmad, F.; Wooi, O.S. Preparation and characterization of activated carbon from gelam wood bark (Melaleuca cajuputi). Malays J. Anal. Sci. 2001, 7, 65–68. [Google Scholar]
- Giannotas, G.; Kamperidou, V.; Barboutis, I. Tree bark utilization in insulating bio-aggregates: A review. Biofuel. Bioprod. Biorefin. 2021, 15, 1989–1999. [Google Scholar] [CrossRef]
- Gabrielli, S.; Pastore, G.; Stella, F.; Marcantoni, E.; Sarasini, F.; Tirillò, J.; Santulli, C. Chemical and mechanical characterization of licorice root and palm leaf waste incorporated into poly (urethane-acrylate) (PUA). Molecules 2021, 26, 7682. [Google Scholar] [CrossRef]
- Watanabe, M.; Kanaguri, Y.; Smith Jr., R.L. Hydrothermal separation of lignin from bark of Japanese cedar. J. Supercrit. Fluids 2018, 133, 696–703. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef]
- Schiavi, D.; Francesconi, S.; Taddei, A.R.; Fortunati, E.; Balestra, G.M. Exploring cellulose nanocrystals obtained from olive tree wastes as sustainable crop protection tool against bacterial diseases. Sci. Rep. 2022, 12, 1–14. [Google Scholar]
- Fortunati, E.; Kenny, J.M.; Torre, L. Lignocellulosic materials as reinforcements in sustainable packaging systems: Processing, properties, and applications. In Biomass, Biopolymer-Based Materials, and Bioenergy; Woodhead Publishing: Sawston, UK, 2019; pp. 87–102. [Google Scholar]
- Le Normand, M.; Moriana, R.; Ek, M. Isolation and characterization of cellulose nanocrystals from spruce bark in a biorefinery perspective. Carbohydr. Polym. 2014, 111, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramanian, P.; Kalimuthu, M.; Palaniappan, M.; Alavudeen, A.; Rajini, N.; Santulli, C. Effect of alkali treatment on the properties of acacia caesia bark fibres. Fibers 2021, 9, 49. [Google Scholar] [CrossRef]
- Palanisamy, S.; Mayandi, K.; Palaniappan, M.; Alavudeen, A.; Rajini, N.; Vannucchi de Camargo, F.; Santulli, C. Mechanical properties of phormium tenax reinforced natural rubber composites. Fibers 2021, 9, 11. [Google Scholar] [CrossRef]
- Bensalah, H.; Gueraoui, K.; Essabir, H.; Rodrigue, D.; Bouhfid, R.; Qaiss, A.E. Mechanical, thermal, and rheological properties of polypropylene hybrid composites-based clay and graphite. J. Compos. Mater. 2017, 51, 3563–3576. [Google Scholar] [CrossRef]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A review of natural fibers used in biocomposites: Plant, animal and regenerated cellulose fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Wang, Z.; Gnanasekar, P.; Sudhakaran Nair, S.; Farnood, R.; Yi, S.; Yan, N. Biobased epoxy synthesized from a vanillin derivative and its reinforcement using lignin-containing cellulose nanofibrils. ACS Sustain. Chem. Eng. 2020, 8, 11215–11223. [Google Scholar] [CrossRef]
- Rwawiire, S.; Luggya, G.W.; Tomkova, B. Morphology, Thermal, and Mechanical Characterization of Bark Cloth from Ficus natalensis. ISRN Text. 2013, 2013, 925198. [Google Scholar] [CrossRef]
- Rwawiire, S.; Tomkova, B. Thermal, static, and dynamic mechanical properties of bark cloth (ficus brachypoda) laminar epoxy composites. Polym. Compos. 2017, 38, 199–204. [Google Scholar] [CrossRef]
