Extraction and Physico-Chemical Characterization of Pineapple Crown Leaf Fibers (PCLF)
Abstract
1. Introduction
2. Materials and Methods
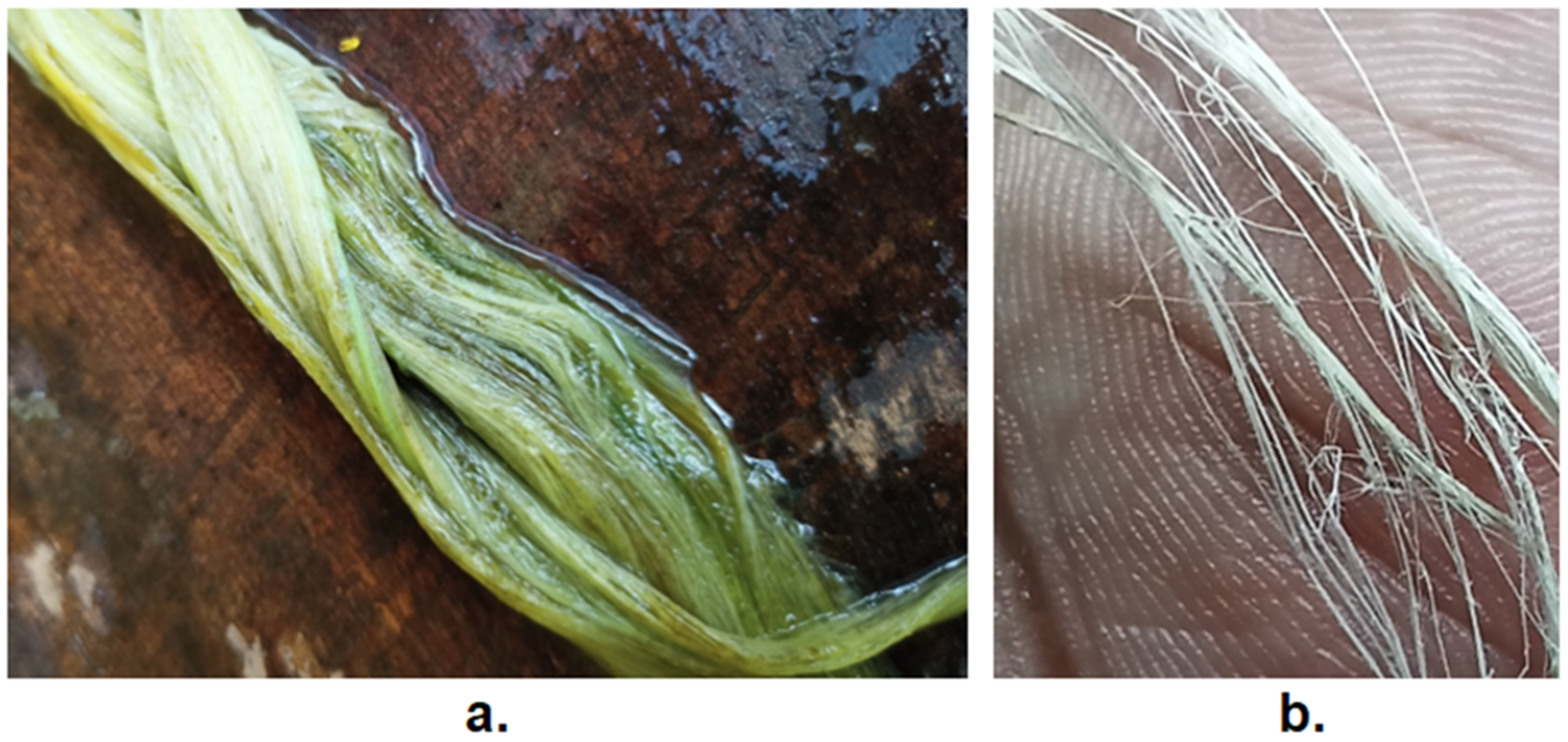
2.1. Extraction of Fiber
2.2. Scraping and Retting Process of Pineapple Leaves
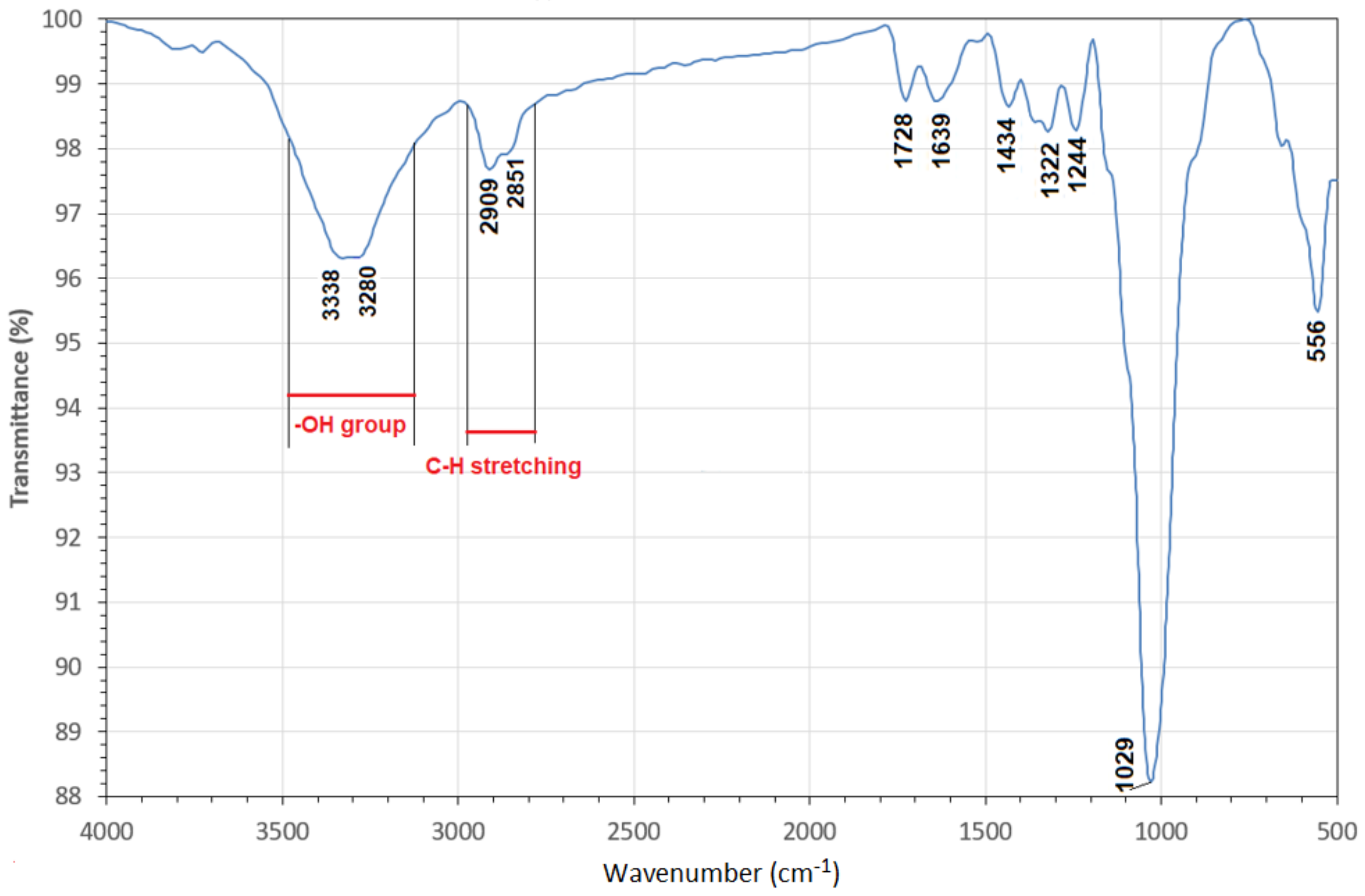
2.3. Scanning Electron Microscopy (SEM)
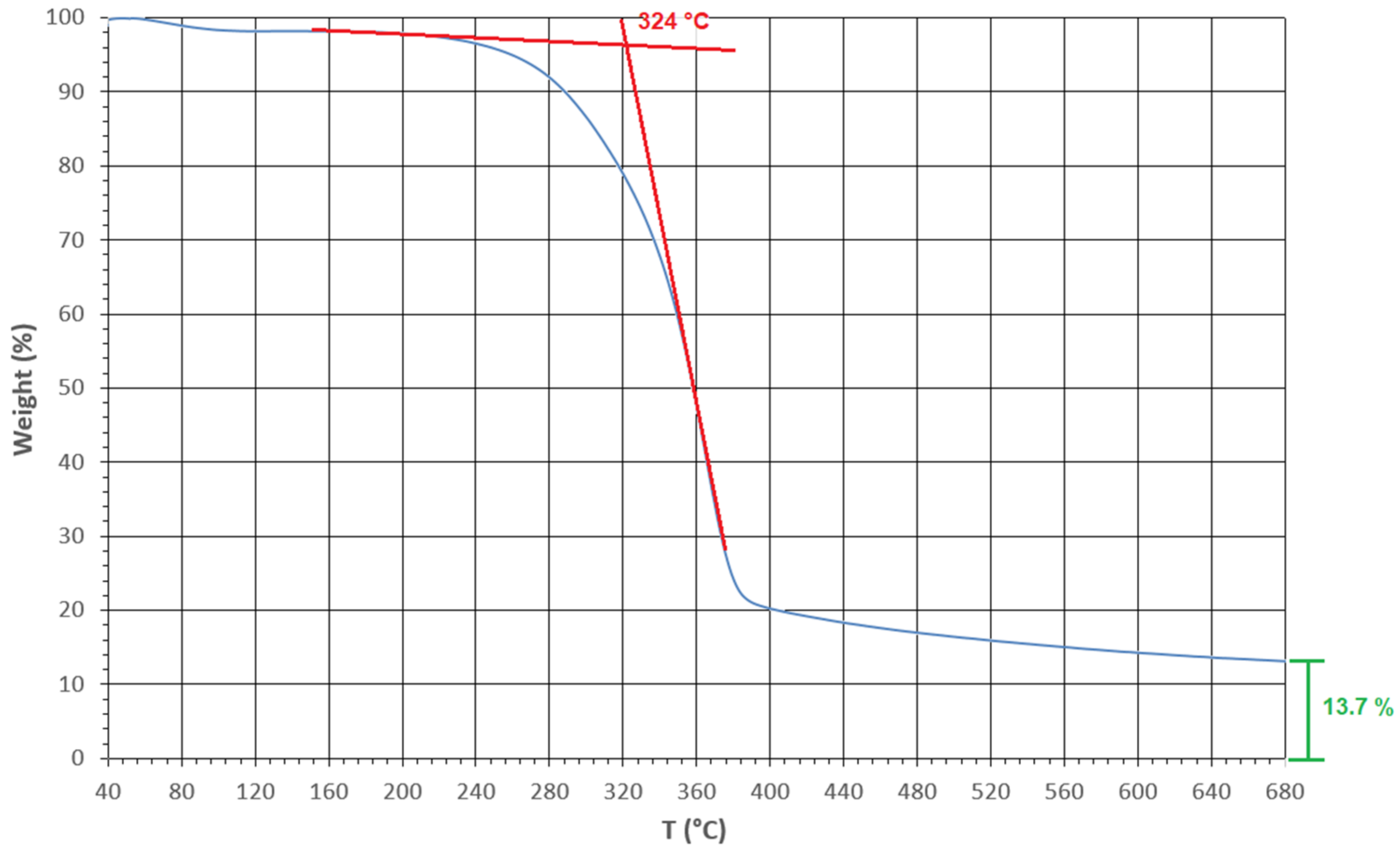
2.4. Thermogravimetric Analysis (TGA)
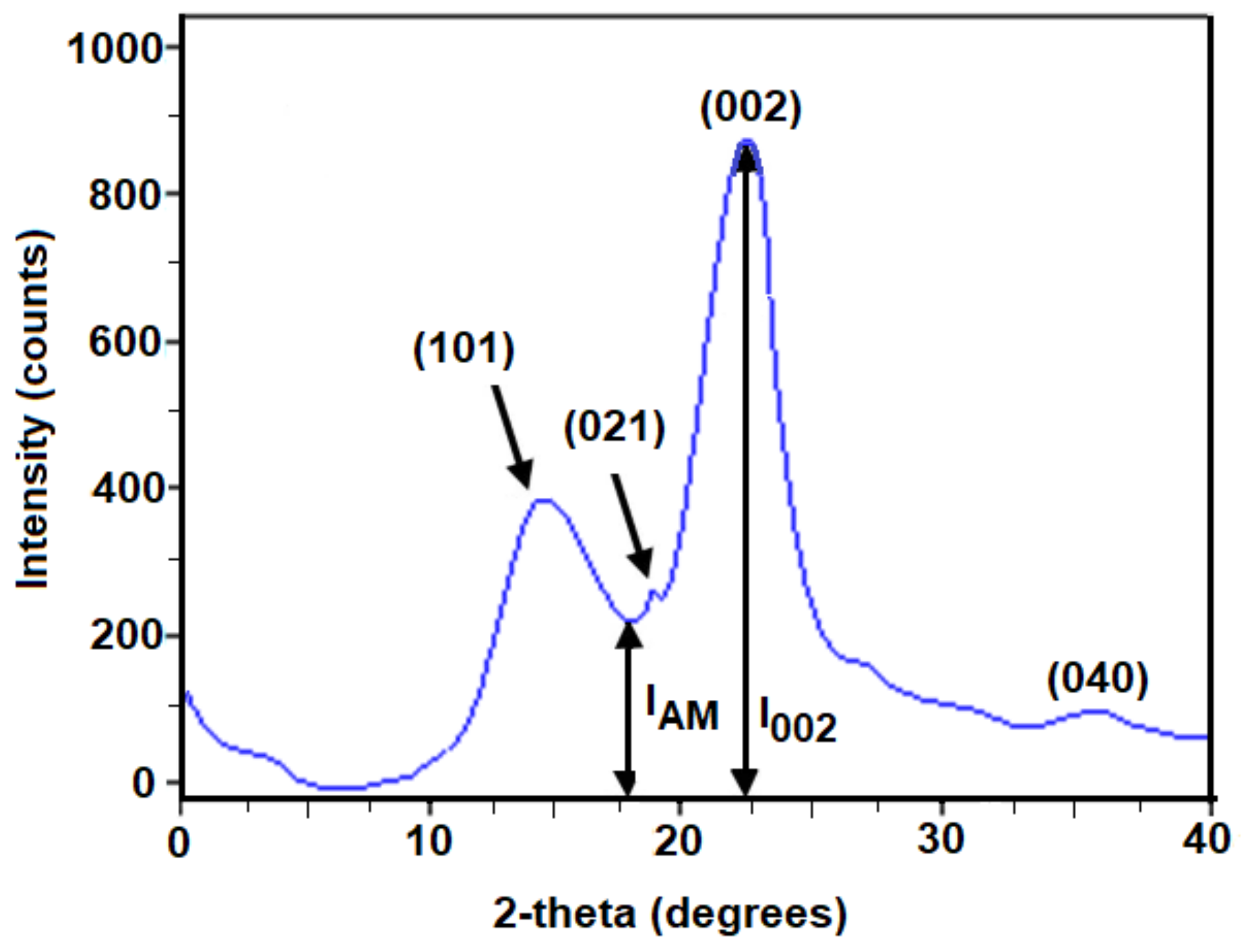
2.5. X-ray Diffraction (XRD)
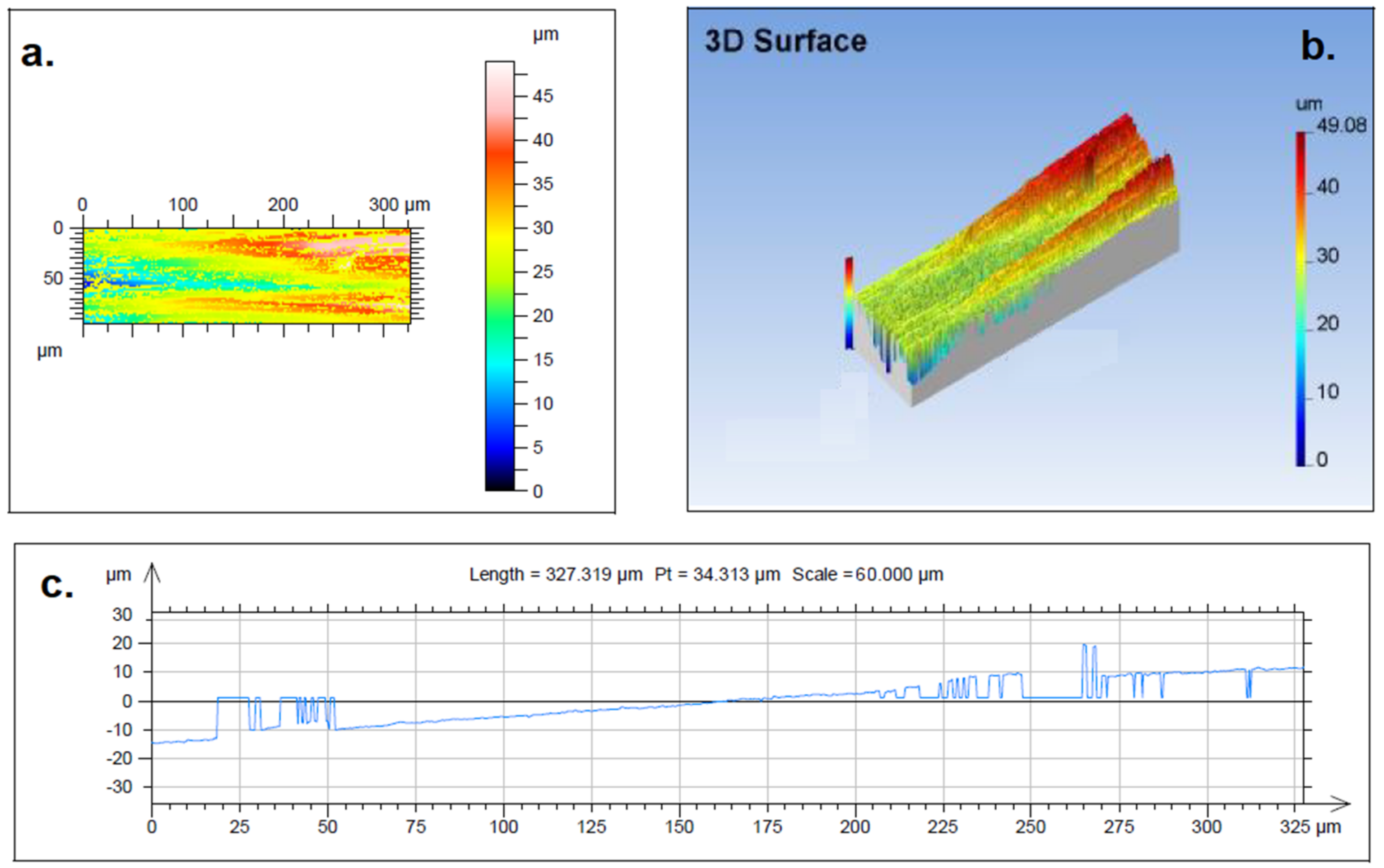
2.6. Atomic Force Microscopy (AFM)
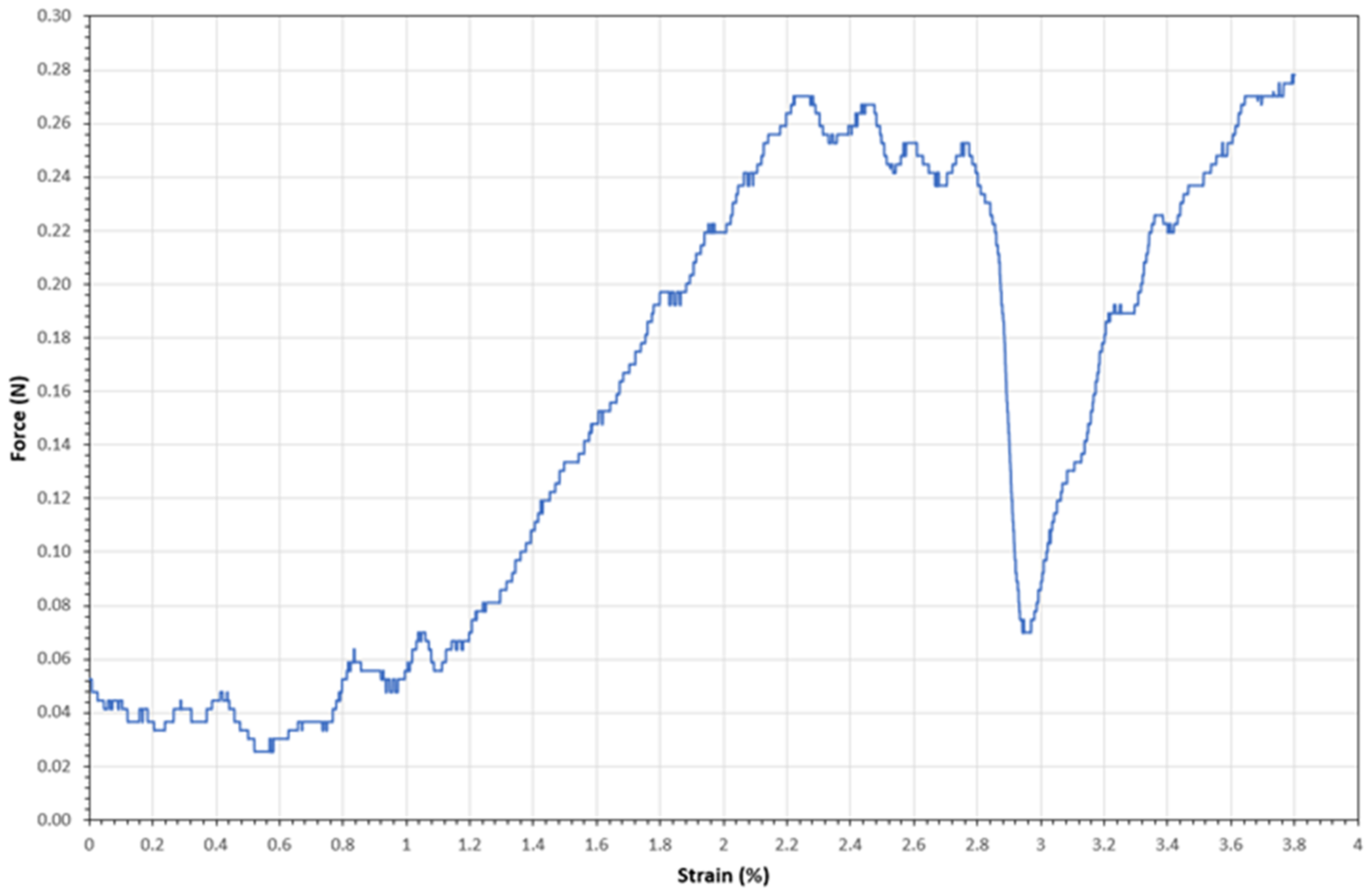
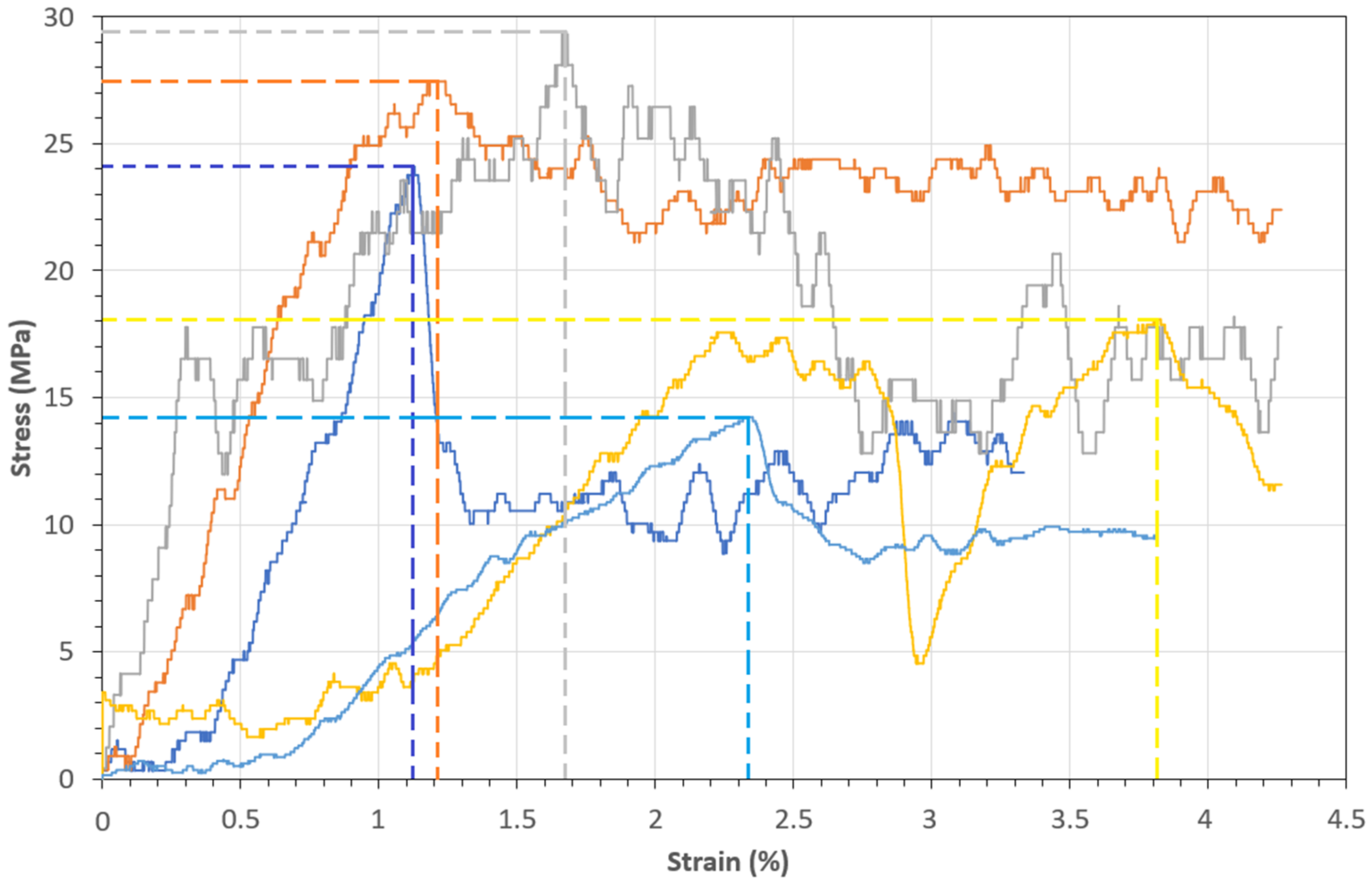
2.7. Single Fiber Tensile Tests (SFTT)
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rao, K.M.M. Extraction and tensile properties of natural fibers: Vakka, date and bamboo. Compos. Struct. 2007, 77, 288–295. [Google Scholar] [CrossRef]
- Ogah, A.O.; Afiukwa, J.N.; Englund, K. Characterization and comparison of thermal stability of agro waste fibers in bio-composites application. J. Chem. Eng. Chem. Res. 2014, 1, 84–93. [Google Scholar]
- Thomsen, A.B.; Rasmussen, S.; Bohn, V.; Nielsen, K.V.; Thygesen, A. Hemp Raw Materials: The Effect of Cultivar, Growth Conditions and Pretreatment on the Chemical Composition of the Fibres; Risø DTU-National Laboratory for Sustainable Energy: Roskilde, Denmark, 2005. [Google Scholar]
- Jumaidin, R.; Diah, N.; Ilyas, R.; Alamjuri, R.; Yusof, F. Processing and Characterisation of Banana Leaf Fibre Reinforced Thermoplastic Cassava Starch Composites. Polymers 2021, 13, 1420. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.A.; Siddiqui, Q.; Khan, M.R.; Mushtaq, M.; Wasim, M.; Farooq, A.; Naveed, T.; Wei, Q. Bacterial cellulose-natural fiber composites produced by fibers extracted from banana peel waste. J. Ind. Text. 2020, 51, 990S–1006S. [Google Scholar] [CrossRef]
- Ferrante, A.; Santulli, C.; Summerscales, J. Evaluation of Tensile Strength of Fibers Extracted from Banana Peels. J. Nat. Fibers 2019, 17, 1519–1531. [Google Scholar] [CrossRef]
- Hassan, A.; Salema, A.A.; Ani, F.N.; Abu Bakar, A. A review on oil palm empty fruit bunch fiber-reinforced polymer composite materials. Polym. Compos. 2010, 31, 2079–2101. [Google Scholar] [CrossRef]
- Momoh, E.O.; Osofero, A.I. Recent developments in the application of oil palm fibers in cement composites. Front. Struct. Civ. Eng. 2020, 14, 94–108. [Google Scholar] [CrossRef]
- Hasan, K.M.F.; Horváth, P.G.; Bak, M.; Alpár, T. A state-of-the-art review on coir fiber-reinforced biocomposites. RSC Adv. 2021, 11, 10548–10571. [Google Scholar] [CrossRef]