- Butler, J.A.; Slate, A.J.; Todd, D.B.; Airton, D.; Hardman, M.; Hickey, N.A.; Scott, K.; Venkatraman, P.D. A traditional Ugandan Ficus natalensis bark cloth exhibits antimicrobial activity against Methicillin-Resistant Staphylococcus aureus. J. Appl. Microbiol. 2021, 131, 2–10. [Google Scholar] [CrossRef]
- Castellano, J.; Marrero, M.D.; Ortega, Z. Opuntia Fiber and its potential to obtain sustainable materials in the composites field: A review. J. Nat. Fibers 2022, 19, 10053–10067. [Google Scholar] [CrossRef]
- Chou, T.N.; Young, W.B. Strength of untreated and alkali-treated bamboo fibers and reinforcing effects for short fiber composites. J. Aeronaut. Astronaut. Aviat. 2018, 50, 237–246. [Google Scholar]
- Reddy, K.O.; Shukla, M.; Maheswari, C.U.; Rajulu, A.V. Evaluation of mechanical behavior of chemically modified Borassus fruit short fiber/unsaturated polyester composites. J. Compos. Mater. 2017, 46, 2987–2998. [Google Scholar] [CrossRef]
- Martin, N.; Mouret, N.; Davies, P.; Baley, C. Influence of the degree of retting of flax fibers on the tensile properties of single fibers and short fiber/polypropylene composites. Ind. Crops Prod. 2013, 49, 755–767. [Google Scholar] [CrossRef]
- Wang, K.; Addiego, F.; Laachachi, A.; Kaouache, B.; Bahlouli, N.; Toniazzo, V.; Ruch, D. Dynamic behavior and flame retardancy of HDPE/hemp short fiber composites: Effect of coupling agent and fiber loading. Compos. Struct. 2014, 113, 74–82. [Google Scholar] [CrossRef]
- Jagadish; Rajakumaran, M.; Ray, A. Investigation on mechanical properties of pineapple leaf–based short fiber–reinforced polymer composite from selected Indian (northeastern part) cultivars. J. Compos. Mater. 2020, 33, 324–342. [Google Scholar] [CrossRef]
- Singha, A.S.; Kumar Thakur, V. Saccaharum cilliare fiber reinforced polymer composites. E-J. Chem. 2008, 5, 782–791. [Google Scholar] [CrossRef]
- Kathirselvam, M.; Kumaravel, A.; Arthanarieswaran, V.P.; Saravanakumar, S.S. Assessment of cellulose in bark fibers of Thespesia populnea: Influence of stem maturity on fiber characterization. Carbohydr. Polym. 2019, 15, 439–449. [Google Scholar] [CrossRef]
- Chan, J.M.; Day, P.; Feely, J.; Thompson, R.; Little, K.M.; Norris, C.H. Acacia mearnsii industry overview: Current status, key research and development issues. South 2015, 77, 19–30. [Google Scholar] [CrossRef]
- Rajan, B.S.; Saibalaji, M.A.; Mohideen, S.R. Tribological performance evaluation of epoxy modified phenolic FC reinforced with chemically modified Prosopis juliflora bark fiber. Mater. Res. Expr. 2019, 6, 075313. [Google Scholar] [CrossRef]
- Moussaoui, N.; Rokbi, M.; Osmani, H.; Jawaid, M.; Atiqah, A.; Asim, M.; Benhamadouche, L. Extraction and characterization of fiber treatment Inula viscosa fibers as potential polymer composite reinforcement. J. Polym. Environ. 2021, 29, 3779–3793. [Google Scholar] [CrossRef]
- Arul Marcel Moshi, A.; Ravindran, D.; Sundara Bharathi, S.R.; Padma, S.R.; Indran, S.; Divya, D. Characterization of natural cellulosic fiber extracted from Grewia damine flowering plant′s stem. Int. J. Biol. Macromol. 2020, 164, 1246–1255. [Google Scholar]