- Muhammad, A.; Rahman, M.; Hamdan, S.; Sanaullah, K. Recent developments in bamboo fiber-based composites: A review. Polym. Bull. 2018, 76, 2655–2682. [Google Scholar] [CrossRef]
- Valvez, S.; Maceiras, A.; Santos, P.; Reis, P. Olive Stones as Filler for Polymer-Based Composites: A Review. Materials 2021, 14, 845. [Google Scholar] [CrossRef]
- Han, X.; Ding, L.; Tian, Z.; Wu, W.; Jiang, S. Extraction and characterization of novel ultrastrong and tough natural cellulosic fiber bundles from manau rattan (Calamus manan). Ind. Crops Prod. 2021, 173, 114103. [Google Scholar] [CrossRef]
- Han, X.; Wang, J.; Wang, J.; Ding, L.; Zhang, K.; Han, J.; Jiang, S. Micro- and nano-fibrils of manau rattan and solvent-exchange-induced high-haze transparent holocellulose nanofibril film. Carbohydr. Polym. 2022, 298, 120075. [Google Scholar] [CrossRef]
- Kengkhetkit, N.; Amornsakchai, T. A new approach to “Greening” plastic composites using pineapple leaf waste for performance and cost effectiveness. Mater. Des. 2014, 55, 292–299. [Google Scholar] [CrossRef]
- Arib, R.M.N.; Sapuan, S.M.; Hamdan, M.A.M.M.; Paridah, M.T.; Zaman, H.M.D.K. A Literature Review of Pineapple Fibre Reinforced Polymer Composites. Polym. Polym. Compos. 2004, 12, 341–348. [Google Scholar] [CrossRef]
- Jain, J.; Sinha, S. Pineapple Leaf Fiber Polymer Composites as a Promising Tool for Sustainable, Eco-friendly Composite Material: Review. J. Nat. Fibers 2021, 19, 10031–10052. [Google Scholar] [CrossRef]
- Joshi, S.; Patel, S. Review on Mechanical and Thermal Properties of Pineapple Leaf Fiber (PALF) Reinforced Composite. J. Nat. Fibers 2021, 19, 10157–10178. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. A review on the extraction of pineapple, sisal and abaca fibers and their use as reinforcement in polymer matrix. Express Polym. Lett. 2020, 14, 309–335. [Google Scholar] [CrossRef]
- Todkar, S.S.; Patil, S.A. Review on mechanical properties evaluation of pineapple leaf fibre (PALF) reinforced polymer composites. Compos. Part B Eng. 2019, 174, 106927. [Google Scholar] [CrossRef]
- Laftah, W.A.; Rahman, W.A.W.A. Pulping Process and the Potential of Using Non-Wood Pineapple Leaves Fiber for Pulp and Paper Production: A Review. J. Nat. Fibers 2015, 13, 85–102. [Google Scholar] [CrossRef]
- Van Tran, A. Chemical analysis and pulping study of pineapple crown leaves. Ind. Crops Prod. 2006, 24, 66–74. [Google Scholar] [CrossRef]
- Prado, K.S.; Spinacé, M.A. Isolation and characterization of cellulose nanocrystals from pineapple crown waste and their potential uses. Int. J. Biol. Macromol. 2018, 122, 410–416. [Google Scholar] [CrossRef] [PubMed]