- Reddy, N.; Yang, Y. Properties and potential applications of natural cellulose fibers from the bark of cotton stalks. Bioresour. Technol. 2009, 100, 3563–3569. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.G.; Kathiresan, M.; Pandiarajan, P. Effect of alkali-treatment on structural, thermal, tensile properties of dichrostachys cinerea bark fiber and its composites. J. Nat. Fibers 2022, 19, 433–449. [Google Scholar] [CrossRef]
- Koohestani, B.; Darban, A.K.; Mokhtari, P.; Yilmaz, E.; Darezereshki, E. Comparison of different natural fiber treatments: A literature review. Int. J. Environ. Sci. Technol. 2019, 16, 629–642. [Google Scholar] [CrossRef]
- Sympson, W.; Nokes, S.E.; Hickman, A.N. Recirculating calcium hydroxide solution: A practical choice for on-farm high solids lignocellulose pretreatment. Ind. Crops Prod. 2017, 97, 492–497. [Google Scholar] [CrossRef]
- Palanisamy, S.; Kalimuthu, M.; Palaniappan, M.; Alavudeen, A.; Rajini, N.; Santulli, C. Morphological characterization of soapbark fibers. J. Mater. Sci. Res. Rev. 2021, 8, 19. [Google Scholar]
- Amutha, V.; Senthilkumar, B. Physical, chemical, thermal, and surface morphological properties of the bark fiber extracted from acacia concinna plant. J. Nat. Fibers 2021, 18, 1661–1674. [Google Scholar] [CrossRef]
- Kumar, R.; Sivaganesan, S.; Senthamaraikannan, P.; Saravanakumar, S.S.; Khan, A.; Ajith Arul Daniel, S.; Loganathan, L. Characterization of new cellulosic fiber from the bark of Acacia nilotica L. plant. J. Nat. Fibers 2022, 19, 199–208. [Google Scholar] [CrossRef]
- Gopinath, R.; Billigraham, P.; Sathishkumar, T.P. Investigation of physico-chemical, mechanical, and thermal properties of new cellulosic bast fiber extracted from the bark of bauhinia purpurea. J. Nat. Fibers 2022, 19, 9624–9641. [Google Scholar] [CrossRef]
- Ashori, A.; Bahreini, Z. Evaluation of Calotropis gigantea as a promising raw material for fiber-reinforced composite. J. Compos. Mater. 2009, 43, 1297–1304. [Google Scholar] [CrossRef]
- Kumar, R.; Hynes, N.R.; Senthamaraikannan, P.; Saravanakumar, S.; Sanjay, M.R. Physicochemical and thermal properties of ceiba pentandra bark fiber. J. Nat. Fibers 2018, 15, 822–829. [Google Scholar] [CrossRef]
- Baskaran, P.G.; Kathiresan, M.; Senthamaraikannan, P.; Saravanakumar, S.S. Characterization of new natural cellulosic fiber from the bark of dichrostachys cinerea. J. Nat. Fibers 2018, 15, 62–68. [Google Scholar] [CrossRef]
- Manimaran, P.; Saravanan, S.P.; Prithiviraj, M. Investigation of physico chemical properties and characterization of new natural cellulosic fibers from the bark of Ficus Racemosa. J. Nat. Fibers 2021, 18, 274–284. [Google Scholar] [CrossRef]
- Almeshaal, M.; Palanisamy, S.; Murugesan, T.M.; Palaniappan, M.; Santulli, C. Physico-chemical characterization of Grewia Monticola Sond (GMS) fibers for prospective application in biocomposites. J. Nat. Fibers 2022, 19, 15276–15290. [Google Scholar] [CrossRef]
- Vinod, A.; Gowda, T.Y.; Vijay, R.; Sanjay, M.R.; Gupta, M.K.; Jamil, M.; Kushvaha, V.; Siengchin, S. Novel Muntingia Calabura bark fiber reinforced green-epoxy composite: A sustainable and green material for cleaner production. J. Clean. Prod. 2021, 294, 126337. [Google Scholar] [CrossRef]
- Ramkumar, R.; Saravanan, P. Characterization of the cellulose fibers extracted from the bark of piliostigma racemosa. J. Nat. Fibers 2022, 19, 5101–5115. [Google Scholar] [CrossRef]
- Saravanakumar, S.S.; Kumaravel, A.; Nagarajan, T.; Sudhakar, P.; Baskaran, R. Characterization of a novel natural cellulosic fiber from Prosopis juliflora bark. Carbohydr. Polym. 2013, 92, 1928–1933. [Google Scholar] [CrossRef]
- Laifa, F.; Rokbi, M.; Amroune, S.; Zaoui, M.; Seki, Y. Investigation of mechanical, physicochemical, and thermal properties of new fiber from Silybum marianum bark fiber. J. Compos. Mater. 2022, 56, 2227–2238. [Google Scholar] [CrossRef]
- Yadav, N.; Hashmi, S.A. Hierarchical porous carbon derived from eucalyptus-bark as a sustainable electrode for high-performance solid-state supercapacitors. Sustain. Energy Fuels 2020, 4, 1730–1746. [Google Scholar] [CrossRef]
- Haghbin, M.R.; Shahrak, M.N. Process conditions optimization for the fabrication of highly porous activated carbon from date palm bark wastes for removing pollutants from water. Powder Technol. 2021, 377, 890–899. [Google Scholar] [CrossRef]
- Casas-Ledón, Y.; Salgado, K.D.; Cea, J.; Arteaga-Pérez, L.E.; Fuentealba, C. Life cycle assessment of innovative insulation panels based on eucalyptus bark fibers. J. Clean. Prod. 2020, 249, 119356. [Google Scholar] [CrossRef]
- Alishiri, M.; Hooman Hemmasi, A.; Khademi Eslam, H.; Basirjafari, S.; Talaeipour, M. Evaluation and Comparison the Properties of Acoustic Boards Made of Date Palm Fiber. BioResources 2021, 16, 7702–7715. [Google Scholar] [CrossRef]
- Hamouda, T.; Aly, N.M. Date Palm Fiber Composites for Automotive Applications. In Date Palm Fiber Composites; Springer: Singapore, 2020; pp. 387–405. [Google Scholar]
- Kabir, M.M.; Wang, H.; Lau, K.T.; Cardona, F. Effects of chemical treatments on hemp fibre structure. Appl. Surf. Sci. 2013, 276, 13–23. [Google Scholar] [CrossRef]
- Muensri, P.; Kunanopparat, T.; Menut, P.; Siriwattanayotin, S. Effect of lignin removal on the properties of coconut coir fiber/wheat gluten biocomposite. Compos. Part A 2011, 42, 173–179. [Google Scholar] [CrossRef]
- Manimaran, P.; Solai Senthil Kumar, K.; Prithiviraj, M. Investigation of physico chemical, mechanical and thermal properties of the albizia lebbeck bark fibers. J. Nat. Fibers 2021, 18, 1151–1162. [Google Scholar] [CrossRef]
- Gopinath, R.; Billigraham, P.; Sathishkumar, T.P. Physicochemical and thermal properties of cellulosic fiber extracted from the bark of albizia saman. J. Nat. Fibers 2021, 1–17. [Google Scholar] [CrossRef]
- Saravana Kumaar, A.; Senthilkumar, A.; Sornakumar, T.; Saravanakumar, S.S.; Arthanariesewaran, V.P. Physicochemical properties of new cellulosic fiber extracted from Carica papaya bark. J. Nat. Fibers 2019, 16, 175–184. [Google Scholar] [CrossRef]
- Azeez, T.O.; Onukwuli, D.O.; Nwabanne, J.T.; Banigo, A.T. Cissus populnea fiber-unsaturated polyester composites: Mechanical properties and interfacial adhesion. J. Nat. Fibers 2020, 17, 1281. [Google Scholar] [CrossRef]