- De Souza, N.; D’Almeida, J. Tensile, Thermal, Morphological and Structural Characteristics of Abaca (Musa Textiles) Fibers. Polym. Renew. Resour. 2014, 5, 47–60. [Google Scholar] [CrossRef]
- Aseer, J.R.; Sankaranarayanasamy, K.; Jayabalan, P.; Natarajan, R.; Dasan, K.P. Morphological, Physical, and Thermal Properties of Chemically Treated Banana Fiber. J. Nat. Fibers 2013, 10, 365–380. [Google Scholar] [CrossRef]
- Yudhanto, F.; Jamasri; Rochardjo, H.S.B. Application of taguchi method for selection parameter bleaching treatments against mechanical and physical properties of agave cantala fiber. IOP Conf. Series Mater. Sci. Eng. 2018, 352, 012002. [Google Scholar] [CrossRef]
- D’Almeida, A.L.F.S.; Barreto, D.W.; Calado, V.; D’Almeida, J.R.M. Thermal analysis of less common lignocellulose fibers. J. Therm. Anal. Calorim. 2007, 91, 405–408. [Google Scholar] [CrossRef]
- De Rosa, I.; Kenny, J.M.; Puglia, D.; Santulli, C.; Sarasini, F. Tensile behavior of New Zealand flax (Phormium tenax) fibers. J. Reinf. Plast. Compos. 2010, 29, 3450–3454. [Google Scholar] [CrossRef]
- Rwawiire, S.; Tomkova, B. Morphological, thermal, and mechanical characterization of Sansevieria trifasciata fibers. J. Nat. Fibers 2015, 12, 201–210. [Google Scholar] [CrossRef]
- Caraschi, J.C.; Leãto, A.L. Characterization of curaua fiber. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 2000, 353, 149–152. [Google Scholar] [CrossRef]
- Han, S.O.; Jung, Y.M. Characterization of henequen natural fiber by using two-dimensional correlation spectroscopy. J. Mol. Struct. 2008, 883, 142–148. [Google Scholar] [CrossRef]
- Hajiha, H.; Sain, M.; Mei, L.H.I. Modification and Characterization of Hemp and Sisal Fibers. J. Nat. Fibers 2014, 11, 144–168. [Google Scholar] [CrossRef]
- Langhorst, A.; Paxton, W.; Bollin, S.; Frantz, D.; Burkholder, J.; Kiziltas, A.; Mielewski, D. Heat-treated blue agave fiber composites. Compos. Part B Eng. 2019, 165, 712–724. [Google Scholar] [CrossRef]
- Réquilé, S.; Le Duigou, A.; Bourmaud, A.; Baley, C. Peeling experiments for hemp retting characterization targeting biocomposites. Ind. Crops Prod. 2018, 123, 573–580. [Google Scholar] [CrossRef]
- Motaleb, K.Z.M.A.; Islam, S.; Hoque, M.B. Improvement of Physicomechanical Properties of Pineapple Leaf Fiber Reinforced Composite. Int. J. Biomater. 2018, 2018, 7384360. [Google Scholar] [CrossRef]