- Wirawan, W.A.; Choiron, M.A.; Siswanto, E.; Widodo, T.D. Morphology, Structure, and Mechanical Properties of New Natural Cellulose Fiber Reinforcement from Waru (Hibiscus Tiliaceus) Bark. J. Nat. Fibers 2022, 19, 12385–12397. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Shukla, M.; Rajulu, A.V. Chemical composition and structural characterization of Napier grass fibers. Mater. Lett. 2012, 67, 35–38. [Google Scholar] [CrossRef]
- Manimaran, P.; Sanjay, M.R.; Senthamaraikannan, P.; Yogesha, B.; Barile, C.; Siengchin, S. A new study on characterization of Pithecellobium dulce fiber as composite reinforcement for light-weight applications. J. Nat. Fibers 2020, 17, 359–370. [Google Scholar] [CrossRef]
- Pejic, B.M.; Kostic, M.M.; Skundric, P.D.; Praskalo, J.Z. The effects of hemicelluloses and lignin removal on water uptake behavior of hemp fibers. Bioresour. Technol. 2008, 99, 7152–7159. [Google Scholar] [CrossRef] [PubMed]
- Senthamaraikannan, P.; Saravanakumar, S.S.; Sanjay, M.R.; Jawaid, M.; Siengchin, S. Physico-chemical and thermal properties of untreated and treated Acacia planifrons bark fibers for composite reinforcement. Mater. Lett. 2019, 240, 221–224. [Google Scholar] [CrossRef]
- Tiwari, Y.M.; Sarangi, S.K. Characterization of raw and alkali treated cellulosic Grewia Flavescens natural fiber. Int. J. Biol. Macromol. 2022, 209, 1933–1942. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Valenza, A. Characterization of a new natural fiber from Arundo donax L. as potential reinforcement of polymer composites. Carbohydr. Polym. 2014, 106, 77–83. [Google Scholar] [CrossRef]
- Lewis, H.F.; Brauns, F.E.; Buchanan, M.A.; Kurth, E.F. Chemical composition of redwood bark. J. Ind. Eng. Chem. 1944, 36, 759–764. [Google Scholar] [CrossRef]
- Umashankaran, M.; Gopalakrishnan, S. Effect of sodium hydroxide treatment on physico-chemical, thermal, tensile and surface morphological properties of Pongamia Pinnata L. bark fiber. J. Nat. Fibers 2021, 18, 2063–2076. [Google Scholar] [CrossRef]
- Meddah, M.; Rokbi, M.; Zaoui, M. Extraction and characterization of novel natural lignocellulosic fibers from Malva sylvestris L. J. Compos. Mater. 2022, 00219983221146355. [Google Scholar] [CrossRef]
- Angelini, L.G.; Tavarini, S.; Di Candilo, M. Performance of new and traditional fiber hemp (Cannabis sativa L.) cultivars for novel applications: Stem, bark, and core yield and chemical composition. J. Nat. Fibers 2016, 13, 238–252. [Google Scholar] [CrossRef]
- Ndoumou, R.L.; Soulat, D.; Labanieh, A.R.; Ferreira, M.; Meva’a, L.; Atangana Ateba, J. Characterization of tensile properties of cola lepidota fibers. Fibers 2022, 10, 6. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Reihmane, S.; Gassan, J. Properties and modification methods for vegetable fibers for natural fiber composites. J. Appl. Polym. Sci. 1996, 59, 1329–1336. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Letman, M.; Viksne, A.; Rence, L. A comparison of compounding processes and wood type for wood fibre—PP composites. Compos. Part A 2005, 36, 789–797. [Google Scholar] [CrossRef]