- Custodio, C.L.; Yang, X.; Wilsby, A.E.; Waller, V.F.; Aquino, R.R.; Tayo, L.L.; Senoro, D.B.; Berglund, L.A. Effect of a Chemical Treatment Series on the Structure and Mechanical Properties of Abaca Fiber (Musa textilis). Mater. Sci. Forum 2020, 1015, 64–69. [Google Scholar] [CrossRef]
- Manimaran, P.; Pillai, G.P.; Vignesh, V.; Prithiviraj, M. Characterization of natural cellulosic fibers from Nendran Banana Peduncle plants. Int. J. Biol. Macromol. 2020, 162, 1807–1815. [Google Scholar] [CrossRef]
- Sreenivasan, V.S.; Somasundaram, S.; Ravindran, D.; Manikandan, V.; Narayanasamy, R. Microstructural, physico-chemical and mechanical characterisation of Sansevieria cylindrica fibres—An exploratory investigation. Mater. Des. 2011, 32, 453–461. [Google Scholar] [CrossRef]
- Mylsamy, K.; Rajendran, I. Investigation on Physio-chemical and Mechanical Properties of Raw and Alkali-treated Agave americana Fiber. J. Reinf. Plast. Compos. 2010, 29, 2925–2935. [Google Scholar] [CrossRef]
- Bekele, A.E.; Lemu, H.G.; Jiru, M.G. Experimental study of physical, chemical and mechanical properties of enset and sisal fibers. Polym. Test. 2021, 106, 107453. [Google Scholar] [CrossRef]
- Yahya, S.A.B.; Yusof, Y. Comprehensive Review on the Utilization of PALF. Adv. Mater. Res. 2013, 701, 430–434. [Google Scholar] [CrossRef]
- Armecin, R.B.; Sinon, F.G.; Moreno, L.O. Abaca Fiber: A Renewable Bio-resource for Industrial Uses and Other Applications. In Biomass and Bioenergy; Springer: Cham, Switzerland, 2014; pp. 107–118. [Google Scholar] [CrossRef]
- Mishra, S.; Mohanty, A.K.; Drzal, L.T.; Misra, M.; Hinrichsen, G. A Review on Pineapple Leaf Fibers, Sisal Fibers and Their Biocomposites. Macromol. Mater. Eng. 2004, 289, 955–974. [Google Scholar] [CrossRef]
- Gebremedhin, N.; Rotich, G.K. Manufacturing of bathroom wall tile composites from recycled low-density polyethylene reinforced with pineapple leaf fiber. Int. J. Polym. Sci. 2020, 2020, 2732571. [Google Scholar] [CrossRef]
- Kanimozhi, M. Investigating the physical characteristics of Sansevieria trifasciata fibre. Int. J. Sci. Res. Pub. 2011, 1, 30–33. [Google Scholar]
- Araujo, J.R.; Mano, B.; Teixeira, G.M.; Spinacé, M.A.S.; De Paoli, M.A. Biomicrofibrilar composites of high density polyethylene reinforced with curauá fibers: Mechanical, interfacial and morphological properties. Comp. Sci. Technol. 2010, 70, 1637–1644. [Google Scholar] [CrossRef]
- Hulle, A.; Kadole, P.; Katkar, P. Agave Americana Leaf Fibers. Fibers 2015, 3, 64–75. [Google Scholar] [CrossRef]
- Gnanasekaran, S.; Li, Y.Y.; Shariffuddin, J.H.; Nordin, N.I.A.A. Production of cellulose and microcellulose from pineapple leaf fibre by chemical-mechanical treatment. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012055. [Google Scholar] [CrossRef]