- Reddy, K.H.; Reddy, R.M.; Ramesh, M.; Krishnudu, D.M.; Reddy, B.M.; Rao, H.R. Impact of alkali treatment on characterization of tapsi (Sterculia urens) natural bark fiber reinforced polymer composites. J. Nat. Fibers 2021, 18, 378–389. [Google Scholar] [CrossRef]
- Mwaikambo, L.Y.; Ansell, M.P. Chemical modification of hemp, sisal, jute, and kapok fibers by alkalization. J. Appl. Polym. Sci. 2002, 84, 2222–2234. [Google Scholar] [CrossRef]
- Zeronian, S.H. The mechanical properties of cotton fibers. J. Appl. Polym. Sci. 1991, 47, 445. [Google Scholar] [CrossRef]
- Ugbolue, S.C. Structure/property relationships in textile fibres. Text Prog. 1990, 20, 1–43. [Google Scholar] [CrossRef]
- Bisanda, E.T.; Ansell, M.P. Properties of sisal-CNSL composites. J. Mater. Sci. 1992, 27, 1690–1700. [Google Scholar] [CrossRef]
- Arthanarieswaran, V.P.; Kumaravel, A.; Kathirselvam, M.; Saravanakumar, S.S. Mechanical and thermal properties of Acacia leucophloea fiber/epoxy composites: Influence of fiber loading and alkali treatment. Int. J. Polym. Anal. Charact. 2016, 21, 571–583. [Google Scholar] [CrossRef]
- Gopinath, R.; Billigraham, P.; Sathishkumar, T.P. Characterization studies on new natural cellulosic fiber extracted from the bark of erythrina variegata. J. Nat. Fibers 2021, 1–20. [Google Scholar] [CrossRef]
- Ndazi, B.S.; Nyahumwa, C.W.; Tesha, J. Chemical and thermal stability of rice husks against alkali treatment. BioResources 2008, 3, 1267–1277. [Google Scholar]
- Safdari, V.; Khodadadi, H.; Hosseinihashemi, S.K.; Ganjian, E. The effects of poplar bark and wood content on the mechanical properties of wood-polypropylene composites. BioResources 2011, 6, 5180–5192. [Google Scholar] [CrossRef]
- Lopez, Y.M.; Paes, J.B.; Gonçalves, F.G.; de Alcântara, P.G.; da Silva, L.F.; Nicácio, M.A.; da Silva, E.S.G.; de Medeiros, J.R.; dos Santos Moreira, J.G.; Nantet, A.C.T. Characterization of the production process of wood plastic composite from sawdust of pinus and recycled thermoplastics. Eng. Florest. Desafios Lte. E Potencialidade 2020, 236–247. [Google Scholar] [CrossRef]
- Borysiuk, P.; Boruszewski, P.; Auriga, R.; Danecki, L.; Auriga, A.; Rybak, K.; Nowacka, M. Influence of a bark-filler on the properties of PLA biocomposites. J. Mater. Sci. 2021, 56, 9196–9208. [Google Scholar] [CrossRef]
- Saini, G.; Bhardwaj, R.; Choudhary, V.; Narula, A.K. Poly(vinyl chloride)–Acacia bark flour composite: Effect of particle size and filler content on mechanical, thermal, and morphological characteristics. J. Appl. Polym. Sci. 2010, 117, 1309–1318. [Google Scholar] [CrossRef]
- Banaei, N.; Ahmadi, S. High-density polyethylene surface modification for the attachment of Eggshell and Oak Bark Nanoparticles. Adv. Appl. NanoBio-Technol. 2020, 1, 67–71. [Google Scholar]
- Yemele, M.C.N.; Koubaa, A.; Cloutier, A.; Soulounganga, P.; Wolcott, M. Effect of bark fiber content and size on the mechanical properties of bark/HDPE composites. Compos. Part A 2010, 41, 131–137. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Joshy, M.K.; Mathew, L.; Joseph, R. Effect of alkali treatment on the mechanical properties of short randomly oriented isora fibre-polyester composites. Prog. Rubber Plast. Recycl. Technol. 2008, 24, 255–272. [Google Scholar] [CrossRef]