- Samal, R.K.; Ray, M.C. Effect of chemical modifications on FTIR spectra. II. Physicochemical behavior of pineapple leaf fiber (PALF). J. Appl. Polym. Sci. 1997, 64, 2119–2125. [Google Scholar] [CrossRef]
- Lee, C.H.; Khalina, A.; Lee, S.H.; Padzil, F.N.M.; Ainun, Z.M.A. Physical, morphological, structural, thermal and mechanical properties of pineapple leaf fibers. In Pineapple Leaf Fibers; Jawaid, M., Asim, M., Tahir, M.P., Nasir, M., Eds.; Springer: Singapore, 2020; pp. 91–121. [Google Scholar]
- Das, M.; Chakraborty, D. Influence of alkali treatment on the fine structure and morphology of bamboo fibers. J. Appl. Polym. Sci. 2006, 102, 5050–5056. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Neto, A.R.S.; Araujo, M.A.; Barboza, R.M.; Fonseca, A.S.; Tonoli, G.H.; Souza, F.V.; Mattoso, L.H.; Marconcini, J.M. Comparative study of 12 pineapple leaf fiber varieties for use as mechanical reinforcement in polymer composites. Ind. Crops Prod. 2015, 64, 68–78. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Gaba, E.; Asimeng, B.; Kaufmann, E.; Katu, S.; Foster, E.; Tiburu, E. Mechanical and Structural Characterization of Pineapple Leaf Fiber. Fibers 2021, 9, 51. [Google Scholar] [CrossRef]
- Amirulhakim, H.; Juwono, A.L.; Roseno, S. Isolation and characterization of cellulose nanofiber from subang pineapple leaf fiber waste produced using ultrafine grinding method. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1098, 062067. [Google Scholar] [CrossRef]
- Jalil, M.A.; Moniruzzaman, M.; Parvez, M.S.; Siddika, A.; Gafur, M.A.; Repon, M.R.; Hossain, M.T. A novel approach for pineapple leaf fiber processing as an ultimate fiber using existing machines. Heliyon 2021, 7, e07861. [Google Scholar] [CrossRef]
- Balaji, A.N.; Nagarajan, K.J. Characterization of alkali treated and untreated new cellulosic fiber from Saharan aloe vera cactus leaves. Carbohydr. Polym. 2017, 174, 200–208. [Google Scholar]
- Demosthenes, L.C.D.C.; Nascimento, L.F.C.; Monteiro, S.N.; Costa, U.O.; Filho, F.D.C.G.; da Luz, F.S.; Oliveira, M.S.; Ramos, F.J.H.T.V.; Pereira, A.C.; Braga, F.O. Thermal and structural characterization of buriti fibers and their relevance in fabric reinforced composites. J. Mater. Res. Technol. 2019, 9, 115–123. [Google Scholar] [CrossRef]
- Manimaran, P.; Senthamaraikannan, P.; Sanjay, M.R.; Marichelvam, M.K.; Jawaid, M. Study on characterization of Furcraea foetida new natural fiber as composite reinforcement for lightweight applications. Carbohydr. Polym. 2018, 181, 650–658. [Google Scholar] [CrossRef]