- Mathew, L.; Joseph, K.U.; Joseph, R. Isora fibre: Morphology, chemical composition, surface modification, physical, mechanical and thermal properties–A potential natural reinforcement. J. Nat. Fibers 2007, 3, 13–27. [Google Scholar] [CrossRef]
- Chirayil, C.J.; Joy, J.; Mathew, L.; Mozetic, M.; Koetz, J.; Thomas, S. Isolation and characterization of cellulose nanofibrils from Helicteres isora plant. Ind. Crops Prod. 2014, 59, 27–34. [Google Scholar] [CrossRef]
- Sivaraj Vijaya, G.; Gounder, R.I.; Natarajan, S.S. Characterization of natural cellulose fibers from the barks of ziziphus nummularia as a reinforcement for lightweight composite applications. J. Nat. Fibers 2022, 19, 15663–15679. [Google Scholar] [CrossRef]
- Gurupranes, S.V.; Rajendran, I.; Shanmuga Sundaram, N. Suitability assessment of raw-alkalized Ziziphus nummularia bark fibers and its polymeric composites for lightweight applications. Polym. Compos. 2022, 43, 5059–5075. [Google Scholar]
- Wirawan, W.A.; Setyabudi, S.A.; Widodo, T.D.; Choiron, M.A. Surface modification with silane coupling agent on tensile properties of natural fiber composite. J. Energy Mech. Mater. Manuf. Eng. 2017, 2, 98–103. [Google Scholar] [CrossRef]
- Madhu, P.; Mavinkere Rangappa, S.; Khan, A.; Al Otaibi, A.; Al-Zahrani, S.A.; Pradeep, S.; Gupta, M.K.; Boonyasopon, P.; Siengchin, S. Experimental investigation on the mechanical and morphological behavior of Prosopis juliflora bark fibers/E-glass/carbon fabrics reinforced hybrid polymeric composites for structural applications. Polym. Compos. 2020, 41, 4983–4993. [Google Scholar] [CrossRef]
- Ramesh, M.; Tamil Selvan, M.; Rajeshkumar, L.; Deepa, C.; Ahmad, A. Influence of Vachellia nilotica Subsp. indica tree trunk bark nano-powder on properties of milkweed plant fiber reinforced epoxy composites. J. Nat. Fibers 2022, 19, 13776–13789. [Google Scholar] [CrossRef]
- Khan, A.A.; Parikh, H.; Qureshi, M.R.N. A review on chicken feather fiber (CFF) and its application in Composites. J. Nat. Fibers 2022, 1–21. [Google Scholar] [CrossRef]
- Rangappa, S.M.; Parameswaranpillai, J.; Siengchin, S.; Jawaid, M.; Ozbakkaloglu, T. Bioepoxy based hybrid composites from nano-fillers of chicken feather and lignocellulose Ceiba Pentandra. Sci. Rep. 2022, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
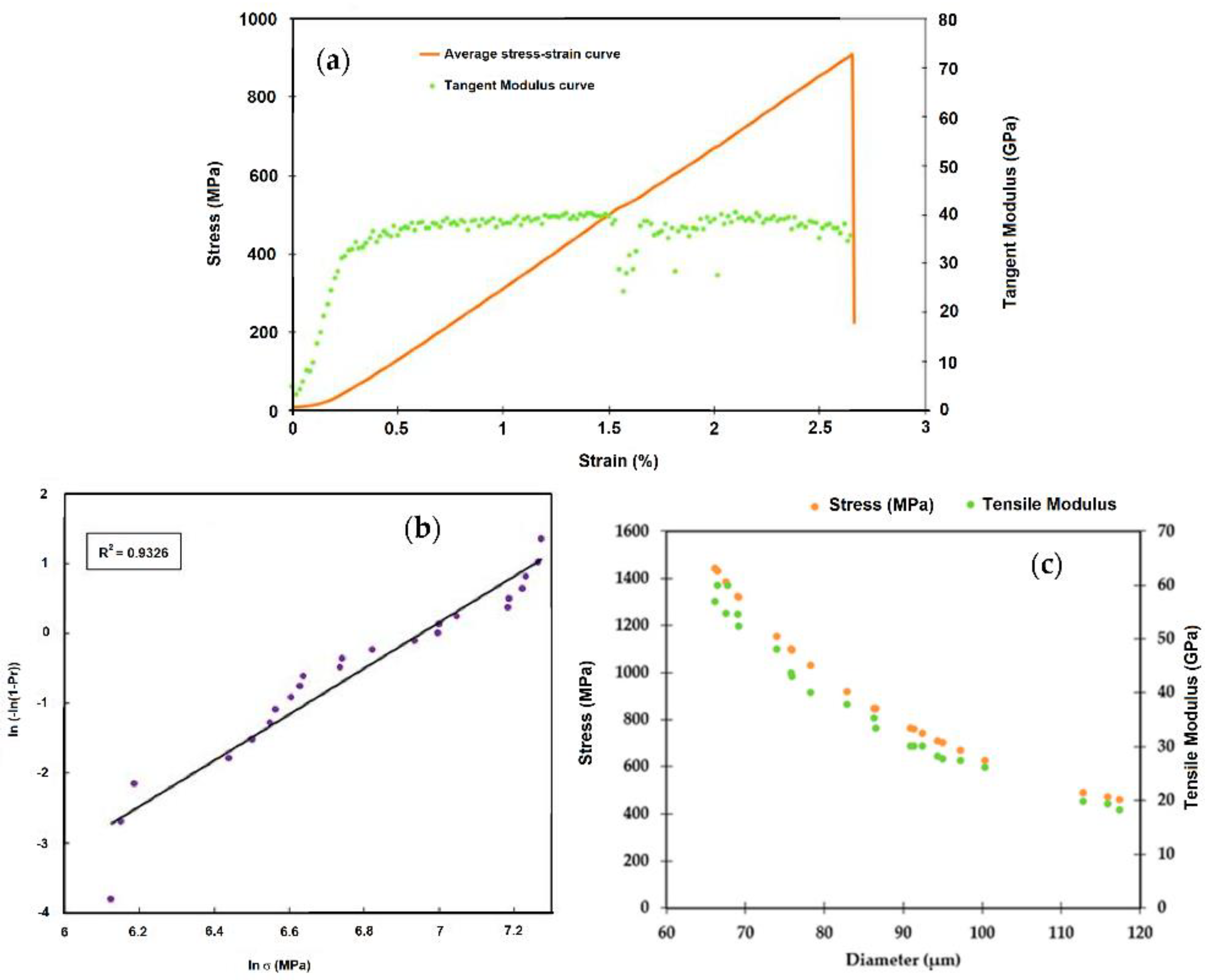

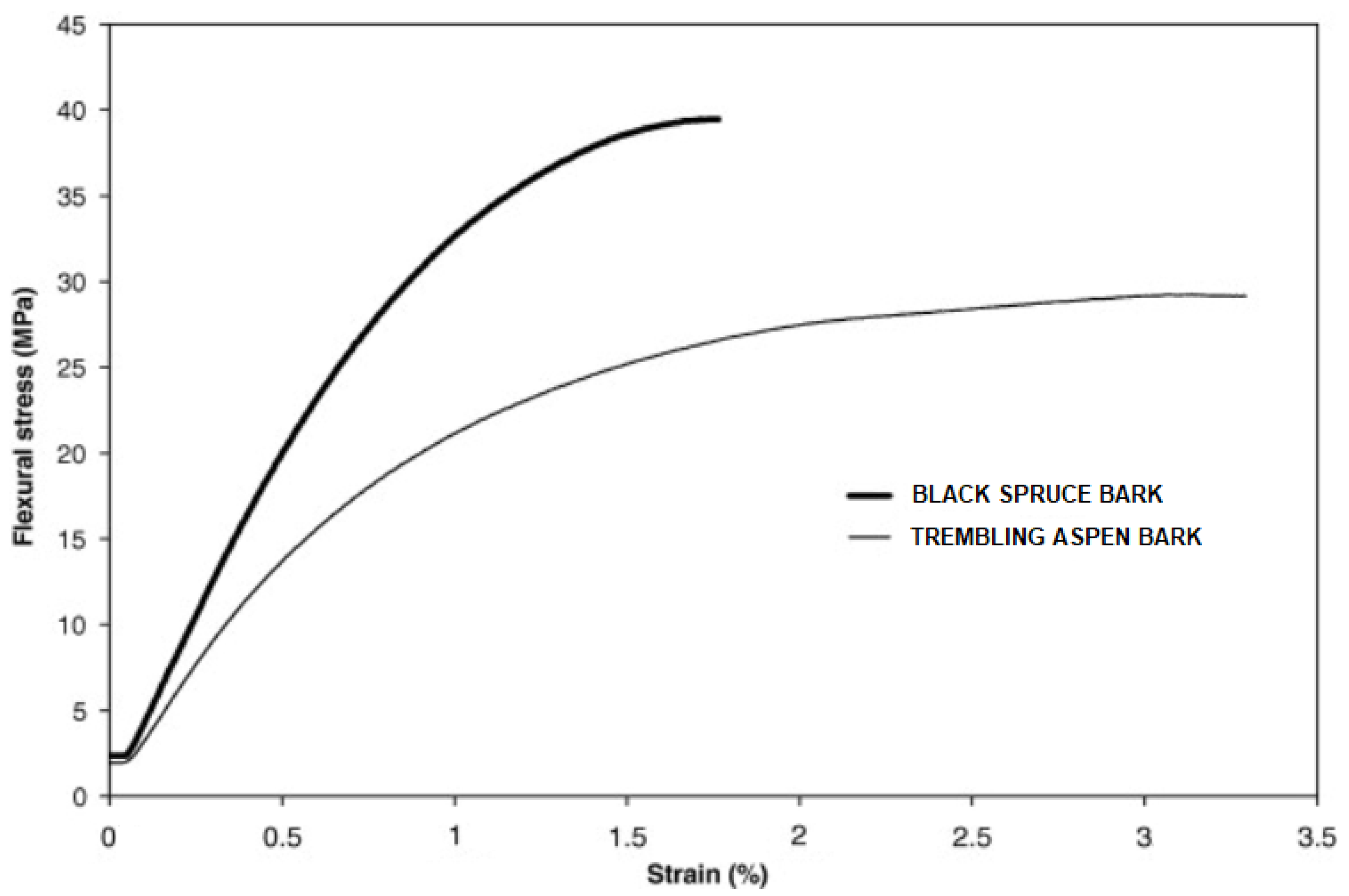
| Fiber | Amount (%) | Length (mm) | Matrix | Ref. |
|---|---|---|---|---|
| Bamboo | 30 wt.% | 40 | Poly(lactic acid) | [57] |
| Borassus | 4, 8, 12, 16, 20, and 24 wt.% | 20–30 | Unsaturated polyester | [58] |
| Flax | 20 vol.% | 3 | Maleated polypropylene | [59] |
| Hemp | 25 and 50 wt.% | 6 | Maleated high density polyethylene | [60] |
| Pineapple leaf | 1, 5, 10, 15, and 20 wt.% | 1–2 | Epoxy | [61] |
| Saccharum ciliare | 30 wt.% | 3 | Urea-formaldehyde | [62] |
| Bark Fiber | Density (kg/m3) | Ref. |
|---|---|---|
| Acacia caesia | 1200 | [72] |
| Acacia concinna | 1365 | [73] |
| Acacia nilotica | 1165 | [74] |
| Bauhinia purpurea | 1190 | [75] |
| Calotropis gigantea | 560 | [76] |
| Ceiba pentandra | 682 | [77] |
| Dichrostachys cinerea | 1240 | [78] |
| Ficus racemosa | 895 | [79] |
| Grewia monticola | 1354 | [80] |
| Muntingia calabura | 924 | [81] |
| Piliostigma racemosa | 1371 | [82] |
| Prosopis juliflora | 580 | [83] |
| Silybum marianum | 1098 | [84] |
| Batch | Cellulose (wt.%) | Hemicellulose (wt.%) | Lignin (wt.%) | Wax (wt.%) | Moisture (%) | Ash (wt.%) |
|---|---|---|---|---|---|---|
| Untreated | 73.1 | 9.41 | 12.04 | 0.57 | 8.21 | 4.06 |
| 2% alkali | 76.32 | 6.31 | 10.53 | 0.32 | 7.84 | 3.54 |
| 5% alkali | 79.13 | 3.02 | 7.41 | 0.25 | 6.42 | 3.10 |
| Batch | Cellulose (wt.%) | Hemicellulose (wt.%) | Lignin (wt.%) | Wax (wt.%) | Moisture (%) | Ash (wt.%) |
| Untreated | 58.46 | 15.32 | 12.51 | 0.86 | 9.42 | 5.12 |
| 15 min | 60.34 | 14.54 | 10.32 | 0.78 | 8.36 | 6.56 |
| 30 min | 64.76 | 13.21 | 8.46 | 0.62 | 7.96 | 7.34 |
| 45 min | 68.31 | 11.54 | 7.85 | 0.53 | 6.55 | 8.81 |
| 60 min | 66.32 | 10.21 | 6.21 | 0.47 | 5.32 | 9.21 |
| 75 min | 64.67 | 8.92 | 5.36 | 0.41 | 4.91 | 10.46 |
| Non Bark Fiber | Elongation (%) | Tensile Strength (MPa) | Stiffness (GPa) | Ref. |
|---|---|---|---|---|
| Cotton | 7–8 | 287–597 | 5.5–12.6 | [111,112] |
| Jute | 1.5–1.8 | 393–773 | 26.5 | [111,113] |
| Flax | 2.7–3.2 | 345–1035 | 27.6 | [111,113] |
| Hemp | 1.6 | 690 | - | [111] |
| Ramie | 3.6–3.8 | 400–938 | 61.4–128 | [111,112] |
| Sisal | 2.0–2.5 | 511–635 | 9.4–22 | [111,114] |
| Coir | 15 | 175 | 4–6 | [111,113] |
| Bark Fiber | Elongation (%) | Tensile Strength (MPa) | Stiffness (GPa) | Ref. |
| Grewia Damine | - | 376 | - | [67] |
| Calotropis gigantea | 2.1 | 381 | 9.7 | [76] |
| Dichrostachys cinerea | - | 873 | - | [78] |
| Grewia flavescens | 3.4 | 276.9 | 10.75 | [101] |
| Prosopis juliflora | 1.8 | 558 | 16 | [83] |
| Acacia Leucophloea | 1.2–2 | 317–1608 | 8.4–69.6 | [115] |
| Erythrina Variegata | - | 301–705 | 4.7–10.5 | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palanisamy, S.; Kalimuthu, M.; Nagarajan, R.; Fernandes Marlet, J.M.; Santulli, C. Physical, Chemical, and Mechanical Characterization of Natural Bark Fibers (NBFs) Reinforced Polymer Composites: A Bibliographic Review. Fibers 2023, 11, 13. https://doi.org/10.3390/fib11020013
Palanisamy S, Kalimuthu M, Nagarajan R, Fernandes Marlet JM, Santulli C. Physical, Chemical, and Mechanical Characterization of Natural Bark Fibers (NBFs) Reinforced Polymer Composites: A Bibliographic Review. Fibers. 2023; 11(2):13. https://doi.org/10.3390/fib11020013
Chicago/Turabian StylePalanisamy, Sivasubramanian, Mayandi Kalimuthu, Rajini Nagarajan, José Maria Fernandes Marlet, and Carlo Santulli. 2023. "Physical, Chemical, and Mechanical Characterization of Natural Bark Fibers (NBFs) Reinforced Polymer Composites: A Bibliographic Review" Fibers 11, no. 2: 13. https://doi.org/10.3390/fib11020013
APA StylePalanisamy, S., Kalimuthu, M., Nagarajan, R., Fernandes Marlet, J. M., & Santulli, C. (2023). Physical, Chemical, and Mechanical Characterization of Natural Bark Fibers (NBFs) Reinforced Polymer Composites: A Bibliographic Review. Fibers, 11(2), 13. https://doi.org/10.3390/fib11020013