- Thomas, E.N.M.; Holmes, L.E. The development and structure of the seedling and young plant of the pineapple (Ananas sativus). New Phytol. 1930, 29, 199–226. [Google Scholar] [CrossRef]
- Teles, M.C.A.; Glória, G.O.; Altoé, G.R.; Netto, P.A.; Margem, F.M.; Braga, F.O.; Monteiro, S.N. Evaluation of the Diameter Influence on the Tensile Strength of Pineapple Leaf Fibers (PALF) by Weibull Method. Mater. Res. 2015, 18, 185–192. [Google Scholar] [CrossRef]
- Najeeb, M.; Sultan, M.; Andou, Y.; Shah, A.; Eksiler, K.; Jawaid, M.; Ariffin, A. Characterization of silane treated Malaysian Yankee Pineapple AC6 leaf fiber (PALF) towards industrial applications. J. Mater. Res. Technol. 2020, 9, 3128–3139. [Google Scholar] [CrossRef]
- Shanmugasundaram, N.; Rajendran, I.; Ramkumar, T. Characterization of untreated and alkali treated new cellulosic fiber from an Areca palm leaf stalk as potential reinforcement in polymer composites. Carbohydr. Polym. 2018, 195, 566–575. [Google Scholar]
- Manimaran, P.; Saravanan, S.; Sanjay, M.; Siengchin, S.; Jawaid, M.; Khan, A. Characterization of new cellulosic fiber: Dracaena reflexa as a reinforcement for polymer composite structures. J. Mater. Res. Technol. 2019, 8, 1952–1963. [Google Scholar] [CrossRef]
- Rathinavelu, R.; Paramathma, B.S. Comprehensive characterization of Echinochloa frumentacea leaf fiber as a novel reinforcement for composite applications. Polym. Compos. 2022, 43, 5031–5046. [Google Scholar] [CrossRef]
- Huda, M.S.; Drzal, L.T.; Mohanty, A.K.; Misra, M. Effect of chemical modifications of the pineapple leaf fiber surfaces on the interfacial and mechanical properties of laminated biocomposites. Compos. Interfaces 2008, 15, 169–191. [Google Scholar] [CrossRef]
- Atalie, D.; Gideon, R.K. Extraction and characterization of Ethiopian palm leaf fibers. Res. J. Text. Appar. 2018, 22, 15–25. [Google Scholar] [CrossRef]
- Gowda, N.K.S.; Vallesha, N.C.; Awachat, V.B.; Anandan, S.; Pal, D.T.; Prasad, C.S. Study on evaluation of silage from pineapple (Ananas comosus) fruit residue as livestock feed. Trop. Anim. Health Prod. 2015, 47, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Meena, L.; Sengar, A.S.; Neog, R.; Sunil, C.K. Pineapple processing waste (PPW): Bioactive compounds, their extraction, and utilisation: A review. J. Food Sci. Technol. 2021, 59, 4152–4164. [Google Scholar] [CrossRef]
- Ketnawa, S.; Chaiwut, P.; Rawdkuen, S. Pineapple wastes: A potential source for bromelain extraction. Food Bioprod. Process. 2012, 90, 385–391. [Google Scholar] [CrossRef]
- Rabiu, Z.; Maigari, F.U.; Lawan, U.; Mukhtar, Z.G. Pineapple waste utilization as a sustainable means of waste management. In Sustainable Technologies for the Management of Agricultural Wastes; Zakaria, Z.A., Ed.; Springer: Singapore, 2018; pp. 143–154. [Google Scholar]
- Lopattananon, N.; Panawarangkul, K.; Sahakaro, K.; Ellis, B. Performance of pineapple leaf fiber–natural rubber composites: The effect of fiber surface treatments. J. Appl. Polym. Sci. 2006, 102, 1974–1984. [Google Scholar] [CrossRef]
- Hariwongsanupab, N.; Thanawan, S.; Amornsakchai, T.; Vallat, M.-F.; Mougin, K. Improving the mechanical properties of short pineapple leaf fiber reinforced natural rubber by blending with acrylonitrile butadiene rubber. Polym. Test. 2017, 57, 94–100. [Google Scholar] [CrossRef]
- Chollakup, R.; Tantatherdtam, R.; Ujjin, S.; Sriroth, K. Pineapple leaf fiber reinforced thermoplastic composites: Effects of fiber length and fiber content on their characteristics. J. Appl. Polym. Sci. 2010, 119, 1952–1960. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Ornaghi, H.L., Jr.; Arantes, V.; Cioffi, M.O.H. Effect of chemical treatment of pineapple crown fiber in the production, chemical composition, crystalline structure, thermal stability and thermal degradation kinetic properties of cellulosic materials. Carbohydr. Res. 2021, 499, 108227. [Google Scholar] [CrossRef]
- Neto, A.R.S.; Araujo, M.A.; Souza, F.V.; Mattoso, L.H.; Marconcini, J.M. Characterization and comparative evaluation of thermal, structural, chemical, mechanical and morphological properties of six pineapple leaf fiber varieties for use in composites. Ind. Crops Prod. 2013, 43, 529–537. [Google Scholar] [CrossRef]
- Saloni, S.; Chauhan, K.; Tiwari, S. Pineapple production and processing in north eastern India. J. Pharmacogn. Phytochem. 2017, 6, 665–672. [Google Scholar]

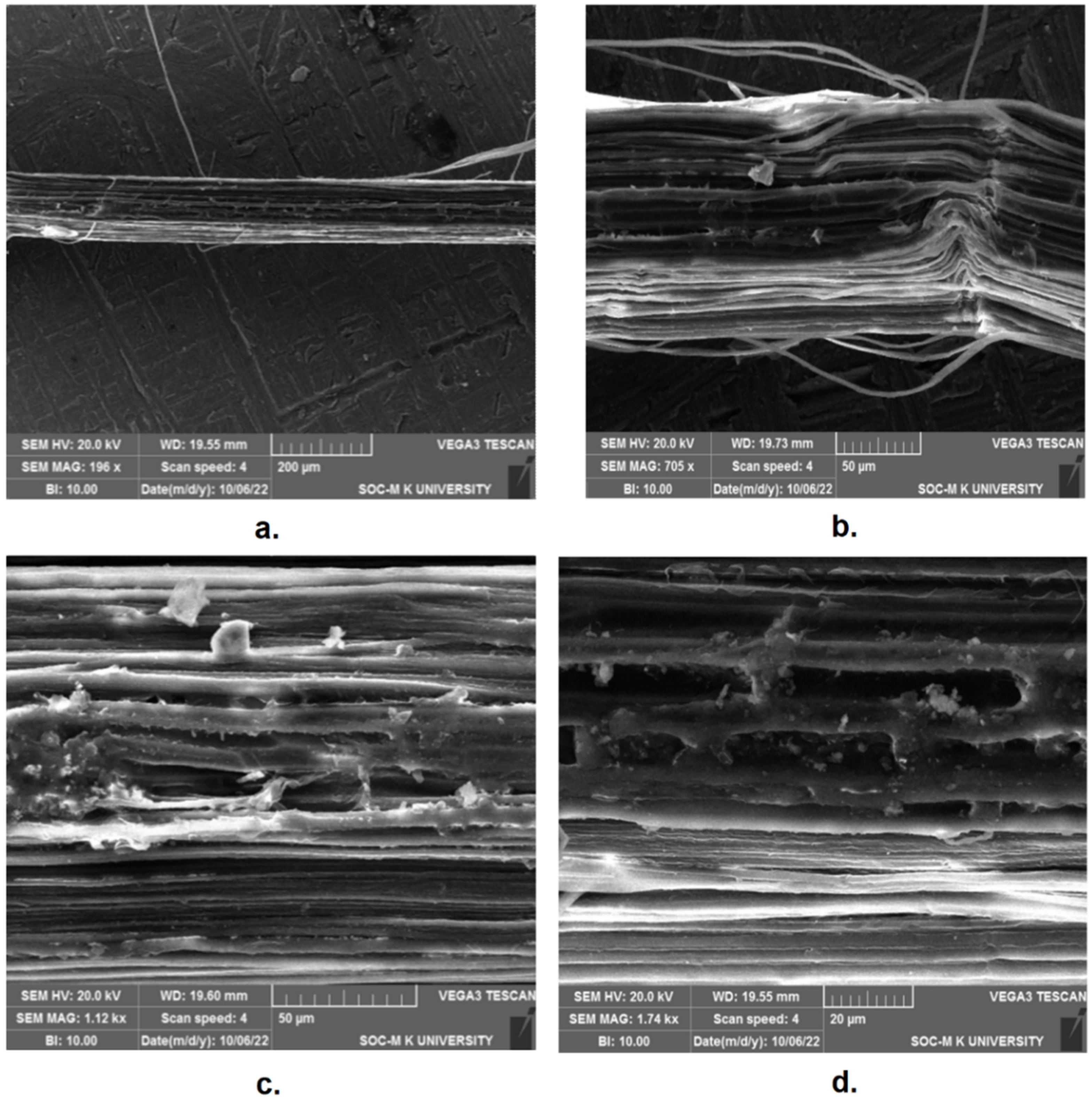
| Fiber | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Wax (%) | Pectin (%) | Ash (%) | Ref. |
|---|---|---|---|---|---|---|---|
| PCLF | 67.3 | 16.9 | 7.4 | 3.8 | 1.3 | 0.8 | - |
| Abaca | 58.3 | 18.3 | 8.3 | 8.3 | 2.8 | - | [35] |
| Banana peduncle | 73.2 | 10.8 | 15.3 | 0.2 | - | 2.6 | [36] |
| Sansevieria cylindrica | 79.7 | 10.1 | 3.8 | 0.1 | - | - | [37] |
| Curaua | 73.6 | 9.9 | 7.5 | - | - | 0.9 | [29] |
| Agave americana | 68.4 | 15.7 | 4.9 | 0.3 | - | - | [38] |
| Sisal | 73.4 | 10 | 8 | 1.1 | 1.5 | [39] | |
| PALF | 68.5 | 18.8 | 6 | 3.2 | 1.1 | 0.9 | [40] |
| Fiber | Diameter (µm) | Length (mm) | Aspect Ratio (×103) | Density (ρ) (kg/m3) | Ref. |
|---|---|---|---|---|---|
| PCLF | 108 | 155 | 1.43 | 1273 | This study |
| PALF | 59.7 | 300.5 | 5.03 | 1440 [42] | [43] |
| Sansevieria trifasciata | 120 | 1090 | 9.83 | 1415 | [44] |
| Curaua | 65 | 1250 | 19.23 | 1100 | [45] |
| Agave americana | 218 | 652 | 2.99 | 1360 | [46] |
| Fiber | Peak Cellulose (101) (Deg) | Peak Cellulose (002) (Deg) | Crystallinity Index (%) | Ref. |
|---|---|---|---|---|
| PCLF | 14.8 | 22.4 | 75.9 | This study |
| PALF | 15.1 | 22 | 76 | [54] |
| Subang PALF | 15.6 | 22.5 | 75 | [55] |
| Queen PALF | 15.5 | 22.9 | 52.2 | [56] |
| Saharan aloe vera | - | 22.6 | 52.6 | [57] |
| Buriti (Mauritia flexuosa) | - | 21.7 | 63.1 | [58] |
| Furcrea foetida | 15 | 22.6 | 52.6 | [59] |
| Parameter | Definition | Value |
|---|---|---|
| Rp | Maximum peak height of the roughness profile | 6.996 µm |
| Rv | Maximum valley depth of the roughness profile | 3.183 µm |
| Rz | Maximum height of the roughness profile | 10.179 µm |
| Rc | Mean height of the maximum profile elements | 7.122 µm |
| Rt | Total height of the roughness profile | 19.129 µm |
| Ra | Arithmetic mean deviation of the roughness profile | 1.344 µm |
| Rq | Root mean square (RMS) deviation of the roughness profile | 1.877 µm |
| Rsk | Skewness of the roughness profile | 2.676 |
| Rku | Kurtosis of the roughness profile | 20.468 |
| Rmr | Relative material ratio of the roughness profile | 0.392% |
| Rdc | Roughness profile section height difference | 1.989 µm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johny, V.; Kuriakose Mani, A.; Palanisamy, S.; Rajan, V.K.; Palaniappan, M.; Santulli, C. Extraction and Physico-Chemical Characterization of Pineapple Crown Leaf Fibers (PCLF). Fibers 2023, 11, 5. https://doi.org/10.3390/fib11010005
Johny V, Kuriakose Mani A, Palanisamy S, Rajan VK, Palaniappan M, Santulli C. Extraction and Physico-Chemical Characterization of Pineapple Crown Leaf Fibers (PCLF). Fibers. 2023; 11(1):5. https://doi.org/10.3390/fib11010005
Chicago/Turabian StyleJohny, Vivek, Ajith Kuriakose Mani, Sivasubramanian Palanisamy, Visakh Kunnathuparambil Rajan, Murugesan Palaniappan, and Carlo Santulli. 2023. "Extraction and Physico-Chemical Characterization of Pineapple Crown Leaf Fibers (PCLF)" Fibers 11, no. 1: 5. https://doi.org/10.3390/fib11010005
APA StyleJohny, V., Kuriakose Mani, A., Palanisamy, S., Rajan, V. K., Palaniappan, M., & Santulli, C. (2023). Extraction and Physico-Chemical Characterization of Pineapple Crown Leaf Fibers (PCLF). Fibers, 11(1), 5. https://doi.org/10.3390/fib11010005








